Microstructure and Properties of Novel Mg-Al-Zn-Mn-Ca-Ni Dissoluble Alloy Fabricated by Industrial Two-Step Extrusion Method
Abstract
:1. Introduction
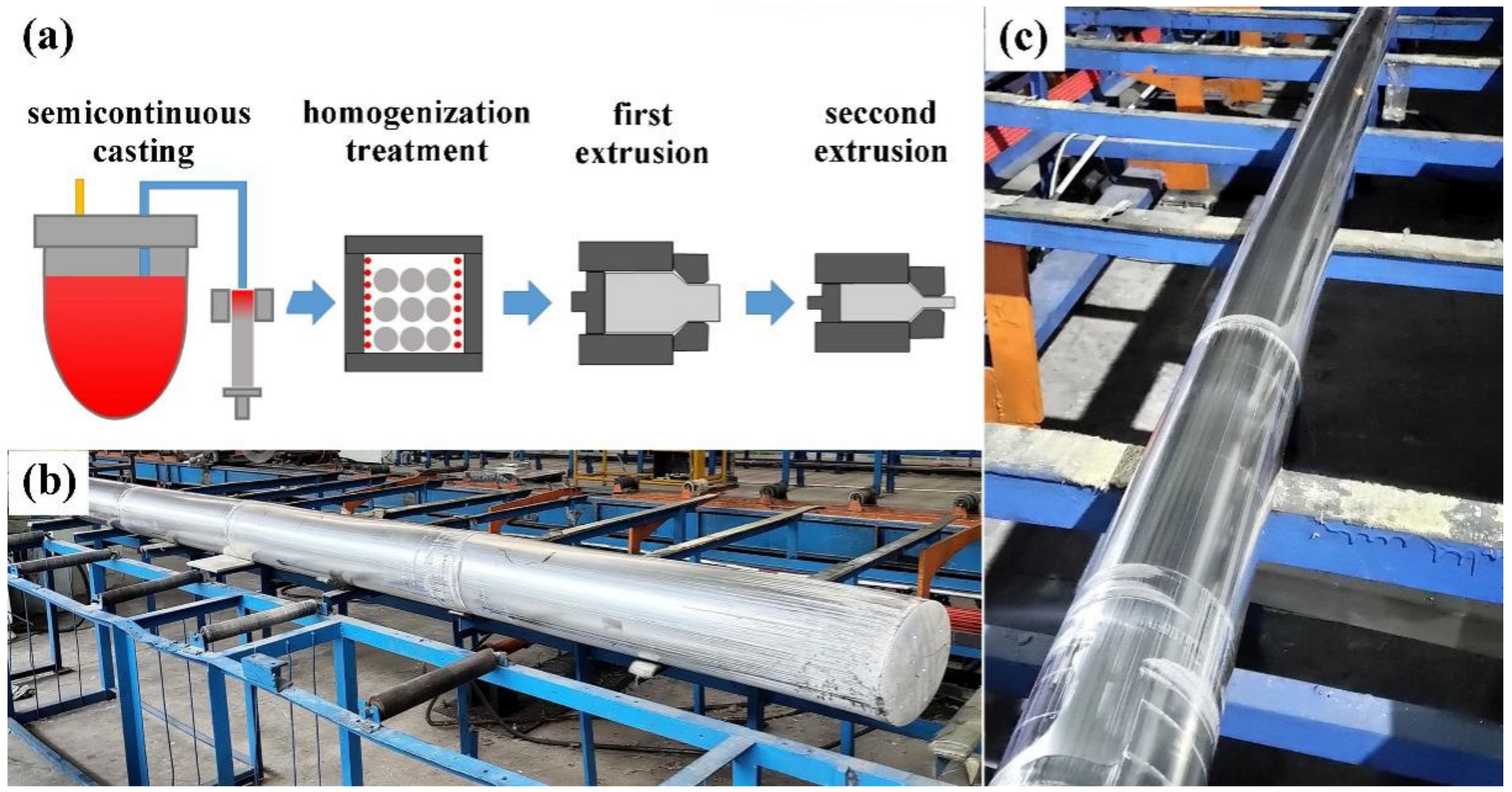
2. Materials and Methods
3. Results and Discussion
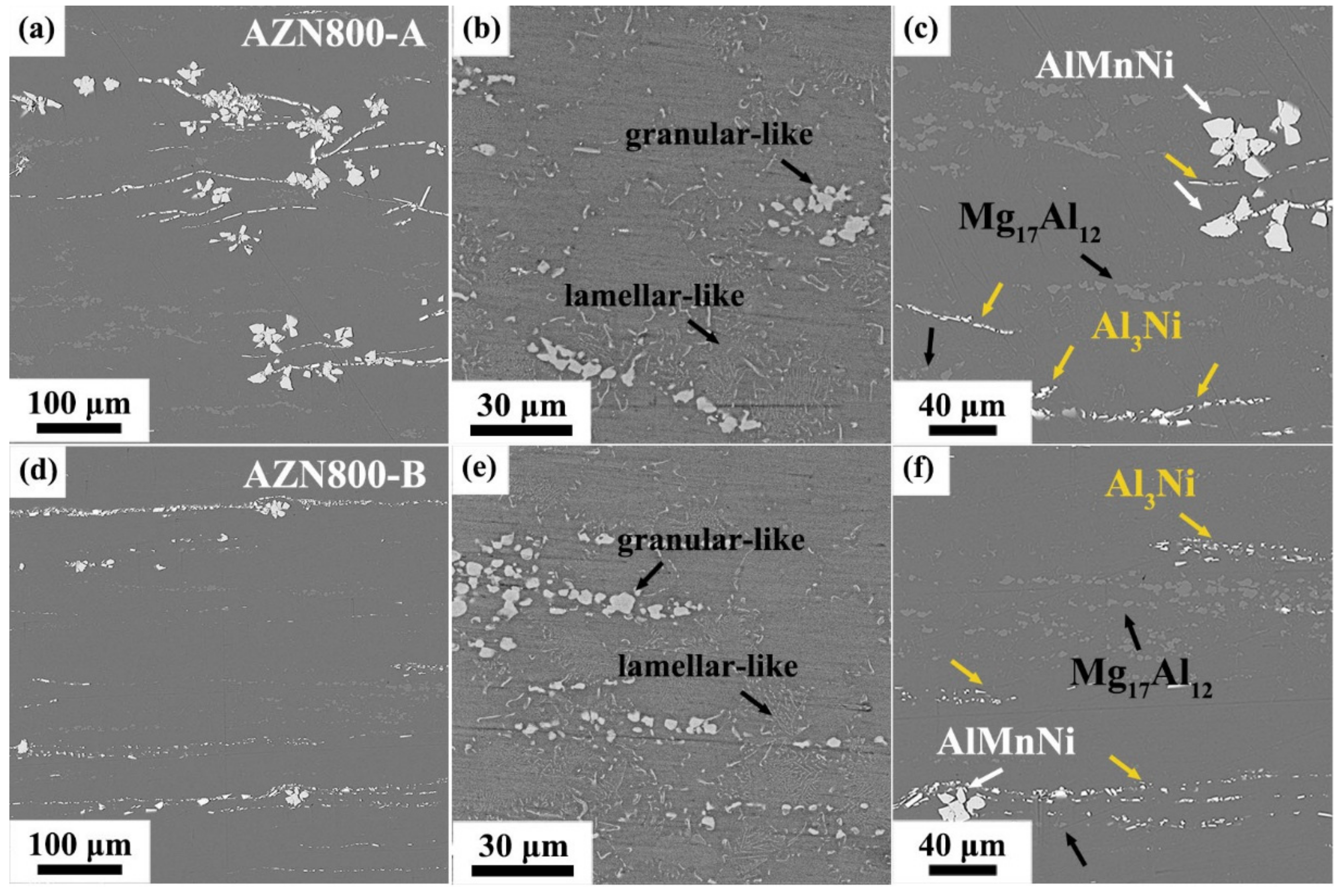
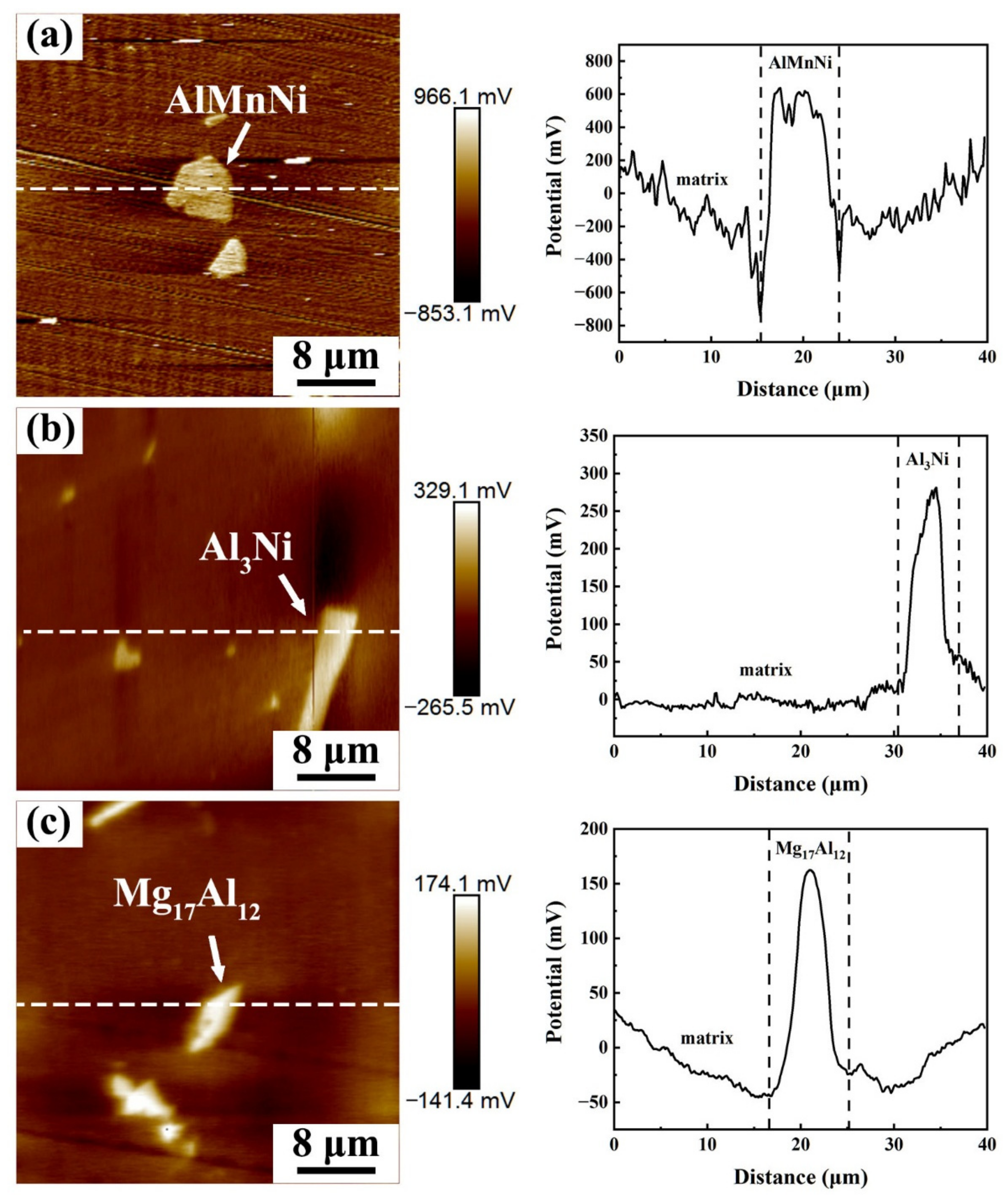
3.1. Microstructure Characterization
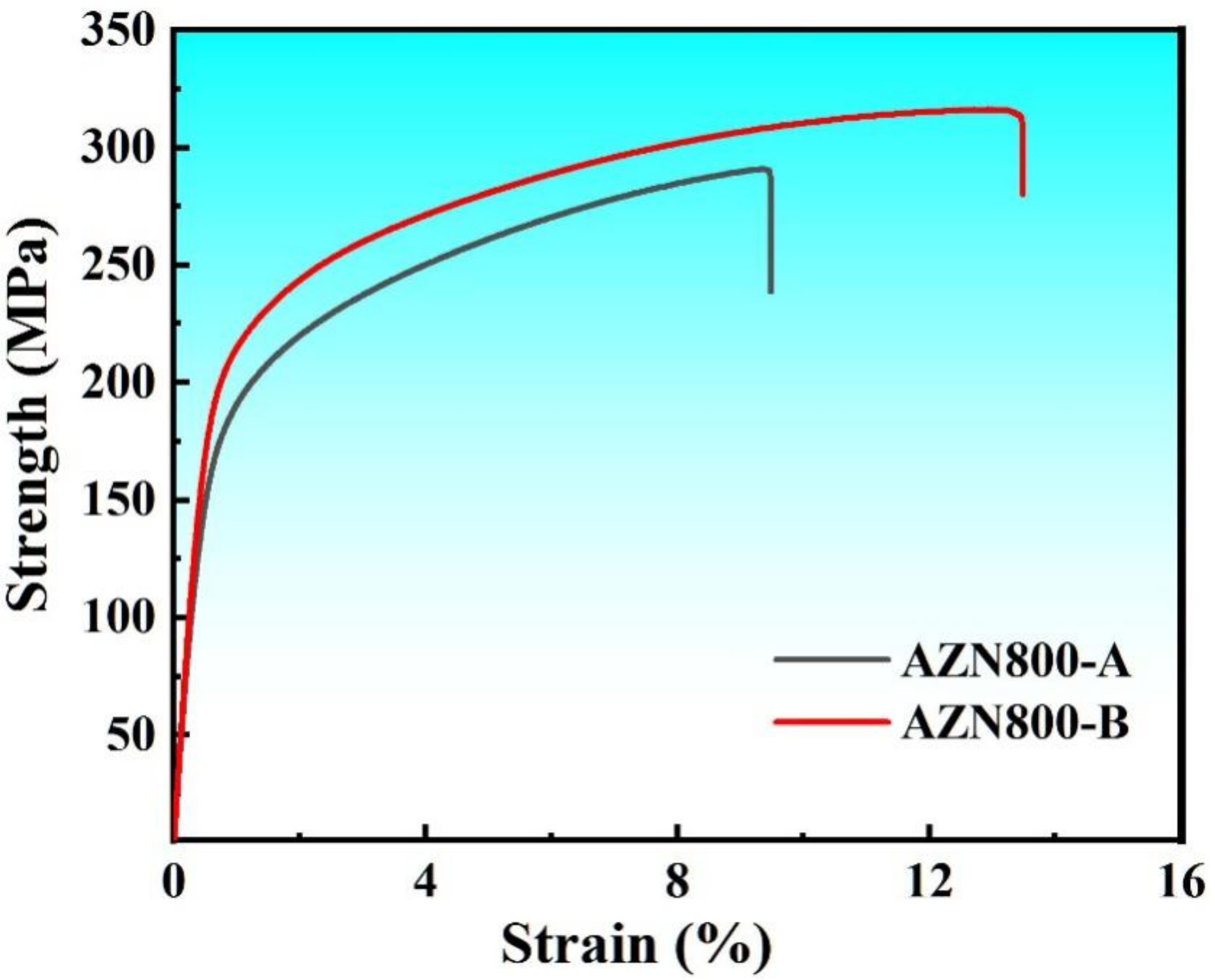
3.2. Mechanical Properties
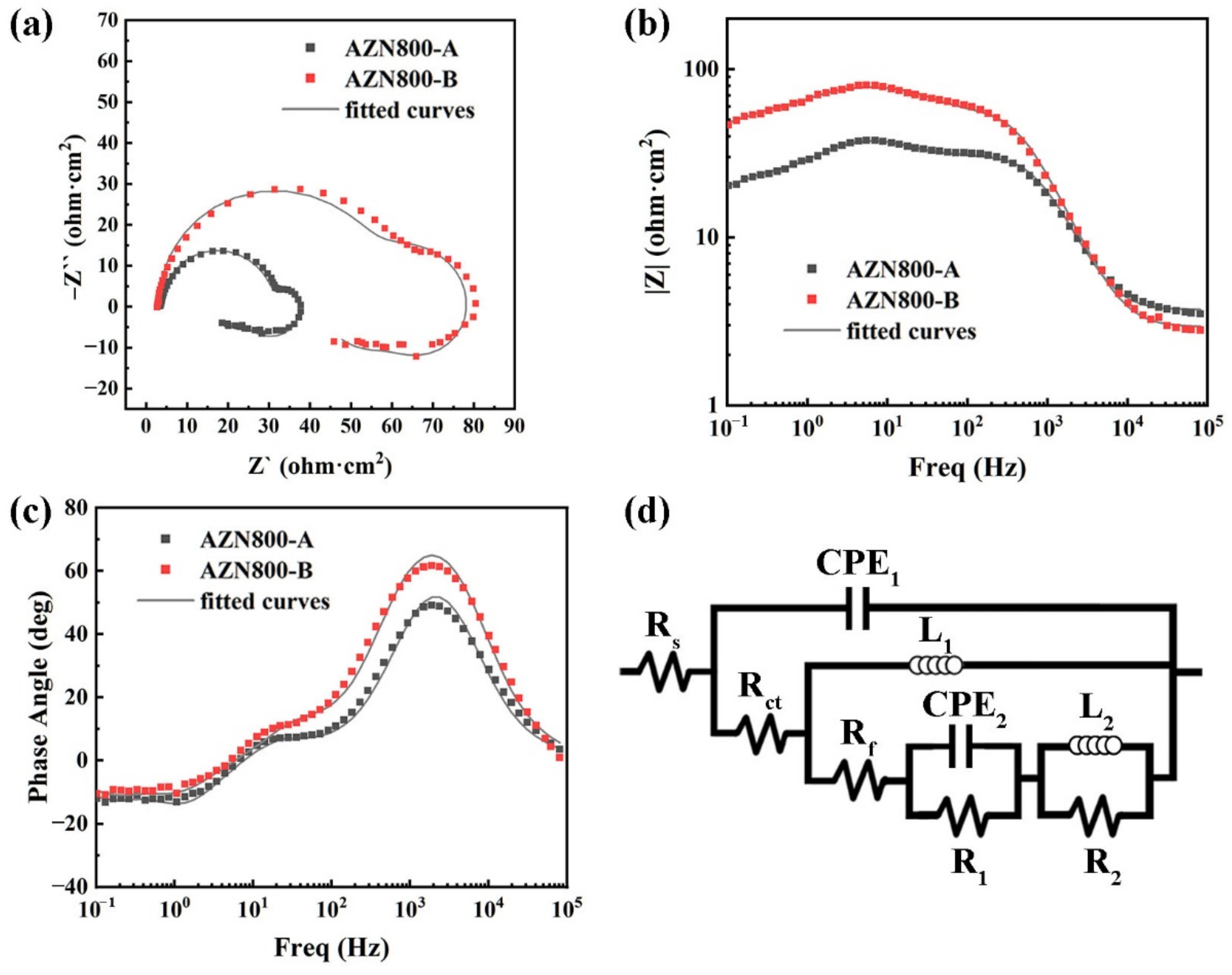
3.3. Corrosion Properties
4. Conclusions
- (1)
- The mainly precipitate phases in the Mg-Al-Zn-Mn-Ca-Ni alloys were bulk-like AlMnNi, strip-like Al3Ni, and granular-like and lamellar-like Mg17Al12. The types of precipitate phases did not change after the two-step extrusion, but the lager-size precipitate phases were obviously broken and refined after the second-step extrusion. In addition to the change in precipitate phases, the two-step extrusion process also had an effect on the texture of the alloy. The texture distribution of the AZN800-B alloy was more dispersed and the texture intensity was increased.
- (2)
- The AZN800-B alloy achieved excellent mechanical properties. The ultimate tensile strength, tensile yield strength, and elongation were 314.6 MPa, 191.2 MPa, and 13.1%, respectively.
- (3)
- Temperature had a great influence on the corrosion susceptibility of the precipitated phase. The corrosion rate of the AZN800-B alloy was 6.04 mg·cm−2·h−1 at 25 °C. However, the AZN800-B alloys exhibited excellent corrosion rates (97.61 mg·cm−2·h−1) at 93 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Serdyukov, S.V.; Degtyareva, N.V.; Patutin, A.V.; Shilova, T.V. Open-hole multistage hydraulic fracturing system. J. Min. Sci. 2016, 52, 1210–1215. [Google Scholar]
- Zheng, C.; Liu, Y.; Wang, H.; Zhu, H.; Liu, Z.; Cai, B.; Shen, Y. Numerical study on improving the erosion life of ball seat for oil and gas reservoir fracturing. Eng. Fail. Anal. 2016, 60, 188–198. [Google Scholar]
- Sampath, K.H.S.M.; Perera, M.S.A.; Ranjith, P.G. Theoretical overview of hydraulic fracturing break-down pressure. J. Nat. Gas Sci. Eng. 2018, 58, 251–265. [Google Scholar]
- Liu, J.; Shao, Z.; Zhang, X. Development of a high-strength soluble aluminum alloy and its application in oil pressure cracking. Chem. Technol. Fuels Oils 2019, 54, 818–823. [Google Scholar]
- Zheng, C.; Wu, X.; Zheng, X.; Jin, H.; Liu, Y. Mechanical properties and wear behavior of a dissolvable magnesium alloy used for multistage fracturing. Wear 2021, 466–467, 203559. [Google Scholar]
- Yang, Y.; Xiong, X.M.; Chen, J.; Peng, X.D.; Chen, D.L.; Pan, F.S. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar]
- Song, J.F.; She, J.; Chen, D.L.; Pan, F.S. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar]
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.F.; Birbilis, N. Magnesium extrusion alloys: A review of developments and prospects. Int. Mater. Rev. 2018, 64, 27–62. [Google Scholar]
- Xiao, D.H.; Geng, Z.W.; Chen, L.; Wu, Z.; Diao, H.Y.; Song, M.; Zhou, P.F. Effects of alloying elements on microstructure and properties of magnesium alloys for tripling ball. Metall. Mater. Trans. A 2015, 46, 4793–4803. [Google Scholar]
- Chen, L.; Huang, G.S.; Zhang, C.; Xia, D.B.; Zhao, Y.C.; Pan, F.S. Corrosion behaviours and mechanical properties of Fe/Mg-8Al-1Zn matrix composite. Mater. Sci. Technol. 2017, 33, 1312–1318. [Google Scholar]
- Han, Z.; Zhang, K.; Yang, J.; Wei, R.; Liu, Y.; Zhang, C. The Anodic Role of Ni-Containing LPSO Phases During the Microgalvanic Corrosion of Mg98Gd1.5Ni0.5 Alloy. J. Mater. Eng. Perform. 2019, 28, 2451–2458. [Google Scholar]
- Han, Z.H.; Zhang, K.; Yang, J.; Wei, R.; Zhang, C.J. Effects of volume fraction of Ni-containing LPSO phase on mechanical and corrosion properties of Mg-Gd-Ni alloys. Mater. Corros. 2019, 70, 537–548. [Google Scholar]
- Wang, J.F.; Gao, S.Q.; Liu, X.Y.; Peng, X.; Wang, K.; Liu, S.J.; Jiang, W.Y.; Guo, S.F.; Pan, F.S. Enhanced mechanical properties and degradation rate of Mg-Ni-Y alloy by introducing LPSO phase for degradable fracturing ball applications. J. Magnes. Alloy. 2020, 8, 127–133. [Google Scholar]
- Chen, L.; Wu, Z.; Xiao, D.H.; Geng, Z.W.; Zhou, P.F. Effects of copper on the microstructure and properties of Mg-17Al-3Zn alloys. Mater. Corros. 2015, 66, 1159–1168. [Google Scholar]
- Geng, Z.W.; Xiao, D.H.; Chen, L. Microstructure, mechanical properties, and corrosion behavior of degradable Mg-Al-Cu-Zn-Gd alloys. J. Alloys Compd. 2016, 686, 145–152. [Google Scholar]
- Wang, M.F.; Xiao, D.H.; Zhou, P.F.; Liu, W.S.; Ma, Y.Z.; Sun, B.R. Effects of rare earth yttrium on microstructure and properties of Mg-Al-Zn alloy. J. Alloys Compd. 2018, 742, 232–239. [Google Scholar]
- Wang, M.; Xiao, D.H.; Liu, W.S. Effect of Si addition on microstructure and properties of magnesium alloys with high Al and Zn contents. Vacuum 2017, 141, 144–151. [Google Scholar]
- Zhang, C.; Wu, L.; Huang, G.S.; Chen, L.; Xia, D.B.; Jiang, B.; Atrens, A.; Pan, F.S. Effects of Fe concentration on microstructure and corrosion of Mg-6Al-1Zn-xFe alloys for fracturing balls applications. J. Mater. Sci. Technol. 2019, 35, 2086–2098. [Google Scholar]
- Liu, B.; Yang, Y.; Zhang, Y.; Du, H.; Hou, L.; Wei, Y. Investigation of rapidly decomposable AZ91-RE-xCu (x=0, 1, 2, 3, 4) alloys for petroleum fracturing balls. J. Phys. Chem. Solids 2020, 144, 109499. [Google Scholar]
- Liu, L.; Yu, S.; Niu, Y.; Liu, E. Preparation and properties of hollow glass microspheres reinforced Mg alloy degradable composites. J. Alloys Compd. 2020, 835, 155198. [Google Scholar]
- Liu, L.; Yu, S.; Liu, E.; Zhao, Y.; Wang, B.; Niu, Y. Effect of Ni addition on the mechanical and degradation properties of hollow glass microsphere/Mg alloy composites. J. Alloys Compd. 2021, 853, 157125. [Google Scholar] [CrossRef]
- Li, R.G.; Li, H.R.; Zhao, D.Y.; Dai, Y.Q.; Fang, D.Q.; Zhang, J.H.; Zong, L.; Sun, J. High strength commercial AZ91D alloy with a uniformly fine-grained structure processed by conventional extrusion. Mater. Sci. Eng. A 2020, 780, 139193. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, J.; Fan, J.; Zhang, H.; Zhang, Q.; Wu, Y.; Li, W.; Dong, H.; Xu, B. Extraordinary mechanical properties of AZ61 alloy processed by ECAP with 160° channel angle and EPT. J. Magnes. Alloy. 2021, 9, 548–559. [Google Scholar] [CrossRef]
- Liu, X.; Xu, R. Achieving ultra-high hardness of Mg-Sm-Ca alloy with the unique nanostructure. Mater. Sci. Eng. A 2021, 825, 141929. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, G.; Wang, L.; Vasilev, E.; Park, J.S.; Sha, G.; Zeng, X.; Knezevic, M. Origins of high ductility exhibited by an extruded magnesium alloy Mg-1.8Zn-0.2Ca: Experiments and crystal plasticity modeling. J. Mater. Sci. Technol. 2021, 84, 27–42. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, Y.; Li, K.; Zhuang, Y.; Li, J.; Yuan, Y.J.; Yin, D. High-ductility fine-grained Mg-1.92Zn-0.34Y alloy fabricated by semisolid and then hot extrusion. J. Magnes. Alloy. 2021. in Press. [Google Scholar] [CrossRef]
- Zhong, F.; Wang, Y.; Wu, R.; Zhang, J.; Hou, L.; Zhang, M. Effect of rolling temperature on deformation behavior and mechanical properties of Mg-8Li-1Al-0.6Y-0.6Ce alloy. J. Alloys Compd. 2020, 831, 154765. [Google Scholar] [CrossRef]
- Nakata, T.; Xu, C.; Ajima, R.; Shimizu, K.; Hanaki, S.; Sasaki, T.T.; Ma, L.; Hono, K.; Kamado, S. Strong and ductile age-hardening Mg-Al-Ca-Mn alloy that can be extruded as fast as aluminum alloys. Acta Mater. 2017, 130, 261–270. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Fu, Q.; Sheng, K.; Liu, M.; Sun, Y.; Mei, D.; Kou, Y.; Zhu, S.; Guan, S. Microstructure, mechanical properties and corrosion fatigue behaviour of biodegradable Mg-Zn-Y-Nd alloy prepared by double extrusion. Corros. Eng. Sci. Technol. 2021, 56, 584–593. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; Huang, G.S.; Huang, Y.; Jiang, B.; Atrens, A.; Pan, F.S. Effect of microalloyed Ca on the microstructure and corrosion behavior of extruded Mg alloy AZ31. J. Alloys Compd. 2020, 823, 153844. [Google Scholar] [CrossRef]
- Woo, S.K.; Blawert, C.; Yasakau, K.A.; Yi, S.; Scharnagl, N.; Suh, B.-C.; Kim, Y.M.; Sun You, B.; Dong Yim, C. Effects of combined addition of Ca and Y on the corrosion behaviours of die-cast AZ91D magnesium alloy. Corros. Sci. 2020, 166, 108451. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, D.; Fan, X.; Guo, F.; Hu, G.; Xue, H.; Pan, F. The effect of Sn addition on aging behavior and mechanical properties of wrought AZ80 magnesium alloy. J. Alloys Compd. 2015, 620, 368–375. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, G.; Atrens, A.; Ding, W. Influence of trace As content on the microstructure and corrosion behavior of the AZ91 alloy in different metallurgical conditions. J. Magnes. Alloy. 2020, 8, 301–317. [Google Scholar] [CrossRef]
- Zhou, X.J.; Liu, C.M.; Gao, Y.H.; Jiang, S.N.; Liu, W.H.; Lu, L.W. Microstructure and mechanical properties of extruded Mg-Gd-Y-Zn-Zr alloys filled with intragranular LPSO phases. Mater. Charact. 2018, 135, 76–83. [Google Scholar] [CrossRef]
- Wang, Q.F.; Liu, K.; Wang, Z.H.; Li, S.B.; Du, W.B. Microstructure, texture and mechanical properties of as-extruded Mg-Zn-Er alloys containing W-phase. J. Alloys Compd. 2014, 602, 32–39. [Google Scholar] [CrossRef]
- Li, Z.T.; Qiao, X.G.; Xu, C.; Kamado, S.; Zheng, M.Y.; Luo, A.A. Ultrahigh strength Mg-Al-Ca-Mn extrusion alloys with various aluminum contents. J. Alloys Compd. 2019, 792, 130–141. [Google Scholar] [CrossRef]
- Xian, J.W.; Peng, L.; Zeng, G.; Wang, D.; Gourlay, C.M. Al11Mn4 formation on Al8Mn5 during the solidification and heat treatment of AZ-series magnesium alloys. Materialia 2021, 19, 101192. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, B.; Xin, Y.; Huang, X.; Liu, C.; Wu, P.; Liu, Q. A quantitative study on mechanical behavior of Mg alloys with bimodal texture components. Acta Mater. 2021, 214, 117013. [Google Scholar] [CrossRef]
- Sánchez-Martín, R.; Pérez-Prado, M.T.; Segurado, J.; Bohlen, J.; Gutiérrez-Urrutia, I.; Llorca, J.; Molina-Aldareguia, J.M. Measuring the critical resolved shear stresses in Mg alloys by instrumented nanoindentation. Acta Mater. 2014, 71, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Cepeda-Jiménez, C.M.; Molina-Aldareguia, J.M.; Pérez-Prado, M.T. Effect of grain size on slip activity in pure magnesium polycrystals. Acta Mater. 2015, 84, 443–456. [Google Scholar] [CrossRef]
- Máthis, K.; Farkas, G.; Garcés, G.; Gubicza, J. Evolution of dislocation density during compression of a Mg-Zn-Y alloy with long period stacking ordered structure. Mater. Lett. 2017, 190, 86–89. [Google Scholar] [CrossRef]
- Xu, C.; Nakata, T.; Qiao, X.G.; Jiang, H.S.; Sun, W.T.; Chi, Y.C.; Zheng, M.Y.; Kamado, S. Effect of extrusion parameters on microstructure and mechanical properties of Mg-7.5Gd-2.5Y-3.5Zn-0.9Ca-0.4Zr (wt%) alloy. Mater. Sci. Eng. A 2017, 685, 159–167. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, F.; Song, L.; Zeng, R.C.; Li, S.Q.; Han, E.H. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Niu, H.Y.; Deng, K.K.; Nie, K.B.; Cao, F.F.; Zhang, X.C.; Li, W.G. Microstructure, mechanical properties and corrosion properties of Mg-4Zn-xNi alloys for degradable fracturing ball applications. J. Alloys Compd. 2019, 787, 1290–1300. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Chang, J.W.; Guo, X.W.; He, S.M.; Fu, P.H.; Peng, L.M.; Ding, W.J. Investigation of the corrosion for Mg-xGd-3Y-0.4Zr (x = 6, 8, 10, 12 wt%) alloys in a peak-aged condition. Corros. Sci. 2008, 50, 166–177. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, A.; Xu, B.; Jiang, J.; Sun, J. Corrosion behavior of AZ91 Mg alloy with a heterogeneous structure produced by ECAP. Corros. Sci. 2021, 187, 109517. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the corrosion mechanism of Mg investigated by electrochemical impedance spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Han, E.H. Corrosion characterization of Mg-8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Ren, J.; Kang, H.; Guo, E.; Li, J.; Wang, T. Corrosion behavior of as-cast Mg–5Sn based alloys with In additions in 3.5 wt% NaCl solution. Corros. Sci. 2020, 164, 108318. [Google Scholar] [CrossRef]
- Liu, J.H.; Song, Y.W.; Chen, J.C.; Chen, P.; Shan, D.Y.; Han, E.H. The special role of anodic second phases in the micro-galvanic corrosion of EW75 mg alloy. Electrochim. Acta 2016, 189, 190–195. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Nyberg, E.A.; Pan, F.S. Effect of Ca addition on the corrosion behavior of Mg-Al-Mn alloy. Appl. Surf. Sci. 2016, 369, 92–100. [Google Scholar] [CrossRef]
- Tan, W.; Li, T.; Li, S.; Fang, D.; Ding, X.; Sun, J. High strength-ductility and rapid degradation rate of as-cast Mg-Cu-Al alloys for application in fracturing balls. J. Mater. Sci. Technol. 2021, 94, 22–31. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Recent insights into the mechanism of magnesium corrosion and research suggestions. Adv. Eng. Mater. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, X.Y.; Kuang, Y.F.; Liu, B.S.; Zhang, K.W.; Fang, D.Q. Enhanced mechanical properties and degradation rate of Mg-3Zn-1Y based alloy by Cu addition for degradable fracturing ball applications. Mater. Lett. 2017, 195, 194–197. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Li, H.X.; Ma, Y.Z.; Zhao, K.N.; Yang, C.L.; Zhang, J.S. Effect of trace Ni addition on microstructure, mechanical and corrosion properties of the extruded Mg-Gd-Y-Zr-Ni alloys for dissoluble fracturing tools. J. Magnes. Alloy. 2021, 9, 1632–1643. [Google Scholar] [CrossRef]
- Ma, K.; Liu, S.; Dai, C.; Liu, X.; Ren, J.; Pan, Y.; Peng, Y.; Su, C.; Wang, J.; Pan, F. Effect of Ni on the microstructure, mechanical properties and corrosion behavior of MgGd1Nix alloys for fracturing ball applications. J. Mater. Sci. Technol. 2021, 91, 121–133. [Google Scholar] [CrossRef]





| Phases | Alloy | Mg | Al | Mn | Ni | Zn |
|---|---|---|---|---|---|---|
| white block-like phase | AZN800-A | 9.75 | 56.20 | 23.03 | 11.02 | - |
| 10.11 | 55.51 | 22.09 | 12.29 | - | ||
| AZN800-B | 10.30 | 56.49 | 21.30 | 11.91 | - | |
| 15.81 | 55.79 | 17.94 | 10.46 | - | ||
| white strip-like phase | AZN800-A | 49.18 | 38.04 | 0.25 | 12.53 | - |
| 50.25 | 37.63 | 0.98 | 11.14 | - | ||
| AZN800-B | 60.79 | 30.58 | 0.23 | 8.40 | - | |
| 52.37 | 34.38 | 0.54 | 12.71 | - | ||
| gray irregular-like phase | AZN800-A | 66.57 | 32.23 | - | - | 1.19 |
| 66.11 | 32.67 | - | - | 1.22 | ||
| AZN800-B | 66.84 | 31.99 | - | - | 1.18 | |
| 66.38 | 32.30 | - | - | 1.32 |
| Alloy | Ecorr (VSCE) | Icorr (μA·cm−2) | βc (mV) | βa (mV) |
|---|---|---|---|---|
| AZN800-A | −1.436 ± 0.022 | 1235.25 ± 66.95 | 296.59 ± 18.97 | 51.00 ± 2.80 |
| AZN800-B | −1.447 ± 0.013 | 878.63 ± 53.38 | 273.62 ± 20.47 | 47.89 ± 1.07 |
| Alloy | Rs (Ω·cm−2) | CPE1 (μF·cm−2) | Rct (Ω·cm−2) | L1 (H·cm−2) | Rf (Ω·cm−2) | CPE2 (sn·μΩ−1·cm−2) | R1 (Ω·cm−2) | L2 (H·cm−2) | R2 (Ω·cm−2) |
|---|---|---|---|---|---|---|---|---|---|
| AZN800-A | 3.715 | 7.85 | 14.07 | 83.32 | 1.726 | 1113.00 | 7.867 | 1.216 | 11.64 |
| AZN800-B | 2.952 | 6.63 | 40.94 | 15.78 | 1.012 | 261.40 | 19.92 | 2.103 | 15.69 |
| Composition (wt.%) | State | UTS (MPa) | TYS (MPa) | ET (%) | UCS (MPa) | CYS (MPa) | EC (%) | Corrosion Rate (mm·day−1) |
|---|---|---|---|---|---|---|---|---|
| Mg-8Al-0.8Zn-0.3Mn-0.1Ca-0.2Ni (this study) | as-extruded | 314.6 | 191.2 | 13.1 | - | - | - | 12.66 (3% KCl 93°C) |
| Mg-17Al-3Zn-5Cu [14] | as-cast | - | - | - | 438 | 295 | 1.6 | ~11.34 (3% KCl 93 °C) * |
| Mg-17Al-3Zn-7Cu-1Gd [15] | as-cast | - | - | - | 442 | 302 | 7.8 | 1.51 (3% KCl 93 °C) |
| Mg-17Al-5Zn-3Si [17] | as-cast | - | - | - | 355 | 251 | 7.3 | 5.39 (3% KCl 93 °C) |
| Mg-15Al-5Zn-0.25Y [16] | as-cast | - | - | - | 417 | 226 | 7.2 | 0.111 (3% KCl 93 °C) |
| Mg-2.5Cu-6Al [53] | as-cast | 215.2 | - | 10.2 | 378.8 | - | 27.3 | 5.69 (3% KCl 93 °C) |
| 7Fe/Mg-6Al-1Zn [18] | as-extruded | - | - | - | ~350 | ~180 | ~9 | ~0.86 (3.5% NaCl 25 °C) * |
| Mg-4Zn-4Ni [44] | as-cast | - | - | - | 264.8 | - | 20.1 | ~8.52 (3% KCl 25 °C) * |
| Mg-3Zn-1Y-4Cu [56] | as-extruded | 302 | 248 | 18.2 | 385 | 224 | 9.6 | 5.52 (3% KCl 93 °C) |
| Mg-10Gd-3Y-0.2Zr-0.8Ni [57] | as-extruded | 342.9 | 257.8 | 15.2 | 596.5 | 296.5 | 17.8 | ~3.35 (3% KCl 93 °C) |
| Mg-6.753Gd-1.66Ni [58] | as-cast | - | - | - | 340 | 128 | - | 5.66 (3% KCl 25 °C) |
| Mg-16.47Gd-2.05Ni [12] | as-cast | - | - | - | 266 | 175 | ~10 | ~3.03 (3.5% NaCl 25 °C) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, H.; Wang, J.; Liu, Y.; Zhang, J. Microstructure and Properties of Novel Mg-Al-Zn-Mn-Ca-Ni Dissoluble Alloy Fabricated by Industrial Two-Step Extrusion Method. Metals 2022, 12, 583. https://doi.org/10.3390/met12040583
Wang J, Li H, Wang J, Liu Y, Zhang J. Microstructure and Properties of Novel Mg-Al-Zn-Mn-Ca-Ni Dissoluble Alloy Fabricated by Industrial Two-Step Extrusion Method. Metals. 2022; 12(4):583. https://doi.org/10.3390/met12040583
Chicago/Turabian StyleWang, Jian, Hongxiang Li, Jinhui Wang, Yaohong Liu, and Jishan Zhang. 2022. "Microstructure and Properties of Novel Mg-Al-Zn-Mn-Ca-Ni Dissoluble Alloy Fabricated by Industrial Two-Step Extrusion Method" Metals 12, no. 4: 583. https://doi.org/10.3390/met12040583
APA StyleWang, J., Li, H., Wang, J., Liu, Y., & Zhang, J. (2022). Microstructure and Properties of Novel Mg-Al-Zn-Mn-Ca-Ni Dissoluble Alloy Fabricated by Industrial Two-Step Extrusion Method. Metals, 12(4), 583. https://doi.org/10.3390/met12040583






