Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials
Abstract
:1. Introduction
2. Experimental
2.1. Anode Material Preparation
2.2. Preparation of Electrode Sheet
2.3. Assembly of Lithium Ion Button Battery
2.4. Physical Properties Characterization of Material
2.5. Electrochemical Performance Test of Materials
3. Results and Discussion
3.1. The Microstructure
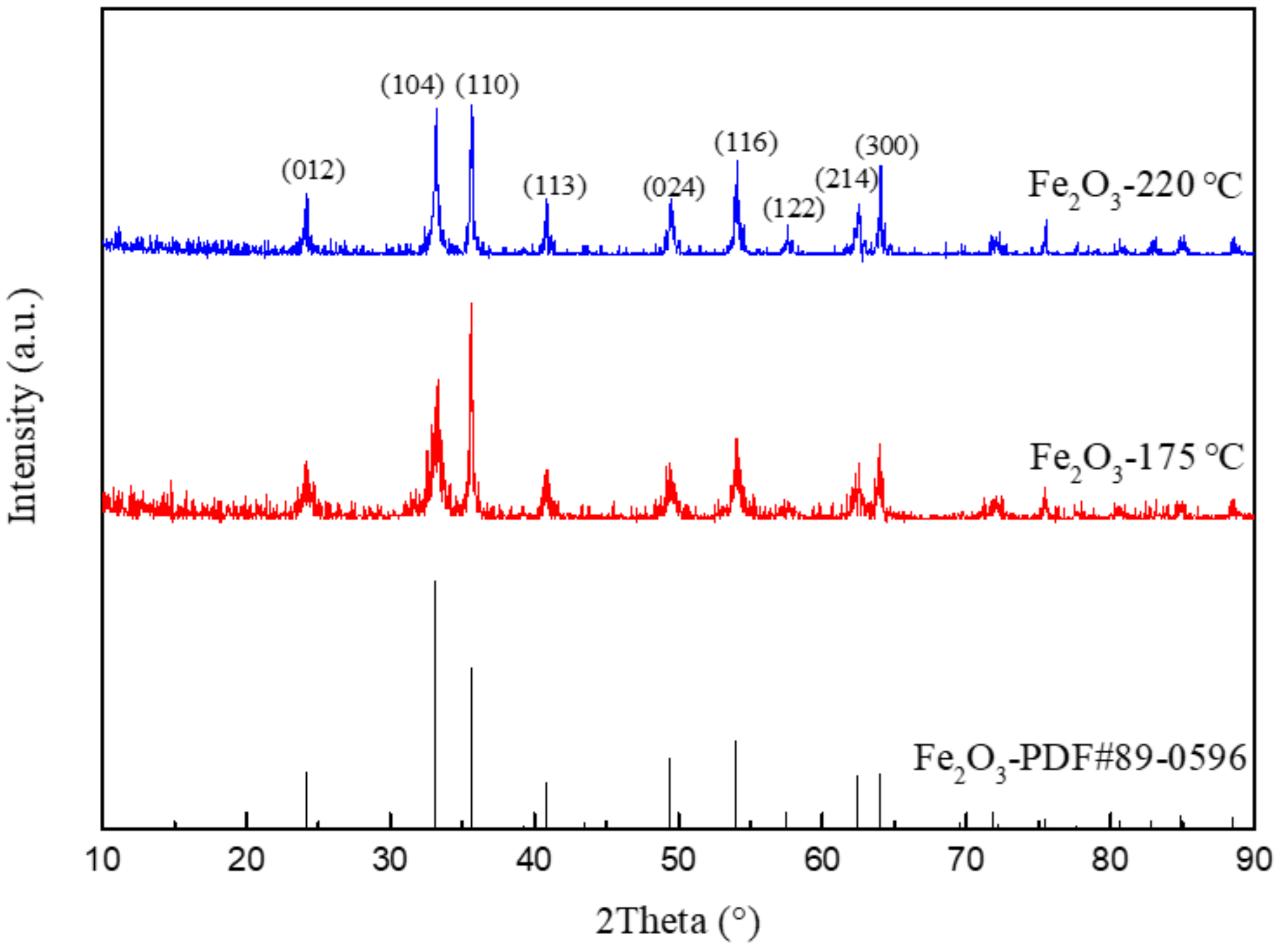
3.2. XRD
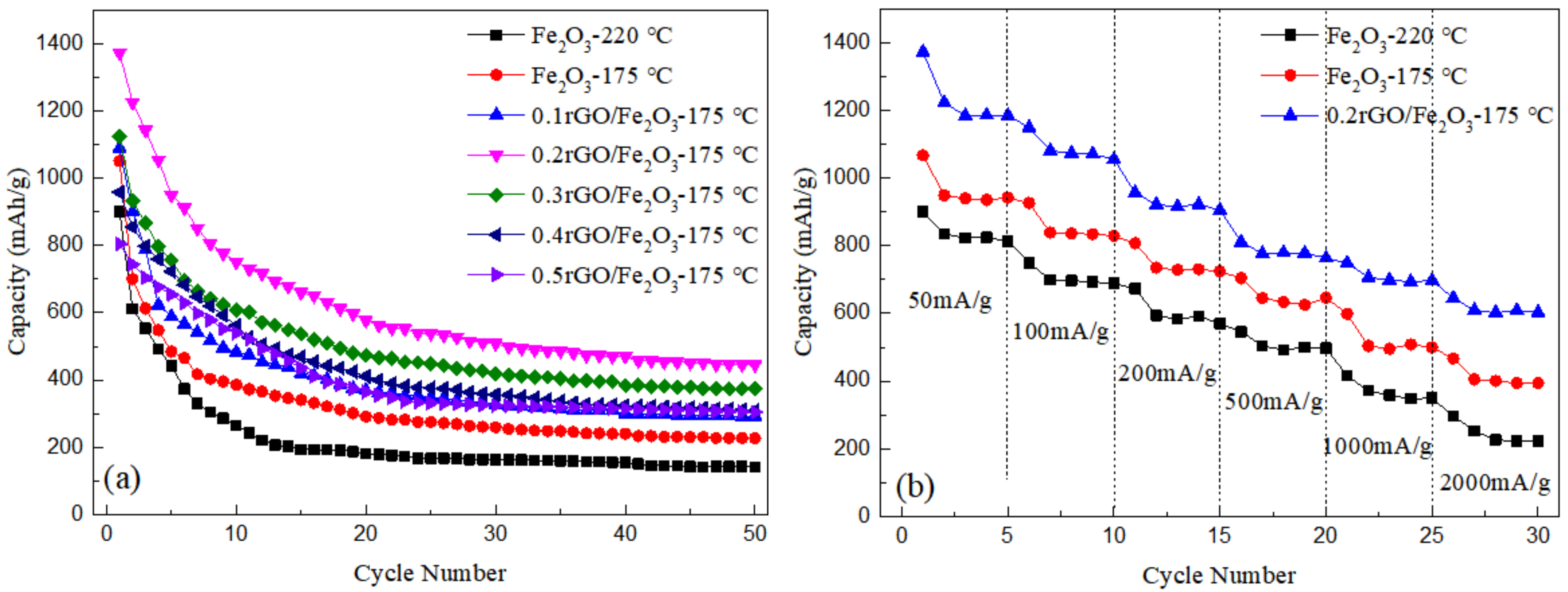
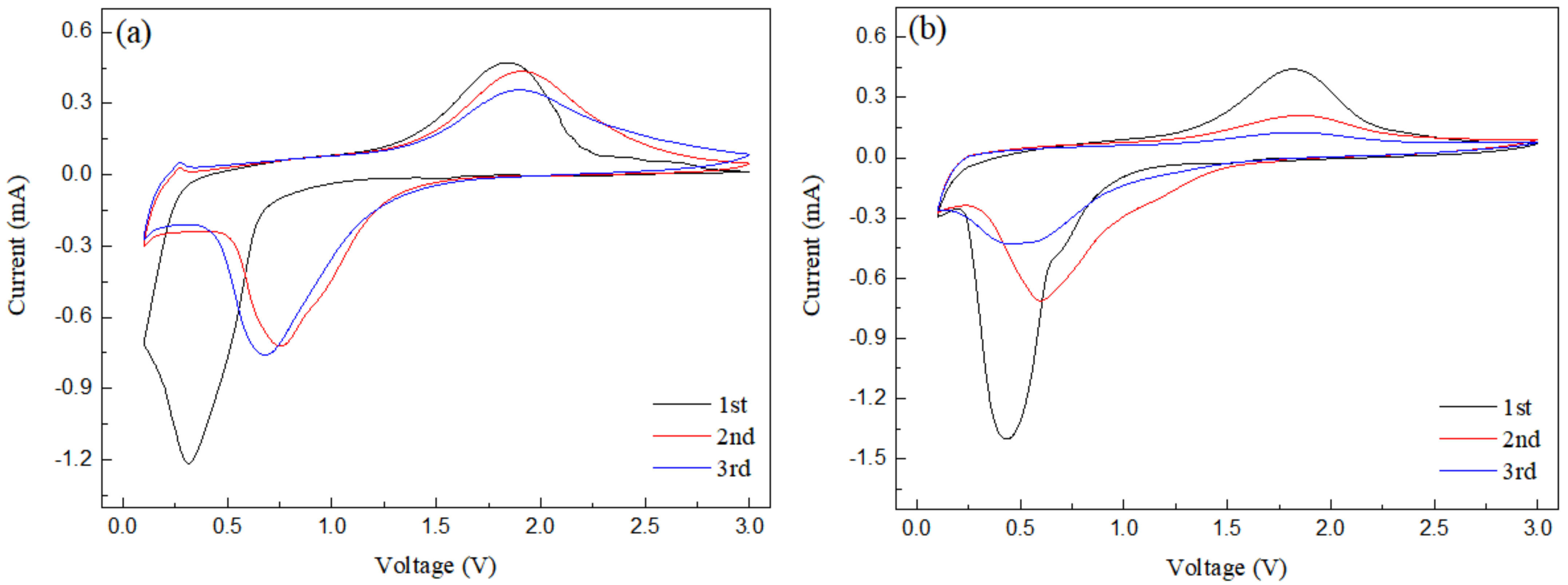
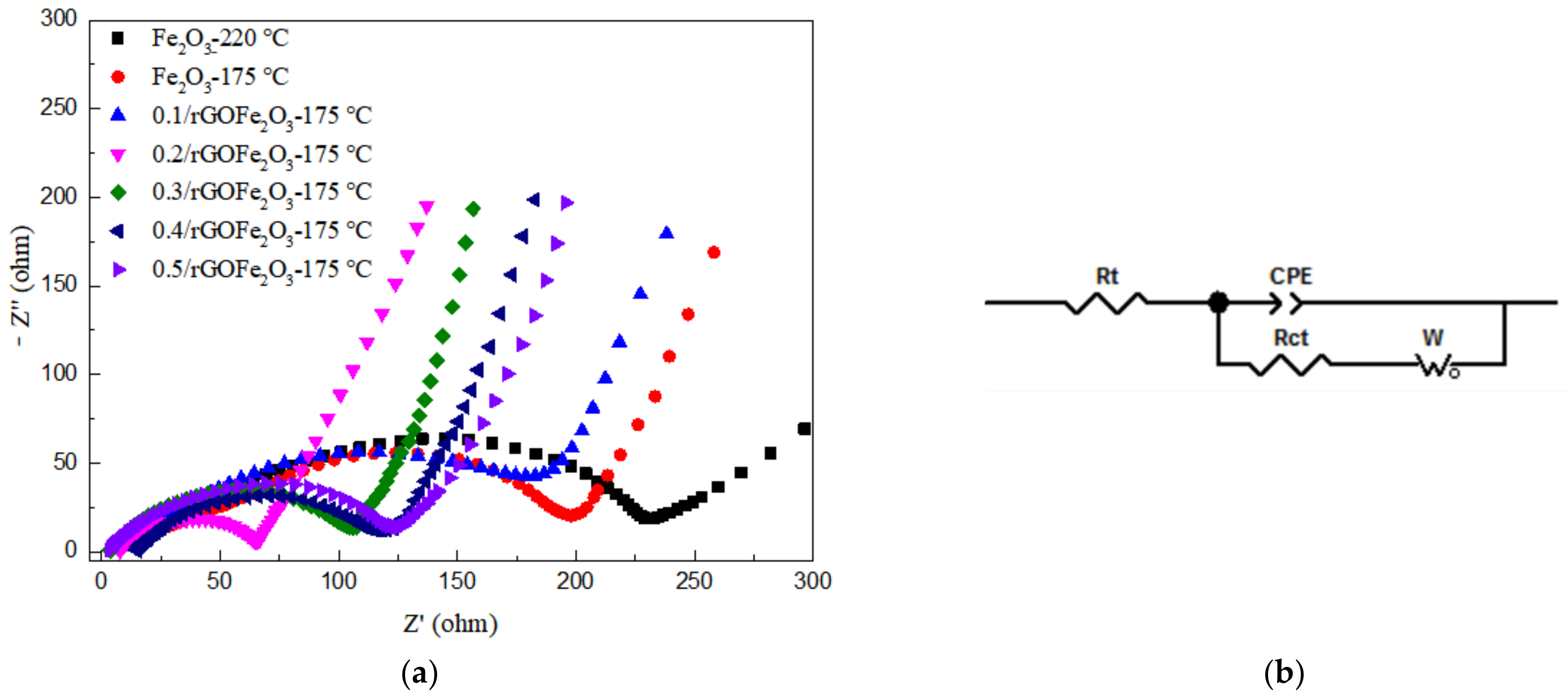
3.3. Electrochemical Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cong, Y.; Ge, Y.; Zhang, T.; Wang, Q.; Shao, M.; Wang, Y. Fabrication of z-scheme Fe2O3-MoS2-Cu2O ternary nanofilm with significantly enhanced photo electro catalytic performance. Ind. Eng. Chem. Res. 2018, 57, 881–890. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X.; Wang, H.; Zhang, Y.; Yang, X.; Shu, H. An Fe3O4@(C-MnO2) core-double-shell composite as a high-performance anode material for lithium ion batteries. RSC Adv. 2015, 5, 14531–14539. [Google Scholar] [CrossRef]
- Li, G.; Han, R.; Xu, X.; Ren, M. Facile synthesis of Mn-doped hollow Fe2O3 nanospheres coated with polypyrrole as anodes for high-performance lithium-ion batteries. RSC Adv. 2016, 6, 48199–48204. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ionatteries. Nature 2000, 407, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Chen, L.; Ju, Z.; Xu, H.; Yang, J.; Qian, Y. Controlled Growth of Porous α-Fe2O3 Branches on β-MnO2 Nanorods for Excellent Performance in Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 4049–4056. [Google Scholar] [CrossRef]
- Luo, J.; Xia, X.; Luo, Y.; Guan, C.; Liu, J.; Qi, X.; Fan, H.J. Rationally designed hierarchical TiO2@ Fe2O3 hollow nanostructures for improved lithium ion storage. Adv. Energy Mater. 2013, 3, 737–743. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, Z.; Li, G. Structural evolution from mesoporous α-Fe2O3 to Fe3O4@C and γ-Fe2O3 nanospheres and their lithium storage performances. CrystEngComm 2011, 13, 4709–4713. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, C.; Liu, J.; Tay, Y.Y.; Jiang, J.; Jia, X.; Zhang, J.; Gong, H.; Hng, H.H.; Yu, T. Epitaxial Growth of Branched α-Fe2O3/SnO2 Nano-Hetero structures with Improved Lithium-Ion Battery Performance. Adv. Funct. Mater. 2011, 21, 2439–2445. [Google Scholar] [CrossRef]
- Wu, M.; Ou, Y.; Lin, P. Iron oxide nanosheets and nanoparticles synthesized by a facile single-step coprecipitation method for lithium-ion batteries. J. Electrochem. Soc. 2011, 158, 231–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Z.; Xu, Z.J. Yolk-shell Fe2O3 circle dot C composites anchored on MWNTs with enhanced lithium and sodium storage. Nanoscale 2015, 7, 9520–9525. [Google Scholar] [CrossRef]
- Zheng, F.; He, M.; Yang, Y.; Chen, Q. Nano electrochemical reactors of Fe2O3 nanoparticles embedded in shells of nitrogen-doped hollow carbon spheres as high-performance anodes for lithium-ion batteries. Nanoscale 2015, 7, 3410–3417. [Google Scholar] [CrossRef]
- Yu, A.; Park, W.H.; Davies, A.; Higgins, C.D.; Chen, Z.; Xiao, X. Free-standing layer-by-layer hybrid thin film of graphene-MnO2 nanotube as anode for lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 1855–1860. [Google Scholar] [CrossRef]
- Lv, X.; Deng, J.; Wang, J.; Zhong, J.; Sun, X. Carbon-coated α-Fe2O3 nanostructures for efficient anode of Li-ion battery. J. Mater. Chem. A 2015, 3, 5183–5188. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, R.; Cai, X.; Jiao, Z.; Wu, M.; Ling, X. Nanorod-like Fe2O3/graphene composite as high-performance anode material for lithium ion batteries. J. Appl. Electrochem. 2014, 44, 53–60. [Google Scholar] [CrossRef]
- Zhou, G.W.; Wang, J.; Gao, P.; Yang, X.; He, Y.; Liao, X.; Yang, J.; Ma, Z. Facile spray drying route for the three-dimensional graphene-encapsulated Fe2O3 nanoparticles for lithium ion battery anodes. Ind. Eng. Chem. Res. 2012, 52, 1197–1204. [Google Scholar] [CrossRef]
- Zhen, Y.X.; Arbizzani, C.; Ortolani, L.; Morandi, V.; Bellani, V.; Giambastiani, G. High yield pro-duction of graphene-Fe2O3 nano-composites via electrochemical intercalation of nitromethane and iron chloride, and their application in lithium storage. FlatChem 2017, 3, 8–15. [Google Scholar]
- Wang, G.X.; Shen, X.P.; Yao, J. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, X.; Ivanovici, S.; Mellen, K. Fabrication of graphene-encapsulated oxide nanoparticles: Towards high-performance anode materials for lithium storage. Angew. Chem. 2010, 49, 8408–8411. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2011, 56, 4532–4539. [Google Scholar] [CrossRef]
- Cai, D.; Li, D.; Wang, S.; Zhu, X.; Yang, W.; Zhang, S.; Wang, H. High rate capability of TiO2/nitrogen-doped graphene nanocomposite as an anode material for lithium-ion batteries. J. Alloys Compd. 2016, 561, 54–58. [Google Scholar] [CrossRef]
- Kan, J.; Wang, Y. Large and fast reversible Li-ion storages in Fe2O3-graphene sheet-on-sheet sandwich-like nanocomposites. Sci. Rep. 2013, 3, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Fu, L.; Liu, Y.; Zheng, G.; Zheng, X.; Guan, X.; Zhang, J. Gas-liquid interfacial assembly and electrochemical properties of 3D highly dispersed α-Fe2O3@graphene aerogel composites with a hierarchical structure for applications in anodes of lithium ion batteries. Electrochim. Acta 2017, 224, 40–48. [Google Scholar] [CrossRef]
- Tian, L.; Zhuang, Q.; Li, J.; Shi, Y.; Chen, J.; Lu, F.; Sun, S. Mechanism of intercalation and deintercalation of lithium ions in graphene nanosheets. Chin. Sci. Bull. 2011, 56, 3204–3206. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, Z.; Xie, K.; Li, Y.; Xu, J.; Zheng, C. A facile one-step hydrothermal synthesis of alpha-Fe2O3 nanoplates imbedded in graphene networks with high-rate lithium storage and long cycle life. J. Mater. Chem. A 2014, 2, 13942–13948. [Google Scholar] [CrossRef]
- Yoon, S.; In, I. Role of poly(N-vinyl-2-pyrrolidone) as stabilizer for dispersion of graphene via hydrophobic interaction. Mater. Sci. 2009, 46, 1316–1321. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef]




| Samples | Synthetic Method | Morphology | First Charge Capacity | Capacity after 50 Cycles | Literature |
|---|---|---|---|---|---|
| graphene-encapsulated Fe2O3 | spray drying method | spheroidal | 728 mAh/g | 711 mAh/g | [21] |
| Fe2O3-graphene | solvent hydrothermal method | Wafer-like | 1081 mAh/g | 889 mAh/g | [22] |
| α-Fe2O3@graphene * aerogel composites | Hydrothermal self-assembly and Freeze-drying | spheroidal | 1408 mAh/g | 745 mAh/g | [23] |
| 0.2rGO/Fe2O3-175 °C | PVP solvent assisted hydrothermal method | nanorod-like | 1372 mAh/g | 435 mAh/g | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Gao, B.; Tu, G.; Liu, H.; Wang, M. Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials. Metals 2022, 12, 593. https://doi.org/10.3390/met12040593
Zhu G, Gao B, Tu G, Liu H, Wang M. Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials. Metals. 2022; 12(4):593. https://doi.org/10.3390/met12040593
Chicago/Turabian StyleZhu, Guanglin, Bo Gao, Ganfeng Tu, Haifeng Liu, and Ming Wang. 2022. "Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials" Metals 12, no. 4: 593. https://doi.org/10.3390/met12040593
APA StyleZhu, G., Gao, B., Tu, G., Liu, H., & Wang, M. (2022). Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials. Metals, 12(4), 593. https://doi.org/10.3390/met12040593






