An Accurate Model of the Corrosion Current Density of Coatings Using an Adaptive Network-Based Fuzzy Inference System
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Synthesis of FGF
2.3. Composite Preparation
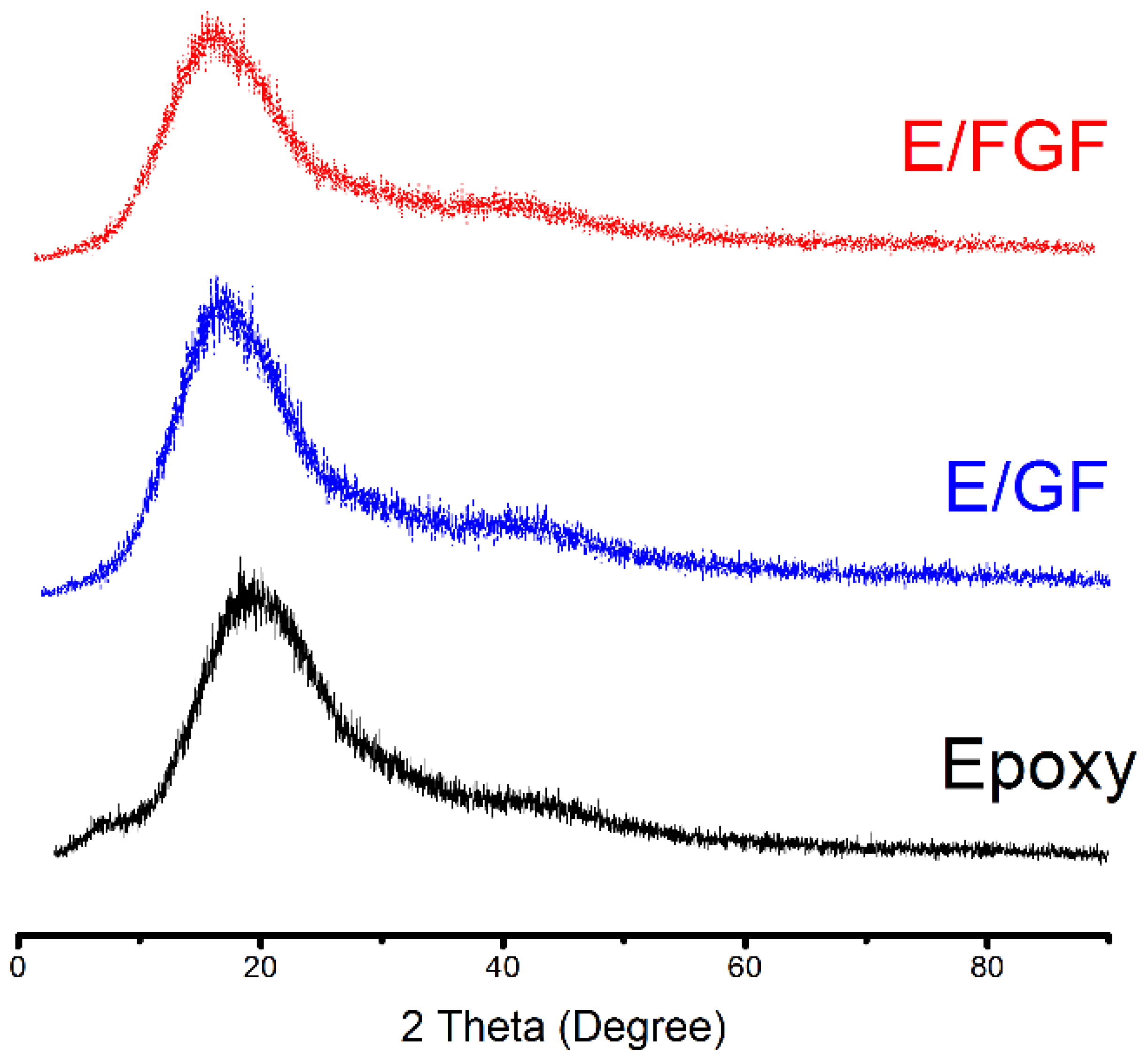
2.4. Characterization
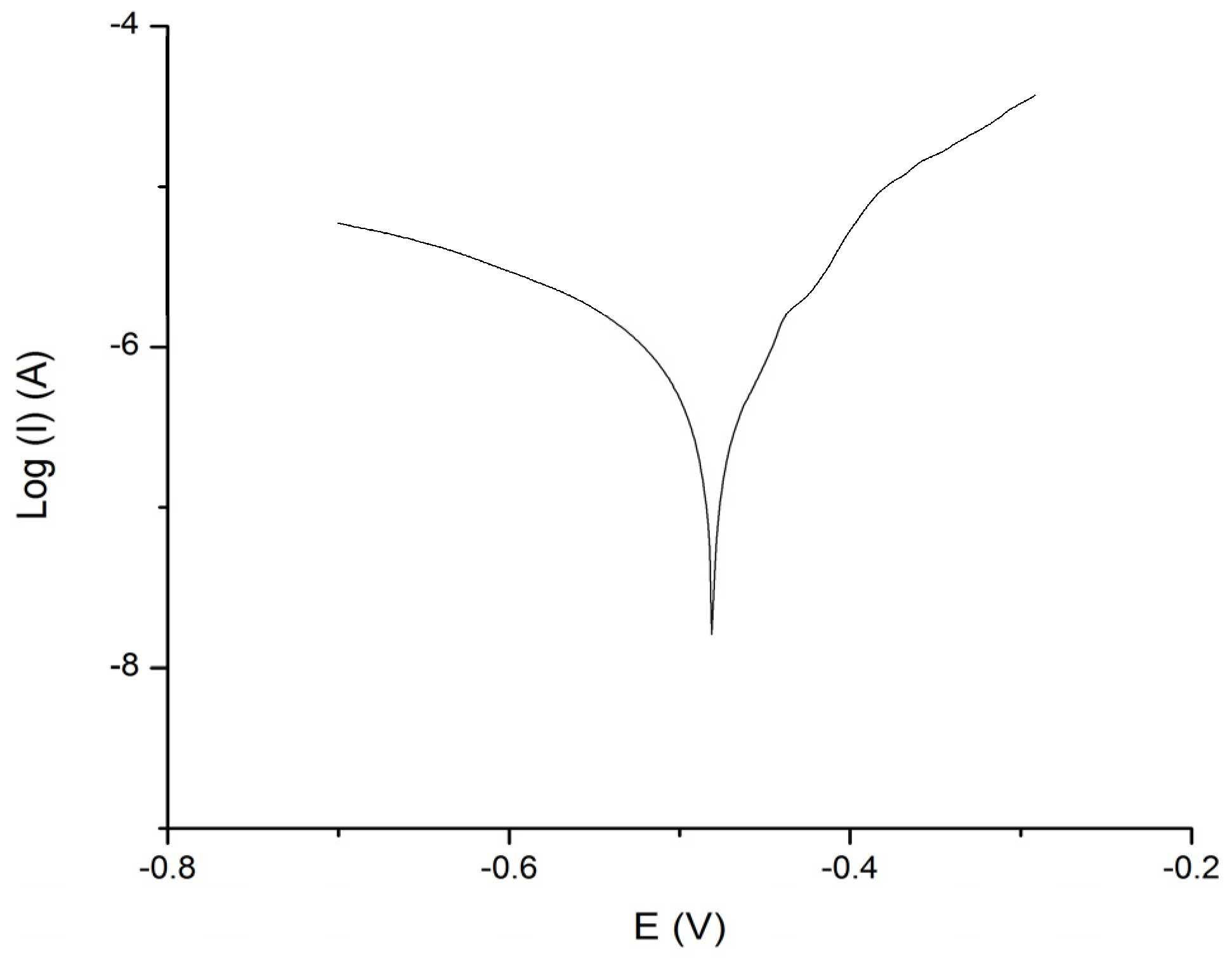
2.5. Polarization Measurements
3. Proposed Modelling of Corrosion Current Density
3.1. ANOVA Model of Corrosion Current Density
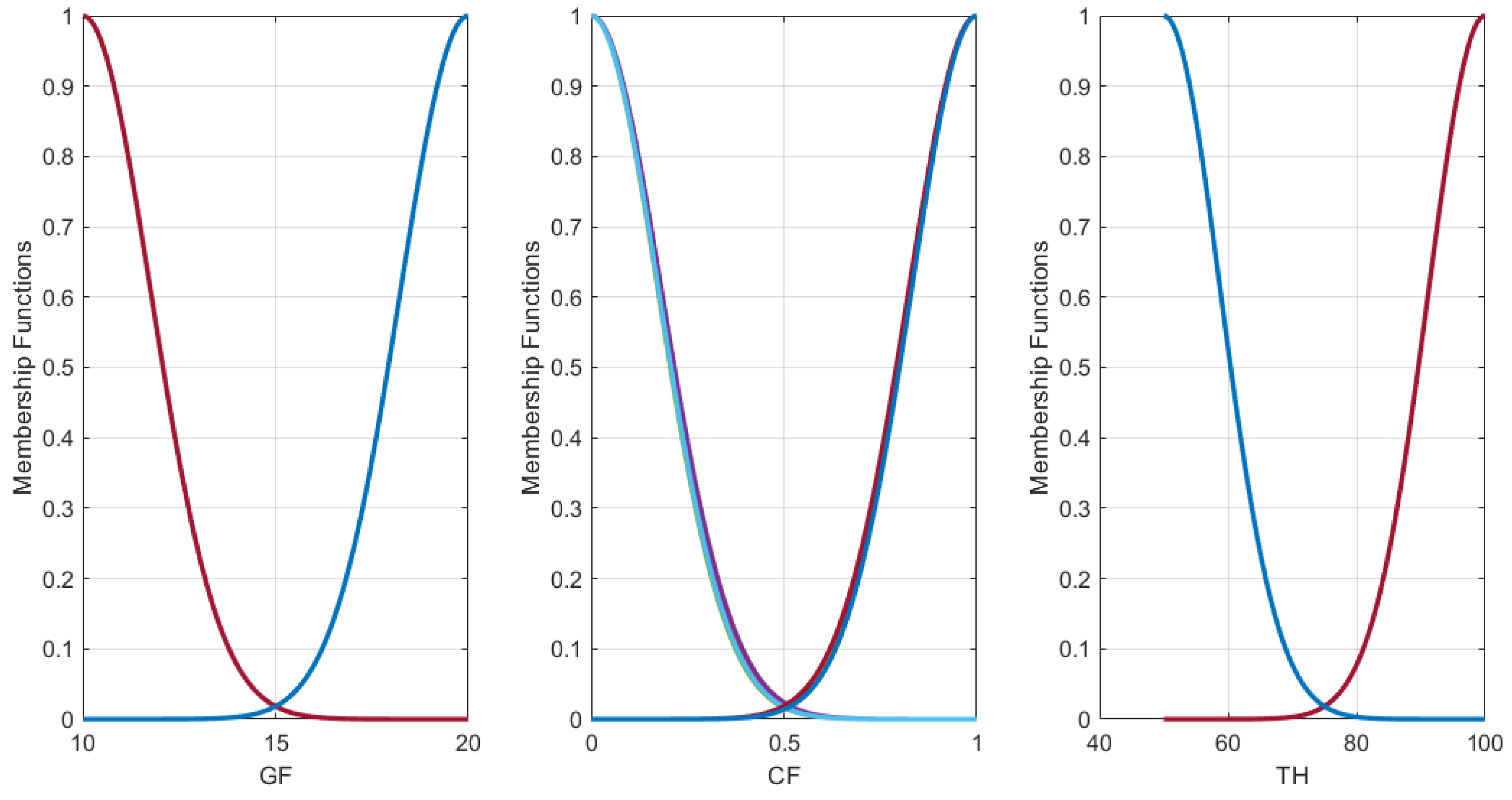
3.2. ANFIS Model of Corrosion Protection System
4. Results and Discussion
4.1. Experimental Results
4.2. ANOVA-Based Modelling Results
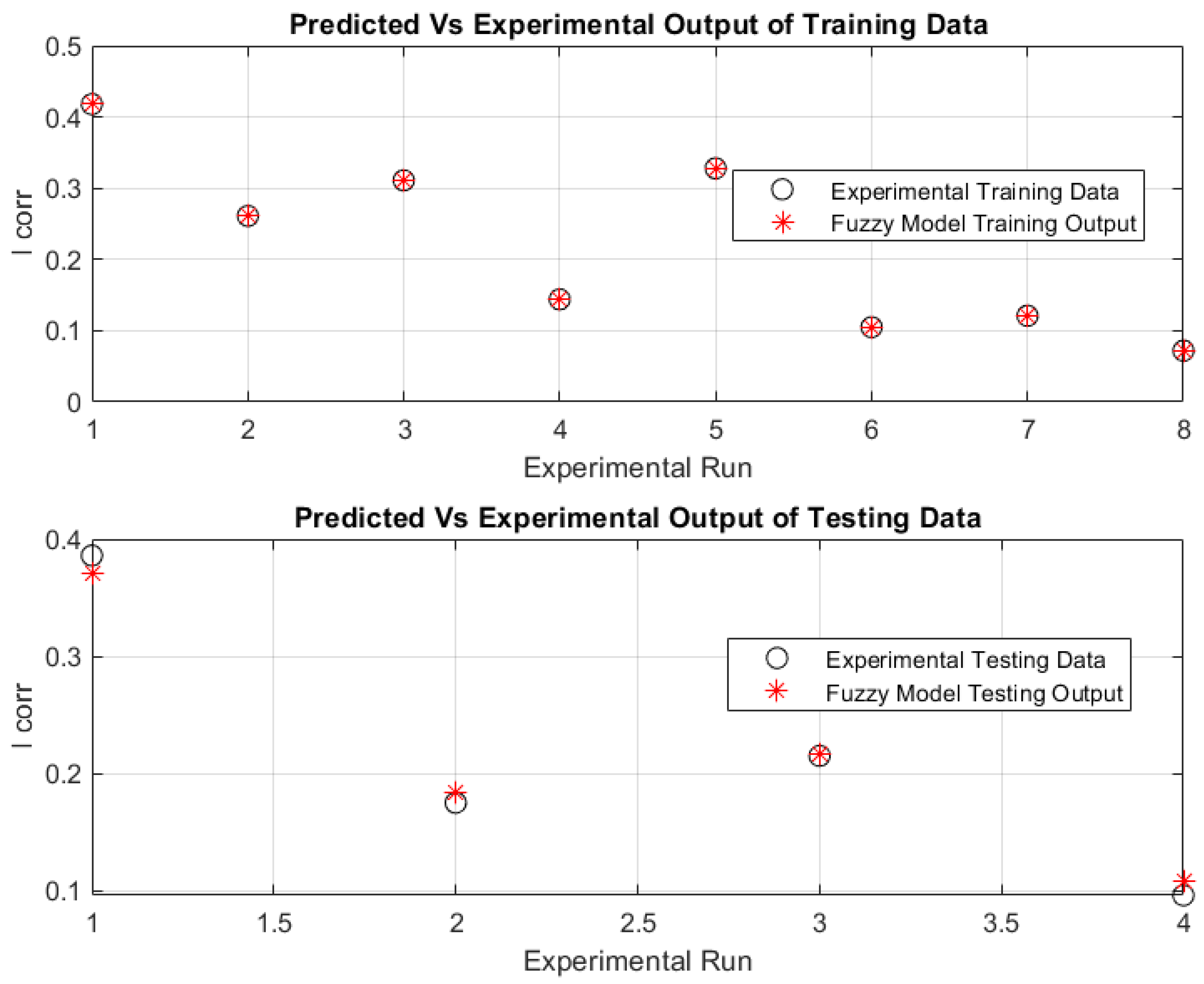
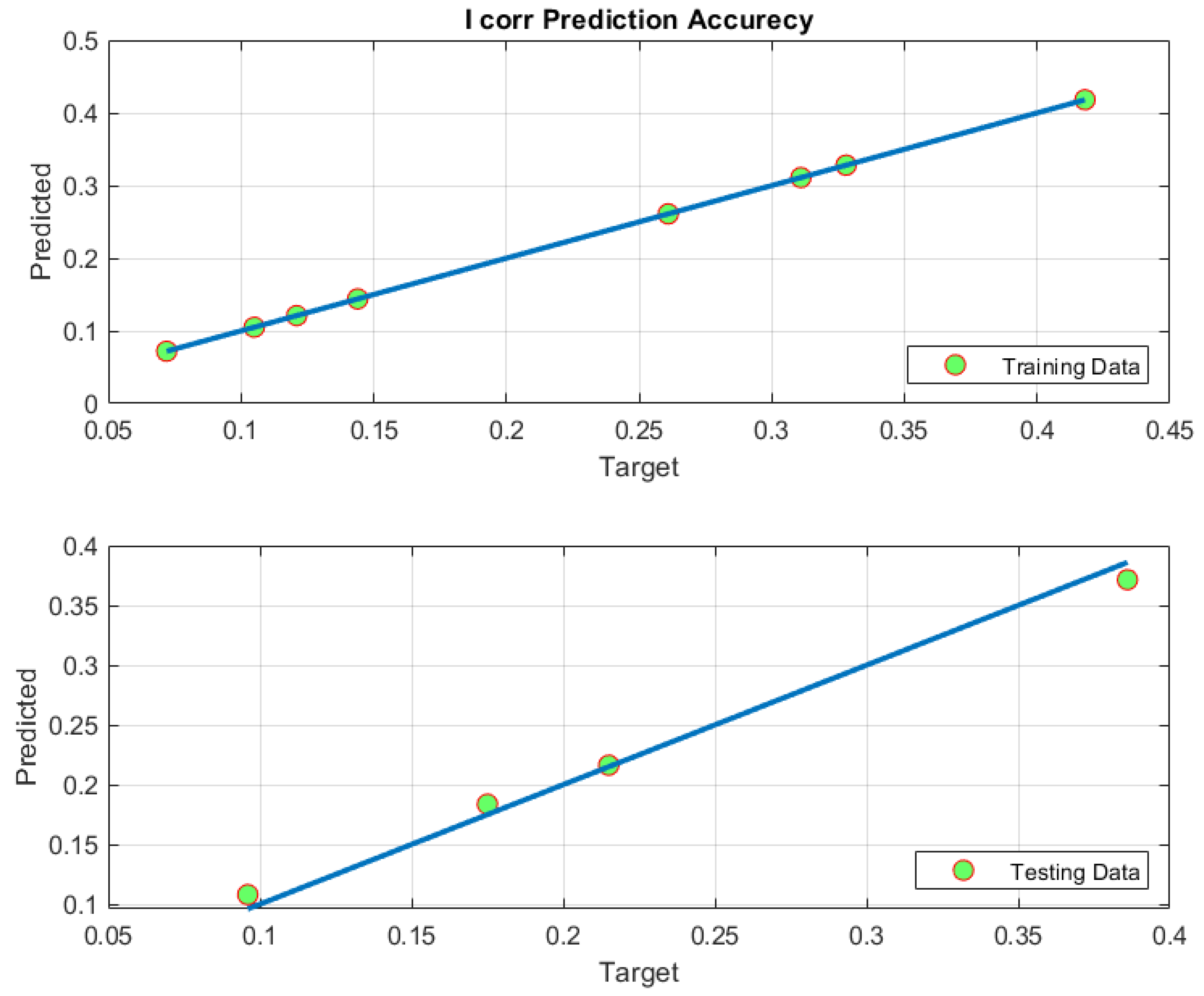
4.3. ANFIS-Based Modelling Results
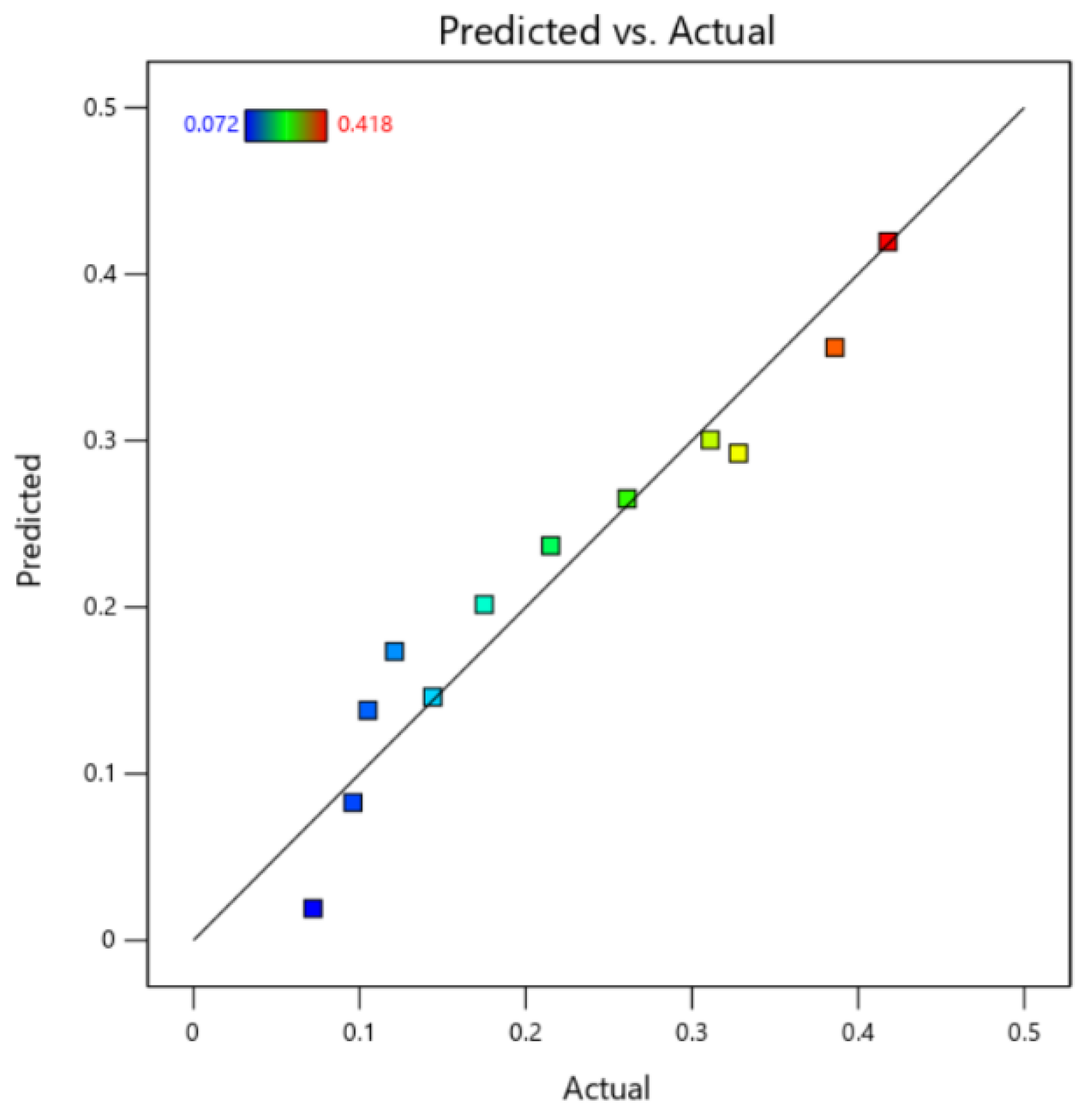
4.4. Model Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casanova, L.; La Padula, M.; Pedeferri, M.; Diamanti, M.V.; Ormellese, M. An insight into the evolution of corrosion resistant coatings on titanium during bipolar plasma electrolytic oxidation in sulfuric acid. Electrochim. Acta 2021, 379, 138190. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kim, M.J.; Kim, Y.G.; Ko, Y.G. Dual-functional crosslinked polymer-inorganic materials for robust electrochemical performance and antibacterial activity. Chem. Eng. J. 2020, 392, 123654. [Google Scholar] [CrossRef]
- Cheng, Y.; Matykina, E.; Skeldon, P.; Thompson, G. Characterization of plasma electrolytic oxidation coatings on Zircaloy-4 formed in different electrolytes with AC current regime. Electrochim. Acta 2011, 56, 8467–8476. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kim, M.J.; Kim, Y.G.; Ko, Y.G. Enhanced chemical stability and boosted photoactivity by transition metal doped-crosslinked polymer-inorganic materials. J. Mol. Liq. 2020, 303, 112700. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Allaf, A.W.; Assfour, B.; Ko, Y.G. Toward two-dimensional hybrid organic-inorganic materials based on a I-PE/UHV-PVD system for exceptional corrosion protection. Appl. Mater. Today 2021, 24, 101142. [Google Scholar] [CrossRef]
- Ying, L.; Wu, Y.; Nie, C.; Wu, C.; Wang, G. Improvement of the Tribological Properties and Corrosion Resistance of Epoxy–PTFE Composite Coating by Nanoparticle Modification. Coatings 2021, 11, 10. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Wang, J.; Zhang, J.; Yin, C.; Fan, L.; Zhang, D. Graphene oxide-cerium oxide hybrids for enhancement of mechanical properties and corrosion resistance of epoxy coatings. J. Mater. Sci. 2021, 56, 10108–10123. [Google Scholar] [CrossRef]
- Alhumade, H.; Nogueira, R.; Yu, A.; Elkamel, A.; Simon, L.; Abdala, A. Role of surface functionalization on corrosion resistance and thermal stability of epoxy/glass flake composite coating on cold rolled steel. Prog. Org. Coat. 2018, 122, 180–188. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, B.; Srivastava, C. ZnCo-carbon nanotube composite coating with enhanced corrosion resistance behavior. Surf. Coat. Technol. 2020, 398, 126083. [Google Scholar] [CrossRef]
- Ehsani, M.; Khonakdar, H.; Ghadami, A. Assessment of morphological, thermal, and viscoelastic properties of epoxy vinyl ester coating composites: Role of glass flake and mixing method. Prog. Org. Coat. 2012, 76, 238–243. [Google Scholar] [CrossRef]
- Suleymani, M.; Bemani, A. Application of ANFIS-PSO algorithm as a novel method for estimation of higher heating value of biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 288–293. [Google Scholar] [CrossRef]
- Alhumade, H.; Rezk, H.; Nassef, A.M.; Al-Dhaifallah, M. Fuzzy Logic Based-Modeling and Parameter Optimization for Improving the Corrosion Protection of Stainless Steel 304 by Epoxy-Graphene Composite. IEEE Access 2019, 7, 100899–100909. [Google Scholar] [CrossRef]
- Chou, J.-S.; Ngo, N.-T.; Chong, W.K. The use of artificial intelligence combiners for modeling steel pitting risk and corrosion rate. Eng. Appl. Artif. Intell. 2017, 65, 471–483. [Google Scholar] [CrossRef]
- Sheikh, M.F.; Kamal, K.; Rafique, F.; Sabir, S.; Zaheer, H.; Khan, K. Corrosion detection and severity level prediction using acoustic emission and machine learning based approach. Ain Shams Eng. J. 2021, 12, 3891–3903. [Google Scholar] [CrossRef]
- Valdez, F.; Vazquez, J.C.; Melin, P.; Castillo, O. Comparative study of the use of fuzzy logic in improving particle swarm optimization variants for mathematical functions using co-evolution. Appl. Soft Comput. 2017, 52, 1070–1083. [Google Scholar] [CrossRef]
- Gaxiola, F.; Melin, P.; Valdez, F.; Castro, J.R.; Castillo, O. Optimization of type-2 fuzzy weights in backpropagation learning for neural networks using GAs and PSO. Appl. Soft Comput. 2016, 38, 860–871. [Google Scholar] [CrossRef]
- Manna, J.; Roy, B.; Sharma, P. Efficient hydrogen generation from sodium borohydride hydrolysis using silica sulfuric acid catalyst. J. Power Sources 2015, 275, 727–733. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Young, R.; Deng, L.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer 2013, 54, 6437–6446. [Google Scholar] [CrossRef]


| Run | A: Load of Filler (wt%) | B: Type of Filler | C: Coating Thickness (+/−5 µm) | Icorr (µA/cm2) |
| 1 | 10 | GF | 50 | 0.418 |
| 2 | 10 | FGF | 50 | 0.261 |
| 3 | 10 | GF | 100 | 0.311 |
| 4 | 10 | FGF | 100 | 0.144 |
| 5 | 15 | GF | 50 | 0.386 |
| 6 | 15 | FGF | 50 | 0.175 |
| 7 | 15 | GF | 100 | 0.215 |
| 8 | 15 | FGF | 100 | 0.096 |
| 9 | 20 | GF | 50 | 0.328 |
| 10 | 20 | FGF | 50 | 0.105 |
| 11 | 20 | GF | 100 | 0.121 |
| 12 | 20 | FGF | 100 | 0.072 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.1462 | 3 | 0.0487 | 37.79 | <0.0001 | significant |
| A (Load of Filler, wt%) | 0.0323 | 1 | 0.0323 | 25.02 | 0.0011 | |
| B (Type of Filler) | 0.0715 | 1 | 0.0715 | 55.42 | <0.0001 | |
| C (Coating Thickness, µm) | 0.0425 | 1 | 0.0425 | 32.95 | 0.0004 | |
| Residual | 0.0103 | 8 | 0.0013 | |||
| Cor Total | 0.1565 | 11 |
| ANOVA Model of CCD | |||
|---|---|---|---|
| Std. Dev. | 0.0359 | R2 | 0.9341 |
| MSE | 0.2193 | Adjusted R2 | 0.9094 |
| C.V.% | 16.37 | Predicted R2 | 0.8426 |
| RMSE | 0.4683 | Adeq Precision | 19.3102 |
| MSE | RMSE | R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Training | Testing | All | Training | Testing | All | Training | Testing | All |
| 6.6244 × 10−15 | 1.0848 × 10−4 | 3.6161 × 10−5 | 8.1391 × 10−8 | 0.0104 | 0.0060 | 1.000 | 0.9997 | 0.9979 |
| Method | GF (Glass Flake Loading, wt%) | CF (Chemical Functionalization) | TH (Coating Thickness, µm) | Icorr (µA/cm2) | RMSE |
|---|---|---|---|---|---|
| ANFIS model | 12 | 1 | 75 | 0.248 | 0.08 |
| Measured data | 12 | 1 | 75 | 0.241 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhumade, H.; Rezk, H. An Accurate Model of the Corrosion Current Density of Coatings Using an Adaptive Network-Based Fuzzy Inference System. Metals 2022, 12, 392. https://doi.org/10.3390/met12030392
Alhumade H, Rezk H. An Accurate Model of the Corrosion Current Density of Coatings Using an Adaptive Network-Based Fuzzy Inference System. Metals. 2022; 12(3):392. https://doi.org/10.3390/met12030392
Chicago/Turabian StyleAlhumade, Hesham, and Hegazy Rezk. 2022. "An Accurate Model of the Corrosion Current Density of Coatings Using an Adaptive Network-Based Fuzzy Inference System" Metals 12, no. 3: 392. https://doi.org/10.3390/met12030392






