Tailoring of Biodegradable Magnesium Alloy Surface with Schiff Base Coating via Electrostatic Spraying for Better Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
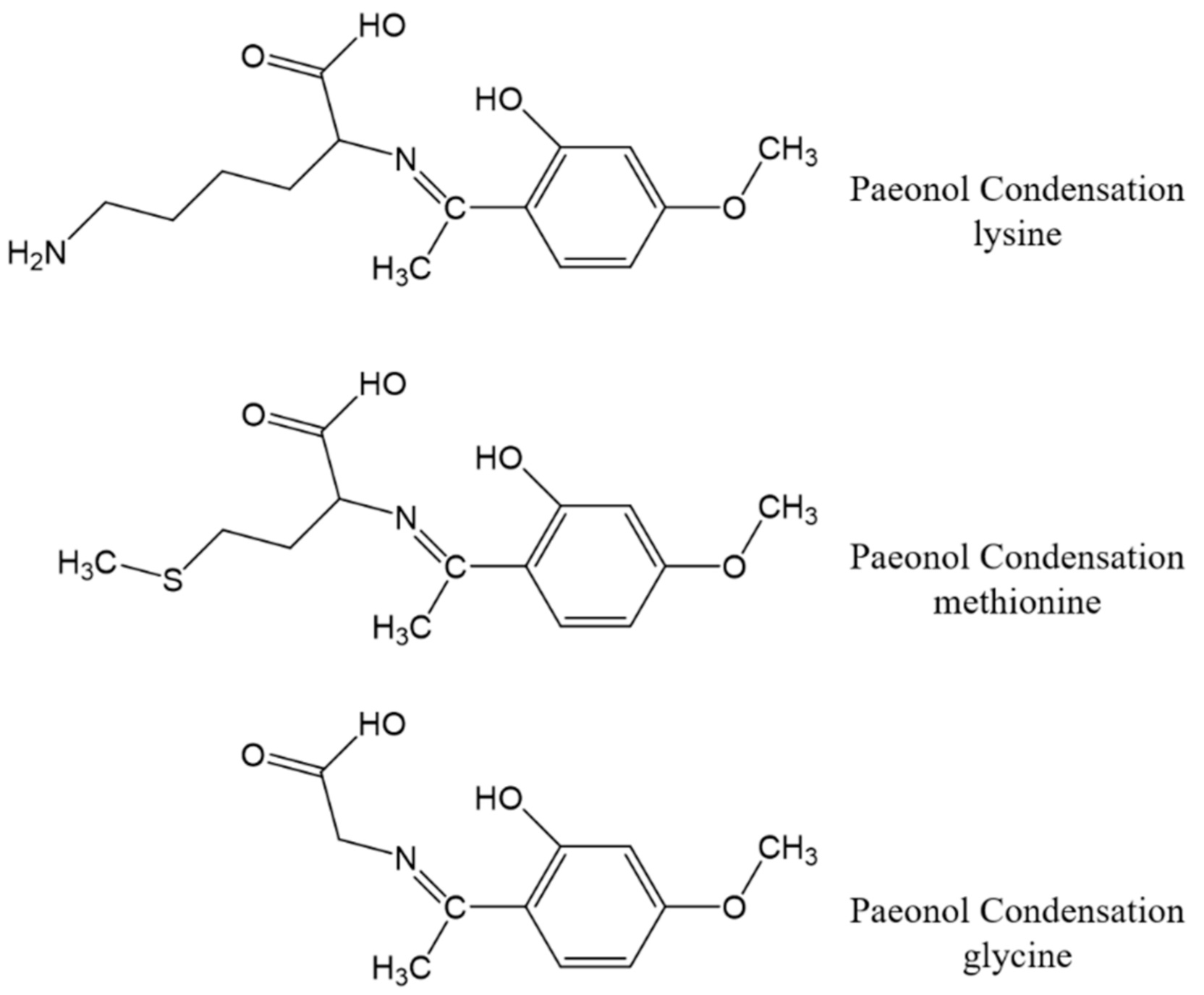
2.2. Synthesis of Schiff Base
2.3. Preparation of Schiff Base Coatings on ZE21B Alloy
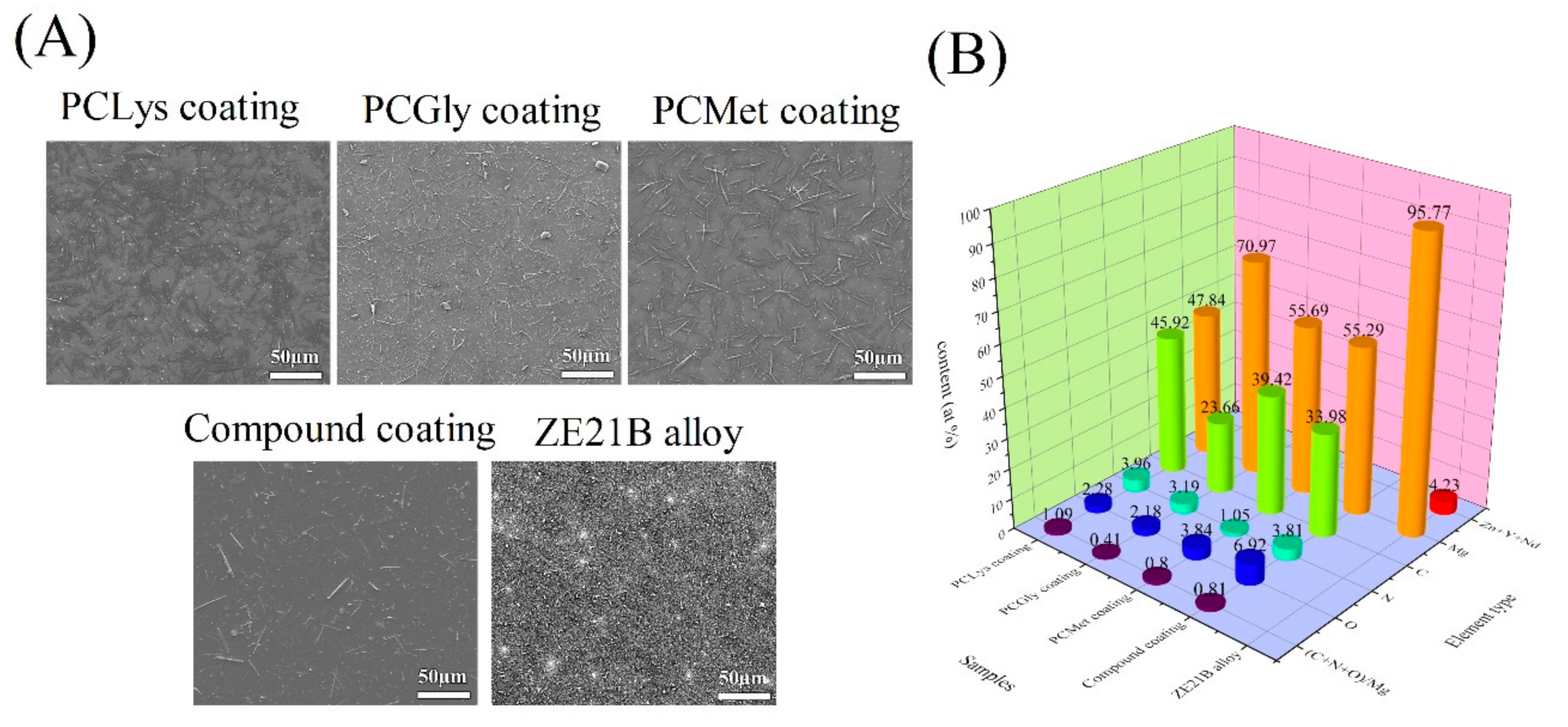
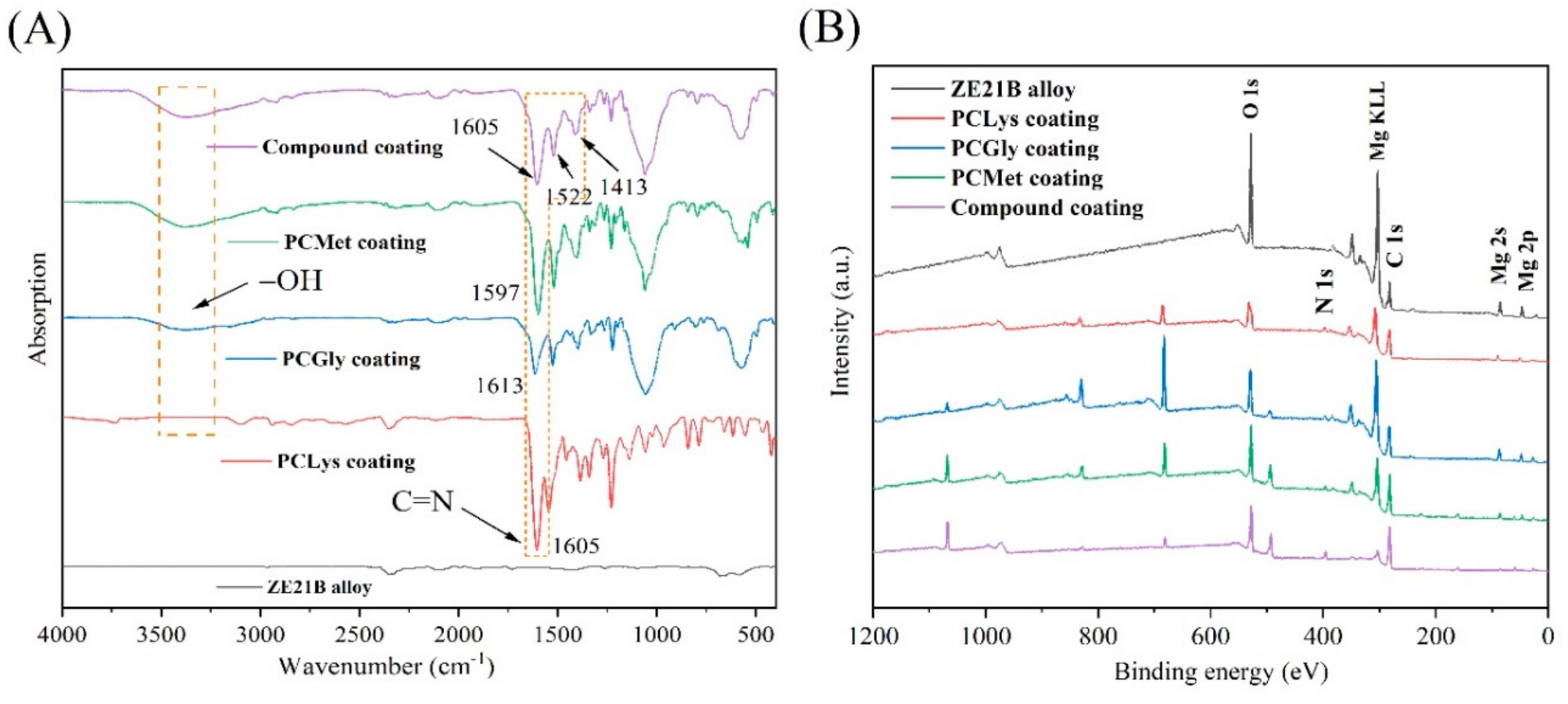
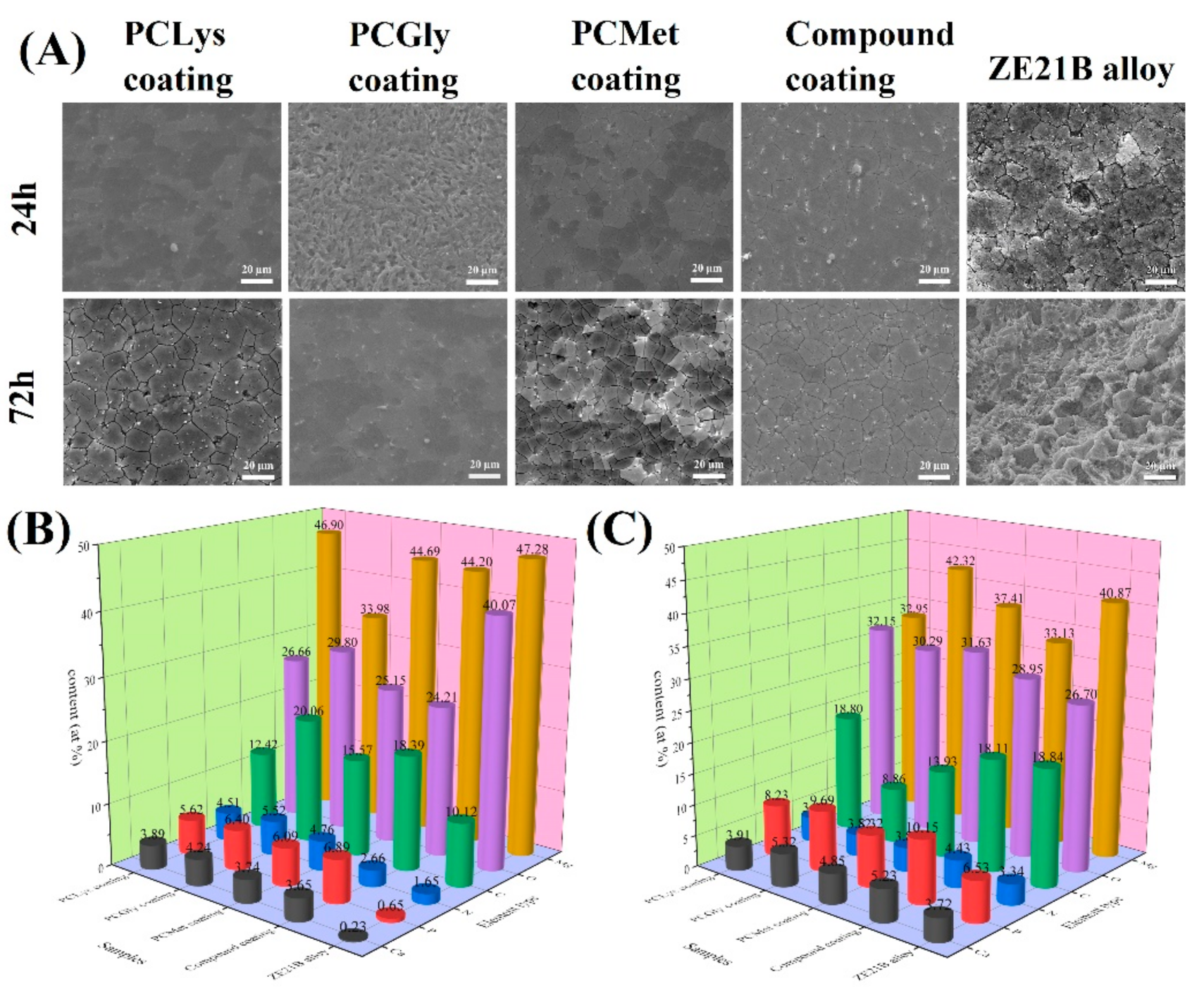
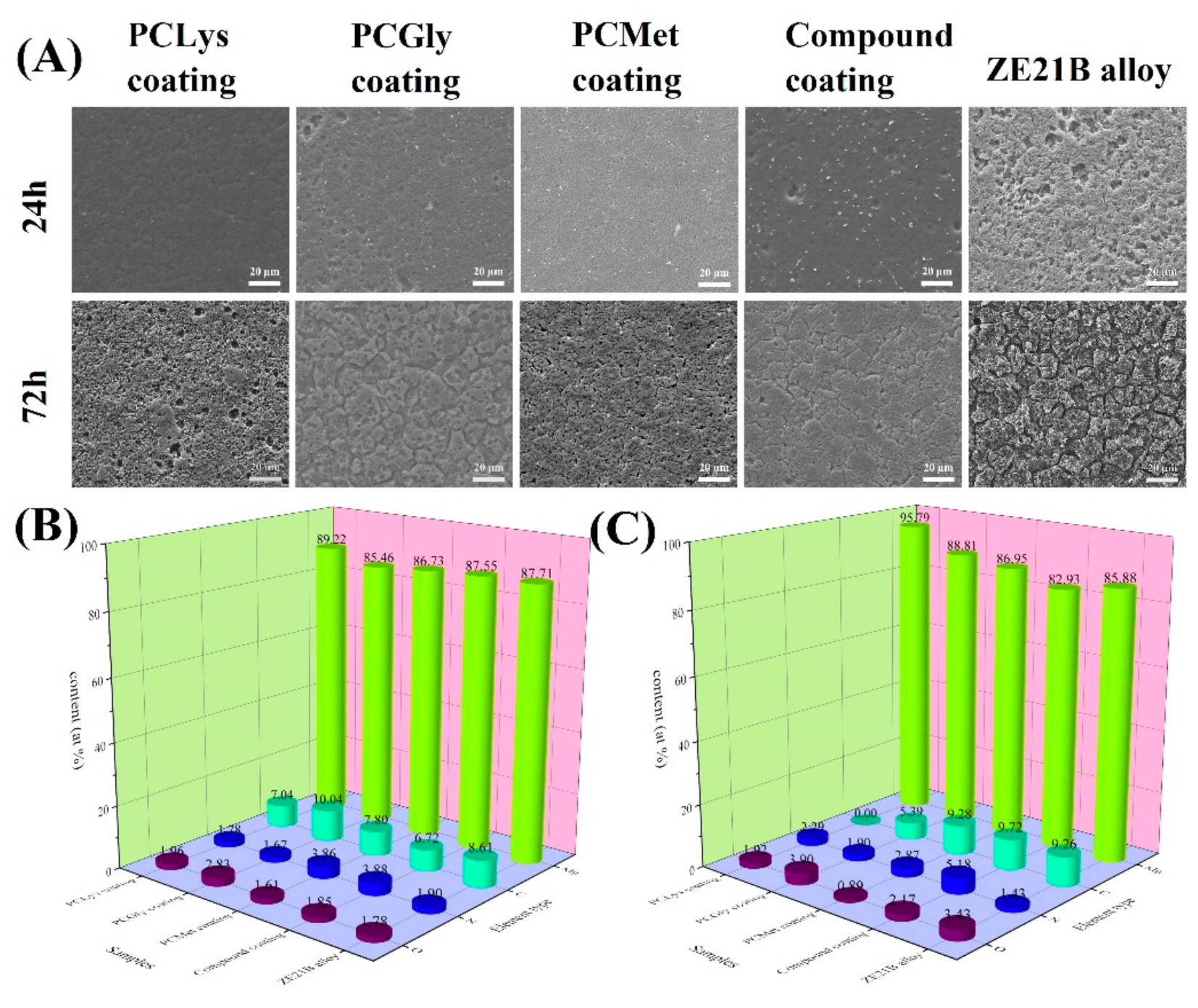
2.4. Characterization of Schiff Base Coatings on ZE21B Alloy
2.5. Immersion Test
2.6. Electrochemical Test
3. Results and Discussion
3.1. Immersion Test
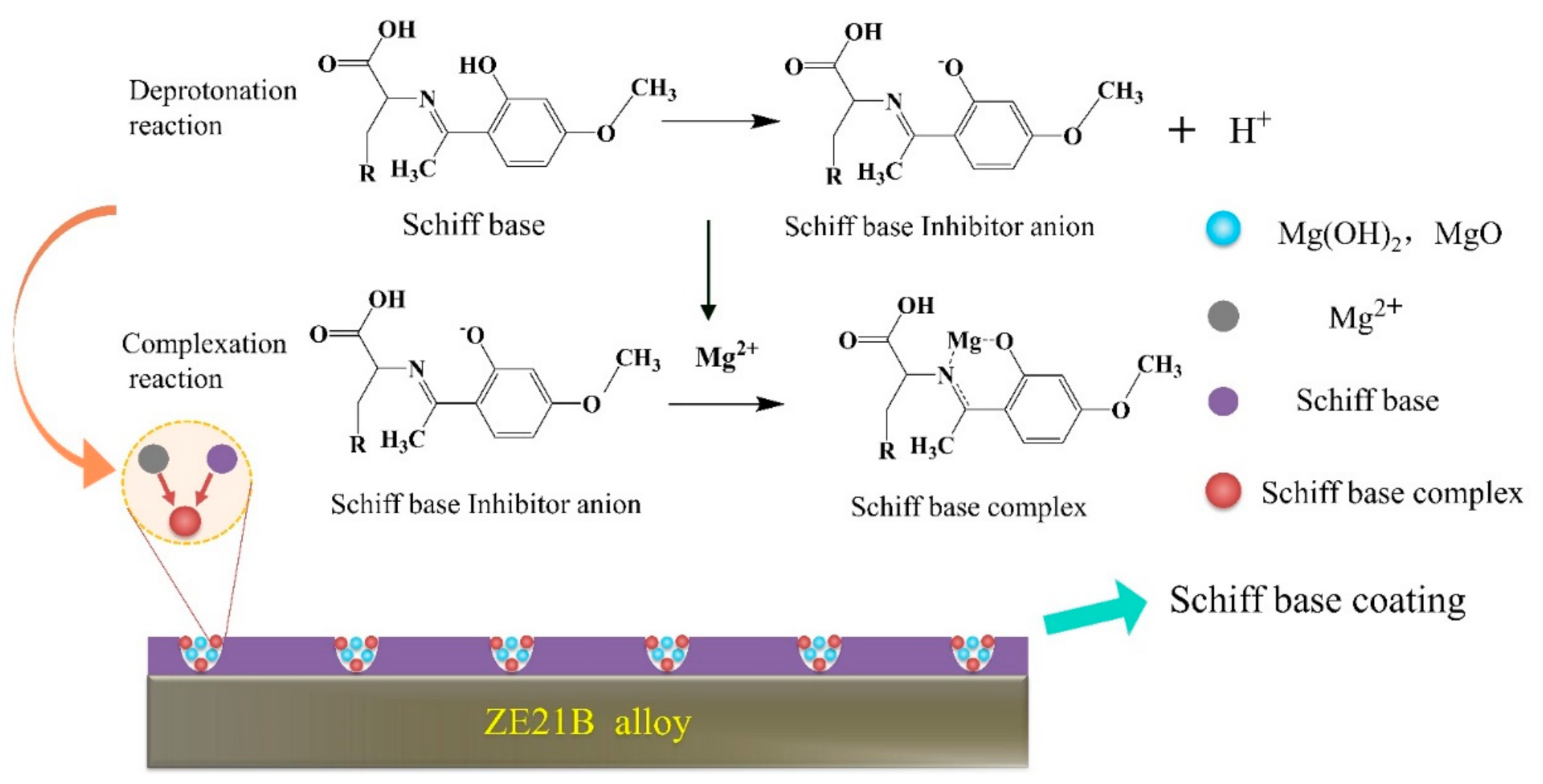
3.2. The Inhibition Mechanism of Schiff-Base Coating
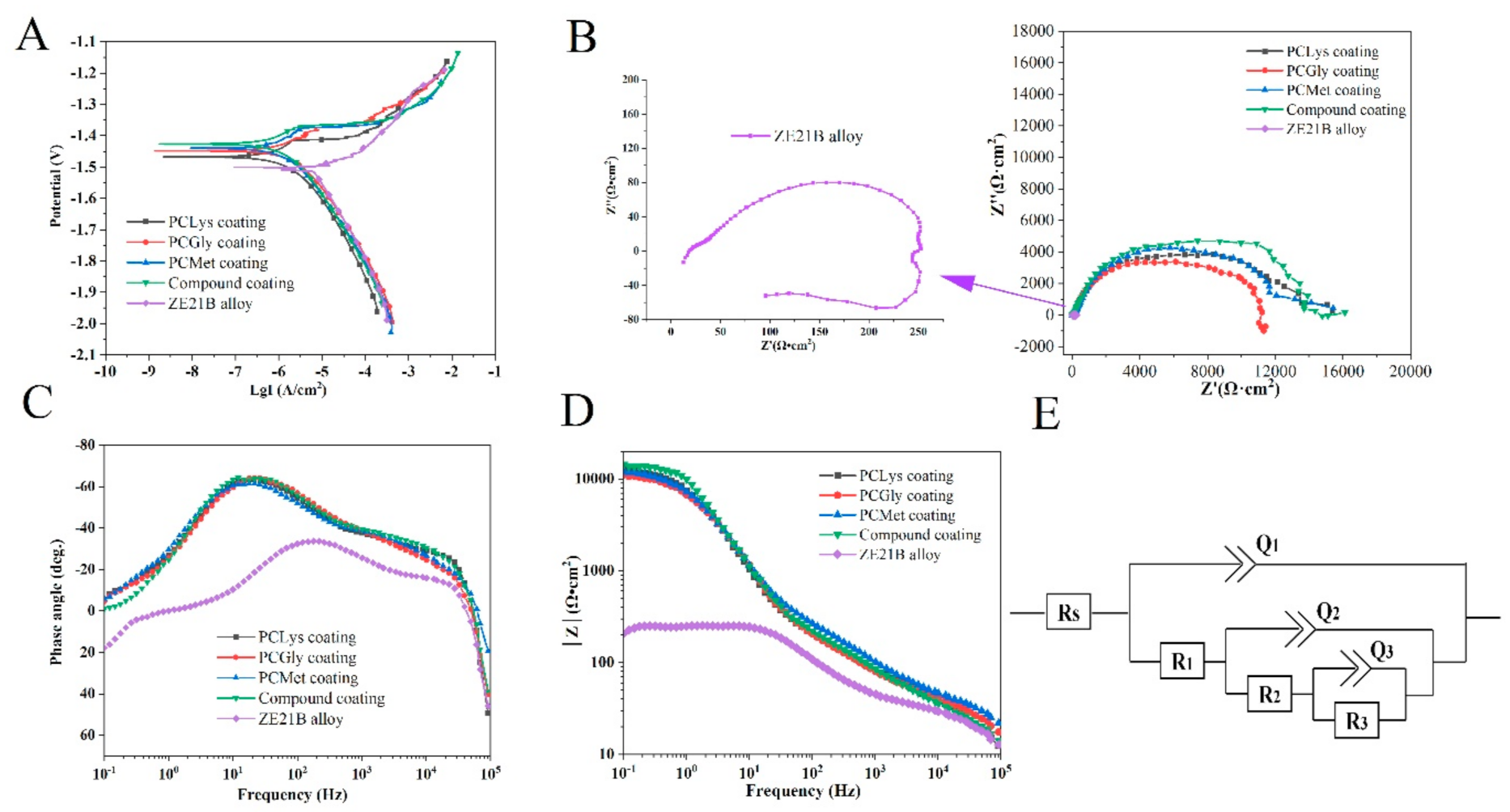
3.3. Electrochemical Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maqbool, A.; Khan, N.Z.; Siddiquee, A.N. Towards Mg Based Light Materials of Future: Properties, Applications, Problems, and Their Mitigation. J. Manuf. Sci. Eng. 2022, 144, 030801. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Song, J.F.; She, J.; Chen, D.L.; Pan, F.S. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Li, S.B.; Yang, X.Y.; Hou, J.T.; Du, W.B. A review on thermal conductivity of magnesium and its alloys. J. Magnes. Alloy. 2020, 8, 78–90. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Stanford, N.; Davies, C.H.J.; Nie, J.F.; Birbilis, N. Magnesium extrusion alloys: A review of developments and prospects. Int. Mater. Rev. 2019, 64, 27–62. [Google Scholar] [CrossRef]
- Chen, J.X.; Tan, L.L.; Yu, X.M.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Chairman, C.A.; Balasundaram, R. Mechanical properties and applications of Magnesium alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Xi, T.F.; Wei, L.N.; Liu, J.; Liu, X.L.; Zhen, Z.; Zheng, Y.F. Research Progress in Bioresorbable Magnesium Scaffolds. Acta Metall. Sin. 2017, 53, 1153–1167. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Yang, H.T. Research Progress in Biodegradable Metals for Stent Application. Acta Metall. Sin. 2017, 53, 1227–1237. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhu, S.; Han, B.Y. Research progress in anodic hydrogen evolution of magnesium electrochemistry corrosion. Cailiao Gongcheng 2020, 48, 17–27. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yang, Y.X.; Li, J.A.; Zeng, R.C.; Guan, S.K. Advances in coatings on magnesium alloys for cardiovascular stents—A review. Bioact. Mater. 2021, 6, 4729–4757. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, B.H.; Cai, Z.X. Recent Progress on Mg- and Zn-Based Alloys for Biodegradable Vascular Stent Applications. J. Nanomater. 2019, 2019, 1310792. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Lu, X.P.; Zheludkevich, M.L. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals—A critical review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Hu, T.; Ouyang, Y.J.; Xie, Z.H.; Wu, L. One-pot scalable in situ growth of highly corrosion-resistant MgAl-LDH/MBT composite coating on magnesium alloy under mild conditions. J. Mater. Sci. Technol. 2021, 92, 225–235. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Zimowska, M.; Ryl, J.; Zielinski, A.; Osipenko, M.A.; Adamiec, J.; Wrzesinska, A.; Claesson, P.M.; Kurilo, I.I. Aqueous molybdate provides effective corrosion inhibition of WE43 magnesium alloy in sodium chloride solutions. Corros. Sci. 2021, 190, 109664. [Google Scholar] [CrossRef]
- Liu, S.Q.; Li, Z.X.; Yu, Q.L.; Qi, Y.M.; Peng, Z.J.; Liang, J. Dual self-healing composite coating on magnesium alloys for corrosion protection. Chem. Eng. J. 2021, 424, 130551. [Google Scholar] [CrossRef]
- Song, Y.W.; Liu, D.; Tang, W.N.; Dong, K.H.; Shan, D.Y.; Han, E.H. Comparison of the corrosion behavior of AM60 Mg alloy with and without self-healing coating in atmospheric environment. J. Magnes. Alloy. 2021, 9, 1220–1232. [Google Scholar] [CrossRef]
- Lu, X.P.; Li, Y.; Ju, P.F.; Chen, Y.; Yang, J.S.; Qian, K.; Zhang, T.; Wang, F.H. Unveiling the inhibition mechanism of an effective inhibitor for AZ91 Mg alloy. Corros. Sci. 2019, 148, 264–271. [Google Scholar] [CrossRef]
- Shin, Y.; Cho, K. Corrosion Behavior and Inhibition Studies of AZ31B Magnesium Alloy With and Without Cl- in the Alkaline Electrolytes in Addition with Various Inhibitor Additives. Corros. Sci. Technol. 2019, 18, 243–252. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach. Phys. Chem. Chem. Phys. 2016, 18, 17898–17911. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Bezaatpour, A.; Shamkhali, A.N.; Basharnavaz, H. Experimental and Theoretical Studies to Examine the Inhibition Effect of a Schiff Base against Magnesium Corrosion. Trans. Indian Inst. Met. 2016, 69, 1545–1555. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yang, S.; Feng, W.; Li, Y.; Cheng, Y. Eelectrochemical Study of Inhibition Effect of a Schiff Base towards Magnesium Alloy Corrosion. Int. J. Electrochem. Sci. 2016, 11, 6043–6051. [Google Scholar] [CrossRef]
- Li, W.; Su, Y.; Ma, L.; Zhu, S.; Zheng, Y.; Guan, S. Sol-gel coating loaded with inhibitor on ZE21B Mg alloy for improving corrosion resistance and endothelialization aiming at potential cardiovascular application. Colloids Surf. B Biointerfaces 2021, 207, 111993. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Corrosion inhibition of Schiff bases for Mg-Zn-Y-Nd alloy in normal saline: Experimental and theoretical investigations. Corros. Sci. 2021, 184, 109268. [Google Scholar] [CrossRef]
- Müller, V.; Balvay, S.; Gaillard, C.; Tadier, S.; Gremillard, L.; Djurado, E. One-step fabrication of single-phase hydroxyapatite coatings on Ti-alloy implants by electrostatic spray deposition: From microstructural investigation to in vitro studies. Surf. Coat. Technol. 2021, 427, 127805. [Google Scholar] [CrossRef]
- Chang, L.; Li, X.; Tang, X.; Zhang, H.; He, D.; Wang, Y.; Zhao, J.; Li, J.; Wang, J.; Zhu, S.; et al. Micro-patterned hydroxyapatite/silk fibroin coatings on Mg-Zn-Y-Nd-Zr alloys for better corrosion resistance and cell behavior guidance. Front. Mater. Sci. 2020, 14, 413–425. [Google Scholar] [CrossRef]
- Jia, Z.Y.; Ma, C.Y.; Zhang, H.B. PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility. Coatings 2021, 11, 1427. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Zou, D.; Kou, F.; Hou, Y.C.; Yasin, A.; Zhang, K. Comparison of conjugating chondroitin sulfate A and B on amine-rich surface: For deeper understanding on directing cardiovascular cells fate. Compos. Part B Eng. 2022, 228, 109430. [Google Scholar] [CrossRef]
- Li, J.A.; Chen, L.; Zhang, X.Q.; Guan, S.K. Enhancing biocompatibility and corrosion resistance of biodegradable Mg-Zn-Y-Nd alloy by preparing PDA/HA coating for potential application of cardiovascular biomaterials. Mater. Sci. Eng. C 2020, 109, 110607. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, S.; Hou, Y.; Li, J.; Guan, S. Sulfur Contents in Sulfonated Hyaluronic Acid Direct the Cardiovascular Cells Fate. ACS Appl. Mater. Interfaces 2020, 12, 46827–46836. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Li, J.A.; Kou, F.; Luo, X.; Yang, P. Reveal crucial subtype of natural chondroitin sulfate on the functionalized coatings for cardiovascular implants. J. Mater. Sci. Technol. 2021, 91, 67–77. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, S.J.; Dong, H.T.; Zhang, X.Q.; Li, J.A.; Guan, S.K. A novel MgF2/PDA/S-HA coating on the bio-degradable ZE21B alloy for better multi-functions on cardiovascular application. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
| Component | Concentration (g/L) |
|---|---|
| NaCl | 8.0 |
| MgSO4·7H2O | 0.1 |
| KCl | 0.4 |
| MgCl2·6H2O | 0.1 |
| CaCl2 | 0.14 |
| Na2HPO4·12H2O | 0.152 |
| KH2PO4·3H2O | 0.06 |
| C6H12O6 | 1.0 |
| NaHCO3 | 0.35 |
| Samples | Corrosion Rate v (mg· cm−2 ·h−1) | Degradation Rate CRw (mm·y−1) | Corrosion Resistance Efficiency η | pH |
|---|---|---|---|---|
| PCLys coating | 0.0961 | 4.729 | 36.36% | 7.09 |
| PCGly coating | 0.0794 | 3.908 | 47.40% | 7.12 |
| PCMet coating | 0.0883 | 4.346 | 41.56% | 7.03 |
| Compound coating | 0.0569 | 2.800 | 62.34% | 7.48 |
| ZE21B alloy | 0.1511 | 7.436 | - | 7.88 |
| Blank control | - | 7.32 | ||
| Samples | Corrosion Rate v (mg· cm−2 ·h−1) | Degradation Rate CRw (mm·y−1) | Corrosion Resistance Efficiency η | pH |
|---|---|---|---|---|
| PCLys coating | 0.0935 | 4.601 | 17.34% | 7.42 |
| PCGly coating | 0.0736 | 3.622 | 34.97% | 7.24 |
| PCMet coating | 0.0288 | 1.417 | 74.57% | 7.69 |
| Compound coating | 0.0281 | 1.383 | 75.14% | 7.81 |
| ZE21B alloy | 0.1131 | 5.566 | - | 8.21 |
| Blank control | - | 7.45 | ||
| Samples | Ecorr (V) | Icorr (µA/cm2) |
|---|---|---|
| PCLys coating | −1.468 | 0.507 |
| PCGly coating | −1.448 | 0.683 |
| PCMet coating | −1.440 | 0.466 |
| Compound coating | −1.427 | 0.440 |
| ZE21B alloy | −1.501 | 5.560 |
| Samples | PCLys Coating | PCGly Coating | PCMet Coating | Compound Coating | ZE21B Alloy |
|---|---|---|---|---|---|
| Rs (Ω·cm2) | 26.1 | 30.7 | 31.0 | 28.4 | 23.3 |
| R1 (Ω·cm2) | 84.4 | 64.7 | 233 | 16,580 | 24.3 |
| Q-Y1 (cm2·sn·Ω) | 0 | 0 | 0 | 0 | 0 |
| n1 | 0.95 | 1 | 0.77 | 0.73 | 1 |
| R2 (Ω·cm2) | 12,660 | 11,000 | 12,430 | 1.3 | 77.3 |
| Q-Y2 (cm2·sn·Ω) | 0 | 0 | 0 | 0 | 0 |
| n2 | 0.80 | 0.78 | 0.83 | 0.78 | 1 |
| R3 (Ω·cm2) | 3037 | 0.7 | 3500 | 53.5 | 115.8 |
| Q-Y3 (cm2·sn·Ω) | 0 | 0.71 | 0 | 0 | 0 |
| n3 | 1 | 0.8 | 1 | 0.67 | 1 |
| Rsum (Ω·cm2) | 15,781.4 | 11,065.4 | 16,163 | 16,634.8 | 217.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Hou, R.; Liu, C.; Xue, Z.; Zhang, K.; Li, J.; Guan, S. Tailoring of Biodegradable Magnesium Alloy Surface with Schiff Base Coating via Electrostatic Spraying for Better Corrosion Resistance. Metals 2022, 12, 471. https://doi.org/10.3390/met12030471
Sheng Y, Hou R, Liu C, Xue Z, Zhang K, Li J, Guan S. Tailoring of Biodegradable Magnesium Alloy Surface with Schiff Base Coating via Electrostatic Spraying for Better Corrosion Resistance. Metals. 2022; 12(3):471. https://doi.org/10.3390/met12030471
Chicago/Turabian StyleSheng, Yulong, Ruiqing Hou, Changsheng Liu, Zhonghua Xue, Kun Zhang, Jingan Li, and Shaokang Guan. 2022. "Tailoring of Biodegradable Magnesium Alloy Surface with Schiff Base Coating via Electrostatic Spraying for Better Corrosion Resistance" Metals 12, no. 3: 471. https://doi.org/10.3390/met12030471






