1. Introduction
The global steel industry is responsible for nearly 20% of global coal consumption, which is mainly used for the reduction in iron ore in the blast furnace (BF) process to generate hot metal. This coal-based reduction process in the BF and the following oxygen steelmaking process leads to specific CO
2 emissions of approximately 2 tons per ton of steel. With a current global production of about 1850 MTPA, 70% via the BF–BOF (basic oxygen furnace) route [
1], the steel industry is the largest industrial CO
2 emitter and accounts for up to 9% of worldwide carbon dioxide emissions. International agreement to achieve net-zero before 2050 for the majority of the steel producing countries has been made. With increasing pressure from governments to reduce CO
2 emissions to fight climate change and ever-increasing emissions trading systems (ETS) and carbon taxes, many steelmakers have announced decarbonization pathways for their steel products [
2]. In
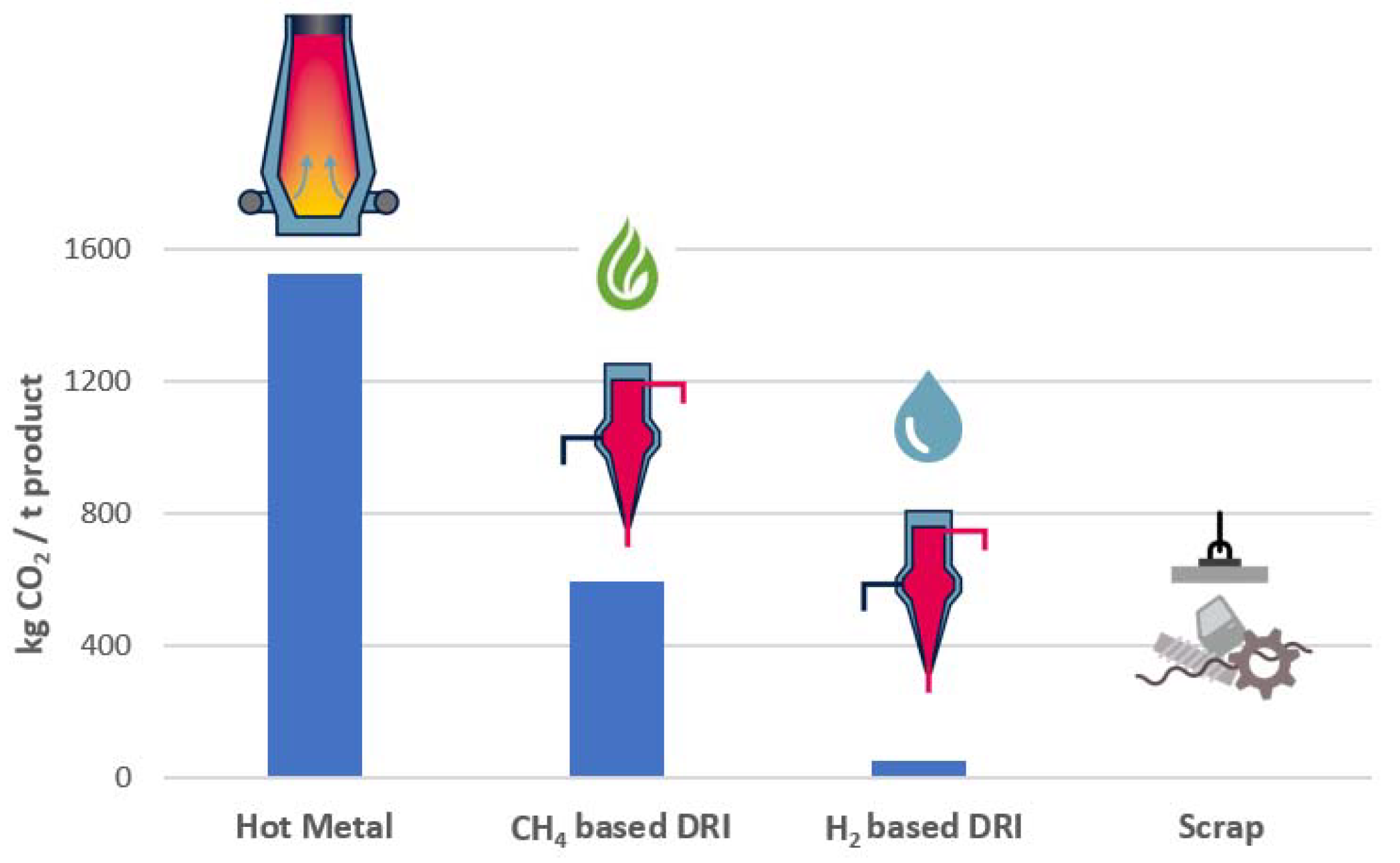
Figure 1 below, on the left side, the specific CO
2 emissions per ton of output product for the typical raw materials such as hot metal, direct reduced iron (DRI), and scrap are shown.
It can be seen that hot metal has by far the largest “backpack” of CO
2 emissions, with around 1.5 tons of CO
2 per ton of liquid steel. Direct reduced iron with natural gas has less than half the emission of hot metal via the BF process. Future hydrogen-based direct reduction is already becoming close to zero, where only some small amount of scope 2 emissions for operating the plant will remain. The only raw material for steelmaking with zero CO
2 emissions is steel scrap. Therefore, an increased usage of steel scrap, which can be recycled infinite times, will play an important role in the decarbonization of the steel industry alongside direct reduction-based steelmaking. CO
2 emissions are grouped into three scopes: scope 1 includes direct emissions, e.g., combustion processes, scope 2 includes indirect emissions, e.g., from electricity, and scope 3 includes other indirect emissions from sources not attributed to the plant, e.g., raw materials [
3].
Increasing global scrap usage for steelmaking shows that all available scrap is currently in use, and this will not change in the future when scrap availability is further increased. Forecasts predict scrap availability from 900 MTPA [
4] to 1300 MTPA [
5] in 2050 (see
Figure 2). Since global steel production is expected to grow far less than scrap availability, forecasts show around 2200 MTPA in 2050 [
1,
6]. The higher scrap availability will lead to new scrap-based electric arc furnace (EAF) steelmaking plants and increased scrap utilization for integrated steelmaking. Processing higher scrap rates, e.g., in the BOF, is an easy and fast measure to reduce the carbon footprint of the integrated plant and therefore will become more popular. Unfortunately, scrap availability and the residual limitation for high-quality steel grades will not allow a switch to entirely scrap-based steelmaking. Therefore, virgin material such as iron ore will remain the dominating raw material long term.
In this paper, the challenges and limitations of processing higher scrap rates will be discussed, which is followed by solutions for scrap processing, higher scrap rates in integrated BOF steel plants, and a hybrid EAF–BOF steelmaking operation as part of the transition toward net-zero carbon emissions. Finally, the CO2 reduction potential of the presented solutions is compared and set into relation to the complexity of implementation.
3. BOF Steelmaking Process: Energy and CO2 Balance
Currently, around 70% of global steel production is based on the BF–BOF route. The remaining 30% is based mainly on scrap-based EAF steelmaking and a smaller but increasing share on DRI–EAF-based steelmaking [
5,
6]. Unlike the electric arc furnace, which uses electric energy as the main source to melt down the charged materials, the BOF process is an autothermal process that does not require external energy input. The main energy input for the LD (BOF) process comes from the sensible heat of the hot metal and chemical reactions such as the oxidation of carbon (C), silicon (Si), and a small amount from the post-combustion of carbon monoxide (CO). While Si is combusted in one step to silicon dioxide (SiO
2), C is combusted in a first step in the steel bath to CO only and during a second step outside the bath to CO
2. The latter step is called post-combustion and generates about two-thirds of the total energy released if carbon is combusted to carbon dioxide (CO
2).
In conventional BOF operation, the post-combustion degree is rather low, only 8–12%. Hence, only a small part of the total energy is used inside the converter. A gas with a higher calorific value is leaving the process, typically collected in a gas holder, and used for heating purposes in the plant later. This process is called BOF gas recovery.
Scrap and other solid charges such as hot-briquetted iron (HBI), DRI, sinter, or iron ore are added as cooling the material to the BOF to equalize the heat balance and compensate for excessive heat from the hot metal. Lime, limestone, and burnt or raw dolomite are added as slag formers.
Figure 4a below shows the energy balance of a BOF converter for a scrap rate of 20% in a simplified manner. The main energy input is around 60%, and the sensible heat of hot metal is typically about 1350 °C, which is followed by the combustion of carbon to CO at approximately 18%. All chemical reactions in the liquid steel and slag account for 34%, and the post-combustion of CO to CO
2 in the BOF process gas provides around 5% of the energy input, assuming a standard post-combustion degree. On the output side, the main energy is the sensible heat of steel at around 1670 °C, consisting of the sensible heat of steel coming from the hot metal with around 56% and the energy of the scrap melting with around 20%. The sensible heat of slag with 12.8%, the sensible heat of the process gas with 8.5%, and the heat losses with about 3% are concluding the energy output. In
Figure 4b, the energy balance for 20% scrap rate is compared with a higher scrap rate of 25%. For a higher scrap rate, the sensible heat of hot metal and heat from the chemical reaction decrease with the lower amount of charged hot metal. In addition, the post-combustion decreases because of the lower amount of CO generated. With the higher scrap rate of 25%, additional energy is required for melting, assuming the same chemical composition and temperature of hot metal and the same tapping conditions. The additional energy required for melting more scrap with less hot metal is shown in red below. The total energy output of steel is the same for the higher scrap rate, but less comes from the hot metal. With a lower hot metal ratio, it is assumed that slag and off-gas decrease. Heat loss will stay relatively constant since the main factors influencing heat loss—the charging and tapping temperatures, refractory, insulation, and the processing time—are not changed. Heat loss is understood as the heat transfer and radiation from the vessel to its surroundings. Since slag and off-gas amount is slightly lower with a higher scrap rate, a similar trend is expected for the total energy compared to the 20% scrap rate.
Since the amount of combustibles in the BOF process is limited and heating agents such as ferrosilicon or coal show limitations regarding process and economics, the share of solid charges such as scrap and HBI/DRI is limited. Depending on the hot metal composition, temperature, plant-specific operation conditions, and corresponding heat losses, the BOF process is well balanced at the scrap rate of 15 to 20%. The possible solid charge rate decreases with the increasing share of HBI and DRI, since these materials have a higher cooling effect than scrap because HBI and DRI contain some percentage of iron oxide that was not reduced in the reduction plant as well as some non-metallic fraction called gangue, which requires more energy for melting. Hence, the maximum share of scrap that can be processed in the BOF is higher than the maximum share of HBI. In the BOF, around 10% of the charge mix can be HBI/DRI, which is either charged with the scrap chute and/or with the material handling system. Furthermore, HBI/DRI have higher CO2 emissions compared to scrap, are more difficult to melt, and special care for the charging step and mixing during processing is required to avoid slopping. Therefore, in the following chapter, the focus will be on scrap as a solid charge.
Another point that restricts the scrap rate in conventional BOF converter steelmaking is bath mixing. Strong mixing of the bath is required to keep temperature gradients small and avoid cold spots to melt down high amounts of scrap per heat quickly. Hence, proper bottom stirring is necessary throughout the vessel lining campaign [
8].
CO
2 balance of integrated BF-BOF steelmaking and specific CO
2 emissions per ton of output product with a scrap rate of 16%, including credits for gas and heat recovery from off-gas and granulated blast furnace slag (GBFS), is shown in
Figure 5 below. The scope 1 emissions from ironmaking and here especially from the blast furnace are dominant along the process chain. At the BOF, the specific scope 1 emissions from the decarburization process are lower by far, with around 160 kg CO
2 per ton of liquid steel, and scope 2 emissions for electric power, oxygen generation, and dry primary dedusting system in total are 35 kg CO
2 per ton of liquid steel. In green, the CO
2 credits are shown in
Figure 5. In the case of a dedusting system with suppressed combustion, BOF off-gas has a good heating value and is typically collected in a gasholder for further usage. Depending on the BOF operation with such gas recovery, CO
2 credits of 30–40 kg per ton of liquid steel can be achieved. Additional credits are possible with heat recovery of BOF off-gas to produce steam and substitute other fossil fuels such as natural gas, which are in a range of 10–30 kg per ton liquid steel. Newly developed solutions for by-product recycling of slags and dust on the BOF, which allows the recovery and reuse of metal and mineral fractions, have the potential for additional credits.
4. Solutions for Increased Scrap Rate for BOF Steelmaking
From the CO2 balance, it can be seen that increasing the scrap rate will reduce the hot metal amount and therefore show promising potential to reduce CO2 emissions of the integrated steelmaking route while in parallel allowing for higher raw material flexibility. Several measures exist to increase the energy input and decrease the energy output at the BOF to allow for operation with higher scrap rates. The next section will discuss efforts such as heat optimization, scrap preheating and premelting, higher post-combustion, combined blowing, and the JET converter.
4.4. Combined Blowing and JET Converter
Stable processing of a high scrap rate is achieved by changing from normal BOF operation to combined blowing converter, known as KOBM, or Klöckner Oxygen Blown Maxhütte. The oxygen blown from the bottom via gas-cooled tuyeres leads to intensive mixing of the bath. This intensive mixing ensures uniform scrap melting and allows for softer blowing with the top lance, which automatically results in a higher post-combustion rate. In addition, the tuyeres installed in the converter bottom can be used for scrap preheating. Such a combined blowing converter is, for example, operated at AM Dofasco, Canada, and an increase in post-combustion and scrap rate has been reported.
Primetals Technologies has several references for such KOBM converter, and the most recent reference of KOBM, which is currently under construction in China, shows the typical advantage of KOBM technology compared to BOF, such as the following:
Faster slag formation and less slopping due to the lime injection, good mixing, and process close to equilibrium—less material loss, less cleaning effort, less maintenance effort
Less iron oxide (FeO) in the slag due to better mixing (FeO in the slag partially reduced again by C from the bath) and therefore higher yield,
Higher post-combustion degree due to increased lance height (more soft blowing).
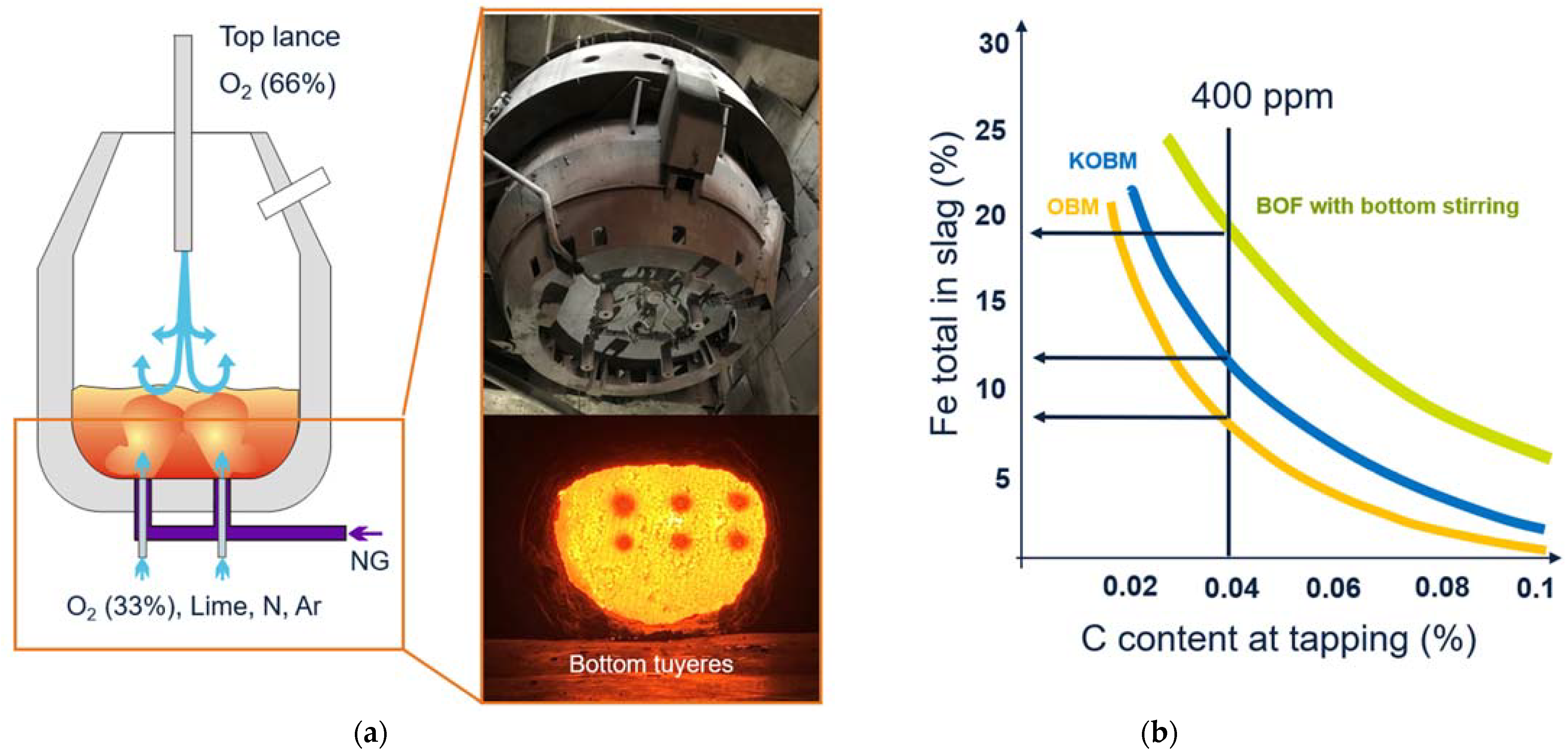
In
Figure 10b, below, the percentage of iron in slag at a specific tapping composition is compared for standard BOF (LD) with bottom stirring, KOBM, and OBM (Oxygen bodenblasen Maxhütte or “Oxygen Bottom-Blowing Furnace”). It clearly shows that the bottom blowing converter has an advantage concerning yield and iron losses due to the very efficient homogenization.
Other advantages of KOBM, such as the shorter process time, better phosphorus removal, and less dust formation are in evaluation and will be reported after completion.
Approximately 30% of the total oxygen is blown through the bottom, accounting for 250 Nm
3 per minute (see
Figure 10a above). The bottom lime injection rate is 700–900 kg/min. The scrap rate is relatively low at this converter, with approximately 10%, since the main target is to maximize iron ore additions and raw material flexibility.
There are also challenges of KOBM operation compared to the BOF, such as the higher maintenance effort for intermediate hot bottom exchange and tuyeres operation. Due to the extensive bath mixing of the bottom and combined blowing converter, vibrations can be observed, which requires stiffer mechanical design and, in some cases, a vibration-damping system.
The JET process was developed to further increase the scrap rate by higher post-combustion degree and the addition of heating agents by the injection of coal directly into the steel bath. The JET process comprises a bottom blowing converter with coal and lime injection combined with a hot blast lance [
8,
9]. The coal injected via the converter bottom as well as the carbon already dissolved in the hot metal is again combusted in two steps—the combustion to CO in the steel bath followed by post-combustion to CO
2 outside the steel bath.
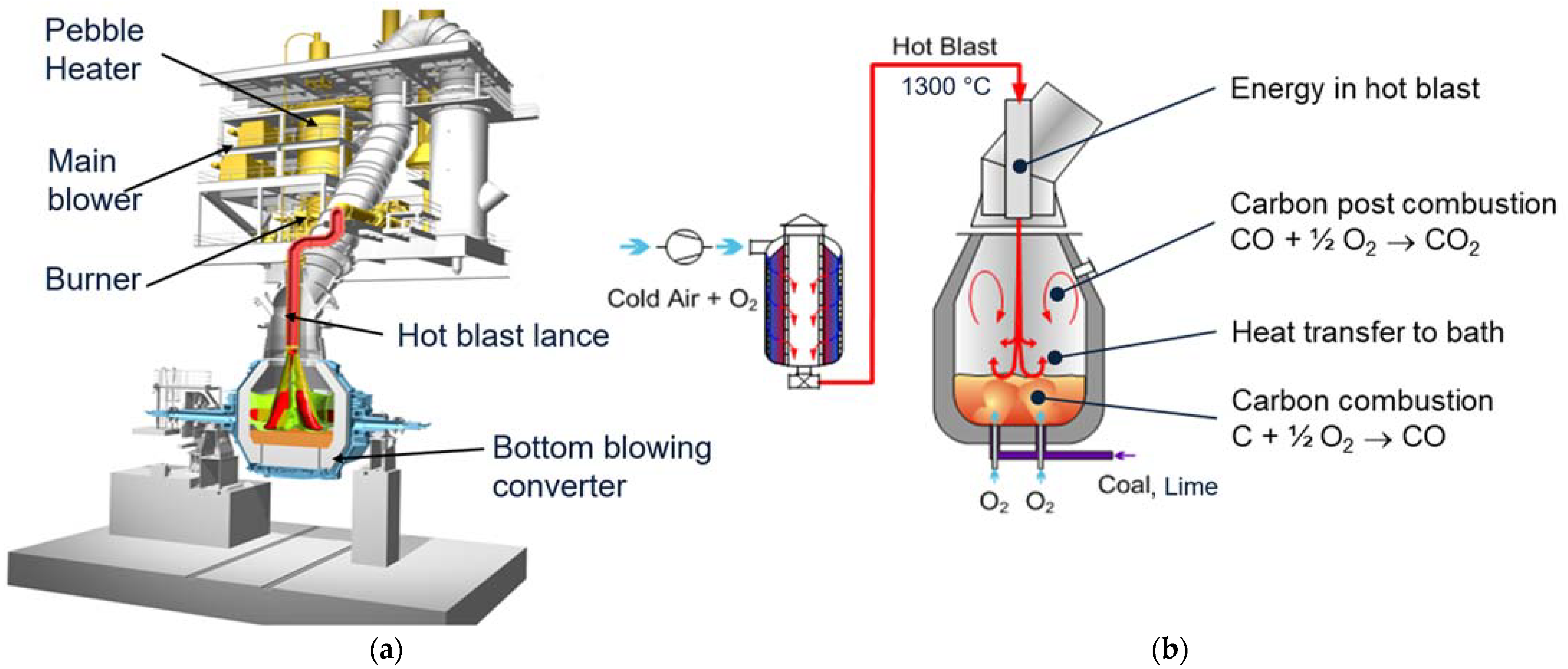
Efficient mixing is required to ensure the highest post-combustion and heat transfer efficiency (I). This is achieved by a hot blast blown with a lance from the top onto the bath. The hot blast consists of air that is enriched with oxygen to about 30% and heated up in a pebble heater to 1300 °C (see the principle in
Figure 11b). Due to the high speed and the high volume of the hot blast, a jet with a very high penetration length is formed, and a lot of surrounding media is sucked into the jet. This leads to an excellent mixing rate inside the converter; the CO coming from the bath is intensively mixed with the oxygen in the hot blast, and combustion to CO
2 takes place. The intensive mixing with the hot blast ensures post-combustion up to 60% and a high HTE leading to an off-gas temperature of about 150 °C above the steel bath temperature.
Figure 11a shows the main components of the JET process, the hot blast system with its core component, the pebble heater, and the bottom blowing converter. The pebble heater is an efficient regenerative heat exchanger that uses pebbles to store energy. Due to the high surface area of the pebbles, they have very high storage power and are ideally suited for short-term heat storage. This, in combination with the high storage density of the pebbles, leads to a very compact design of the pebble heater.
In parallel to the hot blast from the top, oxygen is blown through the converter bottom to decarburize the hot metal. The bottom blowing leads to excellent bath mixing and allows for fast and efficient melting of large scrap amounts.
A full industrial reference for the JET process was implemented in POSCO, Pohang on a 100 t converter (see
Figure 12 and [
8,
9] for details). The principles of the process and performance have been proven in this reference and the design of the equipment. A wide range of scrap rates have been processed successfully, including heats with 50% scrap. In the picture below, a photograph of the converter, the bottom is shown, highlighting the bottom blowing system installed. This system is capable of oxygen blowing as well as coal and lime injection