Influence of Covalent Element B and Si Addition on Magnetocaloric Properties of Gd-Co-Fe-(B,Si) Amorphous Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
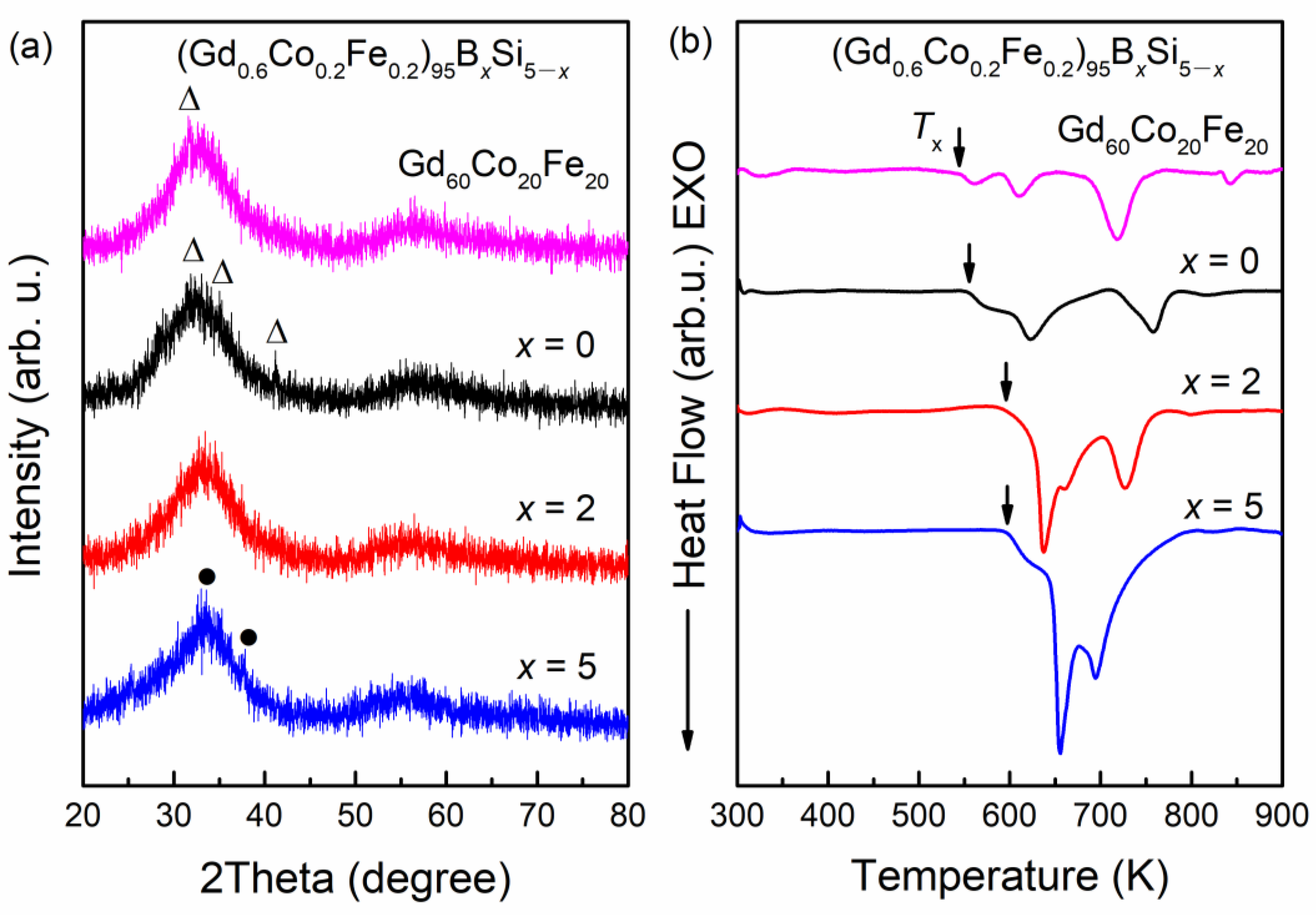
3.1. Structural and Thermal Characterization
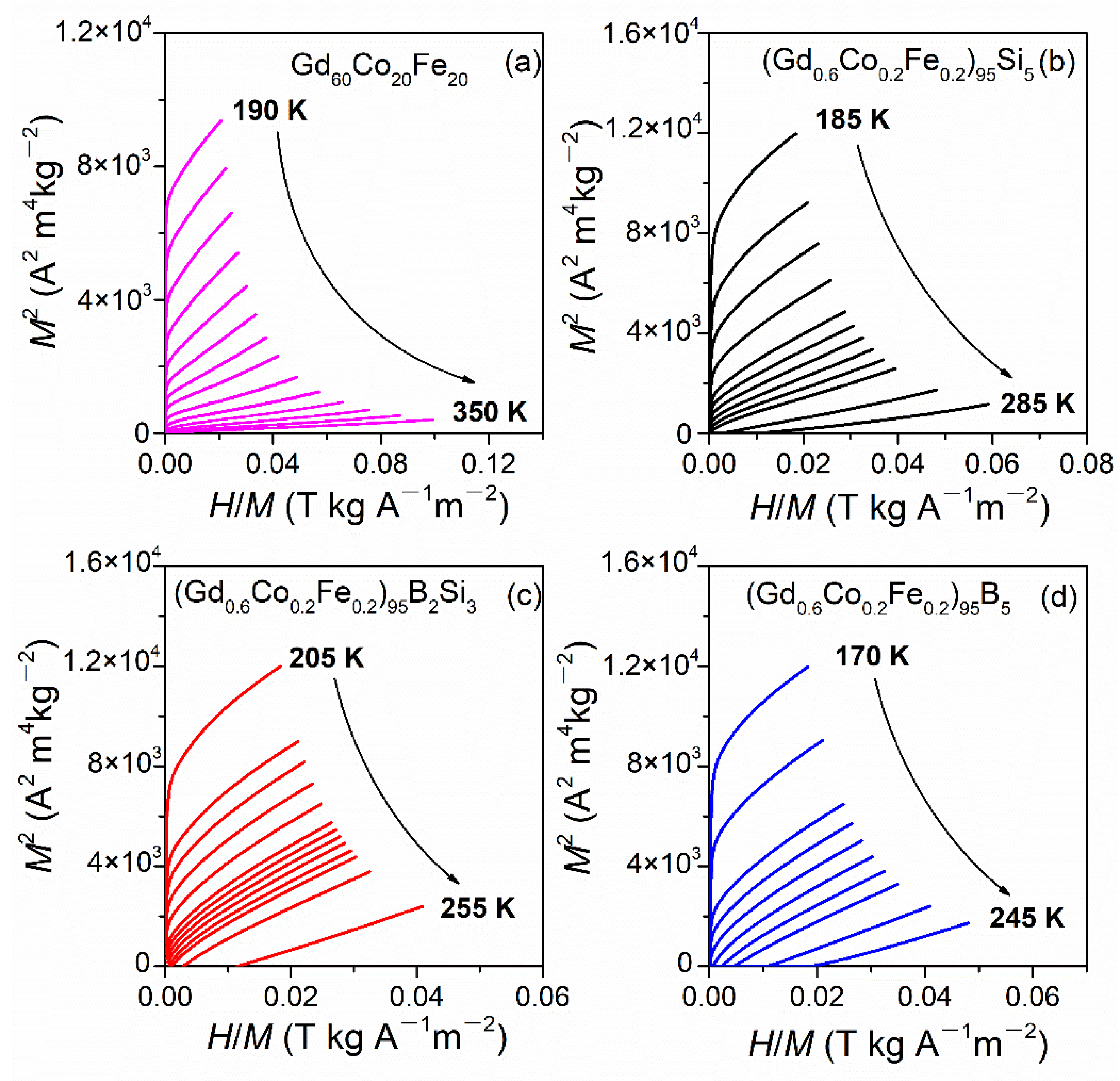
3.2. Magnetic Properties
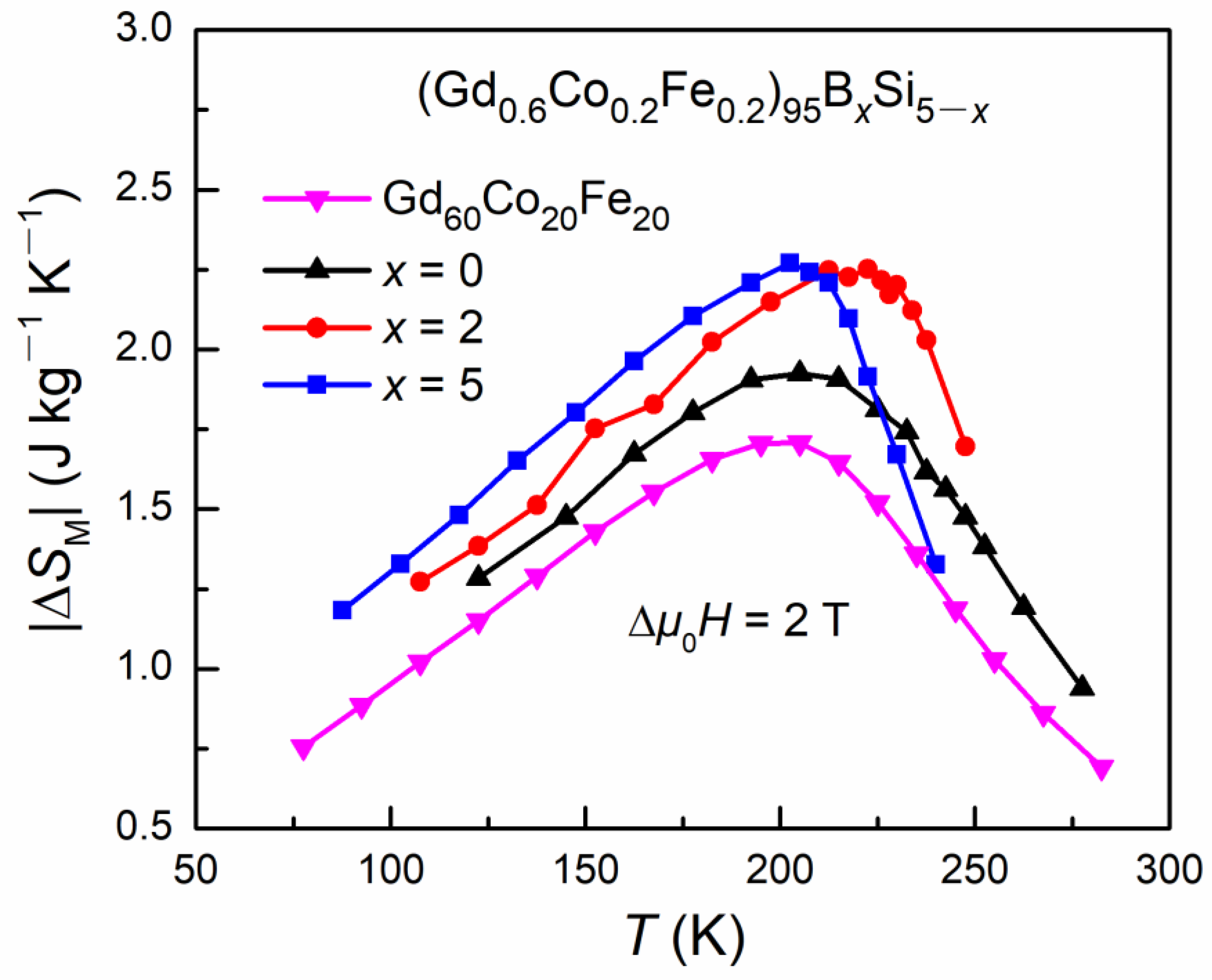
3.3. Magnetocaloric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco, V.; Blazquez, J.S.; Ipus, J.J.; Law, J.Y.; Moreno-Ramirez, L.M.; Conde, A. Magnetocaloric effect: From materials research to refrigeration devices. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Kitanovski, A. Energy applications of magnetocaloric materials. Adv. Energy Mater. 2020, 10, 1903741. [Google Scholar] [CrossRef]
- Gottschall, T.; Skokov, K.P.; Fries, M.; Taubel, A.; Radulov, I.; Scheibel, F.; Benke, D.; Riegg, S.; Gutfleisch, O. Making a cool choice: The materials library of magnetic refrigeration. Adv. Energy Mater. 2019, 9, 1901322. [Google Scholar] [CrossRef] [Green Version]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 1997, 78, 4494–4497. [Google Scholar] [CrossRef]
- Hu, S.Y.; Miao, X.F.; Liu, J.; Ou, Z.Q.; Cong, M.Q.; Haschuluu, O.; Gong, Y.Y.; Qian, F.J.; You, Y.R.; Zhang, Y.J.; et al. Small hysteresis and giant magnetocaloric effect in Nb-substituted (Mn, Fe)2(P,Si) alloys. Intermetallics 2019, 114, 106602. [Google Scholar] [CrossRef]
- Moreno-Ramirez, L.M.; Diaz-Garcia, A.; Law, J.Y.; Giri, A.K.; Franco, V. Hysteresis, latent heat and cycling effects on the magnetocaloric response of (NiMnSi)0.66(Fe2Ge)0.34 alloy. Intermetallics 2021, 131, 107083. [Google Scholar] [CrossRef]
- Morrison, K.; Sandeman, K.G.; Cohen, L.F.; Sasso, C.P.; Basso, V.; Barcza, A.; Katter, M.; Moore, J.D.; Skokov, K.P.; Gutfleisch, O. Evaluation of the reliability of the measurement of key magnetocaloric properties: A round robin study of La(Fe,Si,Mn)Hδ conducted by the SSEEC consortium of European laboratories. Int. J. Refrig. 2012, 35, 1528–1536. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Xie, L.; Liu, C.; Li, Q.; Huo, J.; Chang, C.; Li, H.; Ma, X. Effect of Co/Ni substituting Fe on magnetocaloric properties of Fe-based bulk metallic glasses. Metals 2021, 11, 950. [Google Scholar] [CrossRef]
- Huang, L.W.; Tang, B.Z.; Ding, D.; Wang, X.; Xia, L. Achieving high adiabatic temperature change at room temperature in a Gd48Co50Fe2 amorphous alloy. J. Alloys Compd. 2019, 811, 152003. [Google Scholar] [CrossRef]
- Tang, B.Z.; Xie, H.X.; Li, D.M.; Xia, L.; Yu, P. Microstructure and its effect on magnetic and magnetocaloric properties of the Co50Gd50−xFex glassy ribbons. J. Non-Cryst. Solids 2020, 533, 119935. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhao, D.Q.; Bai, H.Y.; Wang, W.H.; Pan, M.X. Room temperature table-like magnetocaloric effect in amorphous Gd50Co45Fe5 ribbon. J. Phys. D Appl. Phys. 2016, 49, 055004. [Google Scholar] [CrossRef]
- Tang, B.Z.; Guo, D.Q.; Ding, D.; Xia, L.; Chan, K.C. Large adiabatic temperature rise above the water ice point of a minor Fe substituted Gd50Co50 amorphous alloy. J. Non-Cryst. Solids 2017, 464, 30–33. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Ouyang, J.T.; Ding, D.; Li, H.L.; Wang, J.G.; Li, W.H. Influence of Fe substitution on thermal stability and magnetocaloric effect of Gd60Co40−xFex amorphous alloy. J. Alloys Compd. 2018, 769, 186–192. [Google Scholar] [CrossRef]
- Duc, N.T.M.; Shen, H.X.; Clements, E.M.; Thiabgoh, O.; Sanchez Llamazares, J.L.; Sanchez-Valdes, C.F.; Huong, N.T.; Sun, J.F.; Srikanth, H.; Phan, M.H. Enhanced refrigerant capacity and Curie temperature of amorphous Gd60Fe20Al20 microwires. J. Alloys Compd. 2019, 807, 151694. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Q.; Shen, B.L. The effect of Fe/Al ratio on the thermal stability and magnetocaloric effect of Gd55FexAl45−x (x = 15–35) glassy ribbons. J. Appl. Phys. 2012, 111, 07A937. [Google Scholar] [CrossRef]
- Yano, K.; Kita, E. Effect of covalent element boron on exchange interaction and magnetic moment in Fe0.4Gd0.6 binary alloy. J. Magn. Magn. Mater. 2004, 272, 1370–1371. [Google Scholar] [CrossRef]
- Krenke, T.; Duman, E.; Acet, M.; Wassermann, E.F.; Moya, X.; Manosa, L.; Planes, A. Inverse magnetocaloric effect in ferromagnetic Ni-Mn-Sn alloys. Nat. Mater. 2005, 4, 450–454. [Google Scholar] [CrossRef] [Green Version]
- McMichael, R.D.; Ritter, J.J.; Shull, R.D. Enhanced magnetocaloric effect in Gd3Ga5−xFexO12. J. Appl. Phys. 1993, 73, 6946–6948. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Z.; Tan, J.; Zhang, X.; Xu, Y.; Zhang, H.; Li, W. Observation of a Broadened Magnetocaloric Effect in Partially Crystallized Gd60Co40 Amorphous Alloy. Metals 2021, 11, 1741. [Google Scholar] [CrossRef]
- Son, H.; Yoo, G.; Mustaghfiroh, Q.; Kim, D.H.; Choi-Yim, H. Effect of Substituting Hf for Zr on Fe-Co-M-Nb-B (M = Zr, Hf) Amorphous Alloys with High Saturation Magnetization. Metals 2022, 12, 12. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses, 1st ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 188–214. [Google Scholar]
- Yano, K.; Akiyama, Y.; Tokumitsu, K.; Kita, E.; Ino, H. Magnetic moment and Curie temperature for amorphous Fe100−XGdX alloys (18 ≦ X ≦ 60). J. Magn. Magn. Mater. 2000, 214, 217–224. [Google Scholar] [CrossRef]
- Yano, K. Molecular field analysis for melt-spun amorphous Fe100−xGdx alloys (18 ≦ X ≦ 60). J. Magn. Magn. Mater. 2000, 208, 207–216. [Google Scholar] [CrossRef]
- Schwarz, B.; Podmilsak, B.; Mattern, N.; Eckert, J. Magnetocaloric effect in Gd-based Gd60FexCo30−xAl10 metallic glasses. J. Magn. Magn. Mater. 2010, 322, 2298–2303. [Google Scholar] [CrossRef]
- Du, Y.S.; Zhang, C.H.; Lu, Y.M.; Li, L.; Li, J.Q.; Ma, L.; Rao, G.H. Table-like magnetocaloric effect and large refrigerant capacity in Nd6Fe13Pd1−xAgx compounds. Intermetallics 2021, 130, 107062. [Google Scholar] [CrossRef]
- Yu, P.; Chen, L.S.; Xia, L. Phase separation and its effect on the magnetic entropy change profile in an amorphous Gd48Co50Nb2 alloy. J. Non-Cryst. Solids 2018, 493, 82–85. [Google Scholar] [CrossRef]
- Wang, Y.F.; Qin, F.X.; Luo, Y.; Wang, H.; Peng, H.X. Tuning of magnetocaloric effect and optimization of scaling factor for Gd55Ni10Co35 amorphous microwires. J. Alloys Compd. 2018, 761, 1–7. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, Z.; Peng, G.; Feng, T. Near room-temperature magnetocaloric effect in amorphous Fe-Sc alloys: The effect of minor Co additions. J. Non-Cryst. Solids 2019, 505, 211–214. [Google Scholar] [CrossRef]
- Alouhmy, M.; Moubah, R.; Alouhmy, G.; Abid, M.; Lassri, H. Effects of hydrogen implantation on the magnetocaloric properties of amorphous FeZr films. Vacuum 2021, 186, 110063. [Google Scholar] [CrossRef]
- Shishkin, D.A.; Gazizov, A.I.; Volegov, A.S.; Gaviko, V.S.; Baranov, N.V. Magnetic properties and magnetocaloric effect of melt-spun Gd75(Co1−xFex)25 alloys. J. Non-Cryst. Solids 2017, 478, 12–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, Q.; Wang, F.; Zhang, H.; Zhou, Y.; Xia, A.; Li, H.; Chen, S.; Li, W. Tailorable magnetocaloric effect by Fe substitution in Gd-(Co, Fe) amorphous alloy. Intermetallics 2019, 111, 106500. [Google Scholar] [CrossRef]
- Wu, C.; Ding, D.; Xia, L.; Chan, K.C. Achieving tailorable magneto-caloric effect in the Gd-Co binary amorphous alloys. AIP Adv. 2016, 6, 035302. [Google Scholar] [CrossRef] [Green Version]
- Qin, F.X.; Bingham, N.S.; Wang, H.; Peng, H.X.; Sun, J.F.; Franco, V.; Yu, S.C.; Srikanth, H.; Phan, M.H. Mechanical and magnetocaloric properties of Gd-based amorphous microwires fabricated by melt-extraction. Acta Mater. 2013, 61, 1284–1293. [Google Scholar] [CrossRef]
- Oesterreicher, H.; Parker, F.T. Magnetic cooling near Curie temperatures above 300 K. J. Appl. Phys. 1984, 55, 4334–4338. [Google Scholar] [CrossRef]
- Franco, V.; Conde, A.; Kuz’min, M.D.; Romero-Enrique, J.M. The magnetocaloric effect in materials with a second order phase transition: Are TC and Tpeak necessarily coincident? J. Appl. Phys. 2009, 105, 07A917. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Conde, A. Field dependence of the magnetocaloric effect in materials with a second order phase transition: A master curve for the magnetic entropy change. Appl. Phys. Lett. 2006, 89, 222512. [Google Scholar] [CrossRef]
- Duc, N.T.M.; Shen, H.X.; Clements, E.; Thiabgoh, O.; Llamazares, J.L.S.; Sanchez-Valdes, C.F.; Huong, N.T.; Sun, J.F.; Srikanth, H.; Phan, M.H. Critical magnetic and magnetocaloric behavior of amorphous melt-extracted Gd50(Co69.25Fe4.25Si13B13.5)50 microwires. Intermetallics 2019, 110, 106479. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Xu, T.; Liu, F.; Zhang, T. Near room-temperature magnetocaloric effect in FeMnPBC metallic glasses with tunable Curie temperature. J. Magn. Magn. Mater. 2013, 347, 131–135. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Zhong, X.C.; Liu, Z.W.; Zeng, D.C.; Franco, V.; Zhang, J.L. Magnetocaloric effect and critical behavior of amorphous (Gd4Co3)1−xSix alloys. J. Magn. Magn. Mater. 2013, 343, 184–188. [Google Scholar] [CrossRef]




| Alloys | Structure | TC (K) | Tpk (K) | |ΔSMpk| (J kg−1 K−1) | ΔTFWHM (K) | RCP (J kg−1) | Reference |
|---|---|---|---|---|---|---|---|
| μ0H = 0.001 T | μ0H = 2 T | ||||||
| (Gd0.6Co0.2Fe0.2)95B5 | A | 212 | 202.5 | 2.27 | 162 | 367.7 | This work |
| (Gd0.6Co0.2Fe0.2)95B2Si3 | A | 230 | 222.5 | 2.25 | 176 | 396.0 | This work |
| (Gd0.6Co0.2Fe0.2)95Si5 | A | 256 | 205 | 1.92 | 190 | 364.8 | This work |
| Gd60Co20Fe20 | A | 338 | 205 | 1.71 | 178 | 304.4 | This work |
| Gd48Co50Nb2 | A | 220/264 | ~220 | 1.7 | 145 | ~246.5 | [26] |
| Gd55Ni10Co35 (533 K annealing) | A + C | 212 | 212 | 2.03 | 148 | ~300 | [27] |
| Fe89Co1Sc10 | A | 196 | 210 | 1.24 | 182 | 225.7 | [28] |
| (Fe93Zr7)89H11 | A | 200 | ~215 | 1.008 | 145.6 | 146.75 | [29] |
| Gd75(Fe0.75Co0.25)25 | A + C | 223 | ~220 | 2.0 | - | - | [30] |
| Gd60Co30Fe10 | A | 239 | 222.5 | 2.08 | 154 | ~320 | [31] |
| Gd60Co35Fe5 | A | 222 | 222.5 | 2.91 | 104 | ~303 | [31] |
| Gd56Co44 | A | 216 | ~215 | 3.34 | 85 | ~284 | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, Y.; Zhang, Z.; Tan, J.; Zhang, X.; Peng, H.; Xiang, X.; Li, H.; Xia, A.; Li, W. Influence of Covalent Element B and Si Addition on Magnetocaloric Properties of Gd-Co-Fe-(B,Si) Amorphous Alloys. Metals 2022, 12, 386. https://doi.org/10.3390/met12030386
Zhang H, Xu Y, Zhang Z, Tan J, Zhang X, Peng H, Xiang X, Li H, Xia A, Li W. Influence of Covalent Element B and Si Addition on Magnetocaloric Properties of Gd-Co-Fe-(B,Si) Amorphous Alloys. Metals. 2022; 12(3):386. https://doi.org/10.3390/met12030386
Chicago/Turabian StyleZhang, Huiyan, Yafang Xu, Ziyang Zhang, Jia Tan, Xue Zhang, Hui Peng, Xinji Xiang, Hailing Li, Ailin Xia, and Weihuo Li. 2022. "Influence of Covalent Element B and Si Addition on Magnetocaloric Properties of Gd-Co-Fe-(B,Si) Amorphous Alloys" Metals 12, no. 3: 386. https://doi.org/10.3390/met12030386






