Solidification Simulation of Al-Si Alloys with Dendrite Tip Undercooling
Abstract
:1. Nomenclature
| cps | Specific heat of solid as a function of temperature (J Kg−1 K−1); |
| cpl | Specific heat of liquid (J Kg−1 K−1); |
| CL | Liquid concentration (wt%); |
| Co | Average alloy composition (wt%); |
| CS | Solid concentration (wt%); |
| D | Solute diffusivity coefficient of liquid (m2 s−1); |
| G | Temperature gradient in liquid at the mushy zone/liquid interface (°C mm−1); |
| K | Average partition coefficient; |
| Ks | Thermal conductivity of solid as a function of temperature (W m−1 K−1); |
| Kl | Thermal conductivity of liquid (W m−1 K−1); |
| L | Latent heat of fusion (J Kg−1); |
| M | The slope of liquidus line (K wt%−1); |
| P | Pressure (Pa); |
| R | Velocity of mushy zone/liquid interface (mm s−1); |
| t | Time (s); |
| T | Temperature (°C); |
| Tliq | Liquidus temperature (°C); |
| Tini | Initial temperature of liquid (°C); |
| Tm | Melting temperature of pure aluminum (°C); |
| Teut | Eutectic temperature (°C); |
| Instantaneous tip cooling rate = G × R (°C s−1); | |
| ΔT | Undercooling of Tliq (°C); |
| Ur | Velocity in r direction (mm.s−1); |
| uy | Velocity in y direction (mm.s−1); |
| U | Flow velocity in the liquid of mushy zone/liquid interface (mm.s−1); |
| β | Contraction ratio (volumetric shrinkage during solidification); |
| βC | Solute expansion coefficient (K−1); |
| βT | Thermal expansion coefficient (K−1); |
| Γ | Gibbs–Thomson coefficient (K m−1); |
| ϕ | Liquid fraction; |
| ρl | Liquid density (Kg m−3); |
| ρs | Solid density (Kg m−3); |
| µ | Dynamic viscosity (Pa s); |
| Primary arm spacing if no fluid flow effect is considered (µm); | |
| λ1 | Primary arm spacing (µm) |
| Constant parameters | |
| D | 6.25 × 10−9 (m2 s−1) [1]; |
| k | 0.116 [2]; |
| m | −6.675 (K wt%−1) [2]; |
| Tm | (660 °C) [2]; |
| Teut | (578.6 °C) [2]; |
| Γ | 1.97 × 10−7 (K m−1) [3]; |
| βC | −4.26 × 10−4 (K−1) [4]; |
| βT | 1.39 × 10−4 (K−1) [4]; |
| µ | 1.3 × 10−3 (Pa s) [4]; |
2. Introduction
3. Numerical Model
3.1. Background
3.2. Governing Equations
3.3. Domain Definition
3.4. Numerical Simulation Procedure
- At time step t = tm, Equation (3) was used to evaluate the distribution of solute concentration in all of liquid, solid and the two-phase mushy zone.
- Solving Equation (2) and comparing the evaluated T of each CV to (Tliq − ΔT), the CV was determined to be in either the liquid phase or mushy zone.
- Equation 4 was used to evaluate temperature distribution inside the mushy zone or assumed as (Tliq − ΔT) if CL < (Figure 4d–f). Equation (2) was used to evaluate the temperature distribution inside the liquid and solid phases.
- The temperature distribution, evaluated from Equation (2) was used to evaluate ϕ in the mushy zone.
- If there is no re-melting of the solid in any given CV, Equation (5) was used to calculate the mean solute concentration of the solid phase in the CV, and in case of solid phase re-melting in any given CV, the history of prior mean solute concentration in the solid phase was used for the current time step [10].
- All the above steps were repeated until convergence.
4. Results and Discussion
- Comparison of new algorithm with the popular version.
- Transient temperature distribution.
- Effect of ΔT on solidification time.
- Effect of ΔT in the liquid on fluid flow.
- Effect of ΔT in the liquid on G.
- Effect of ΔT in the liquid on R.
- Effect of ΔT in the liquid on λ1.
4.1. Comparison of New Algorithm with the Popular Version
4.2. Transient Temperature Distribution
4.3. Effect of Undercooling on Solidification Time
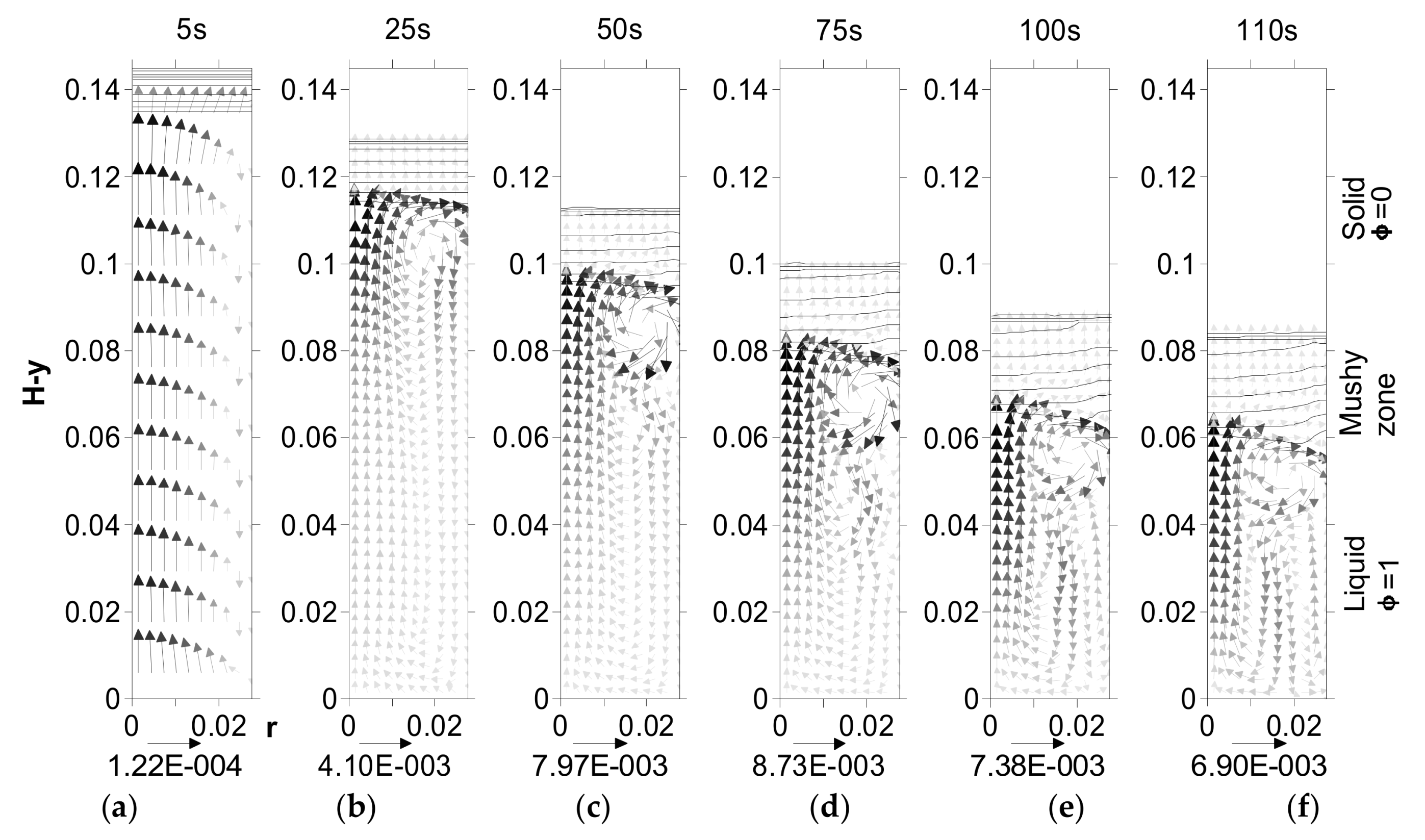
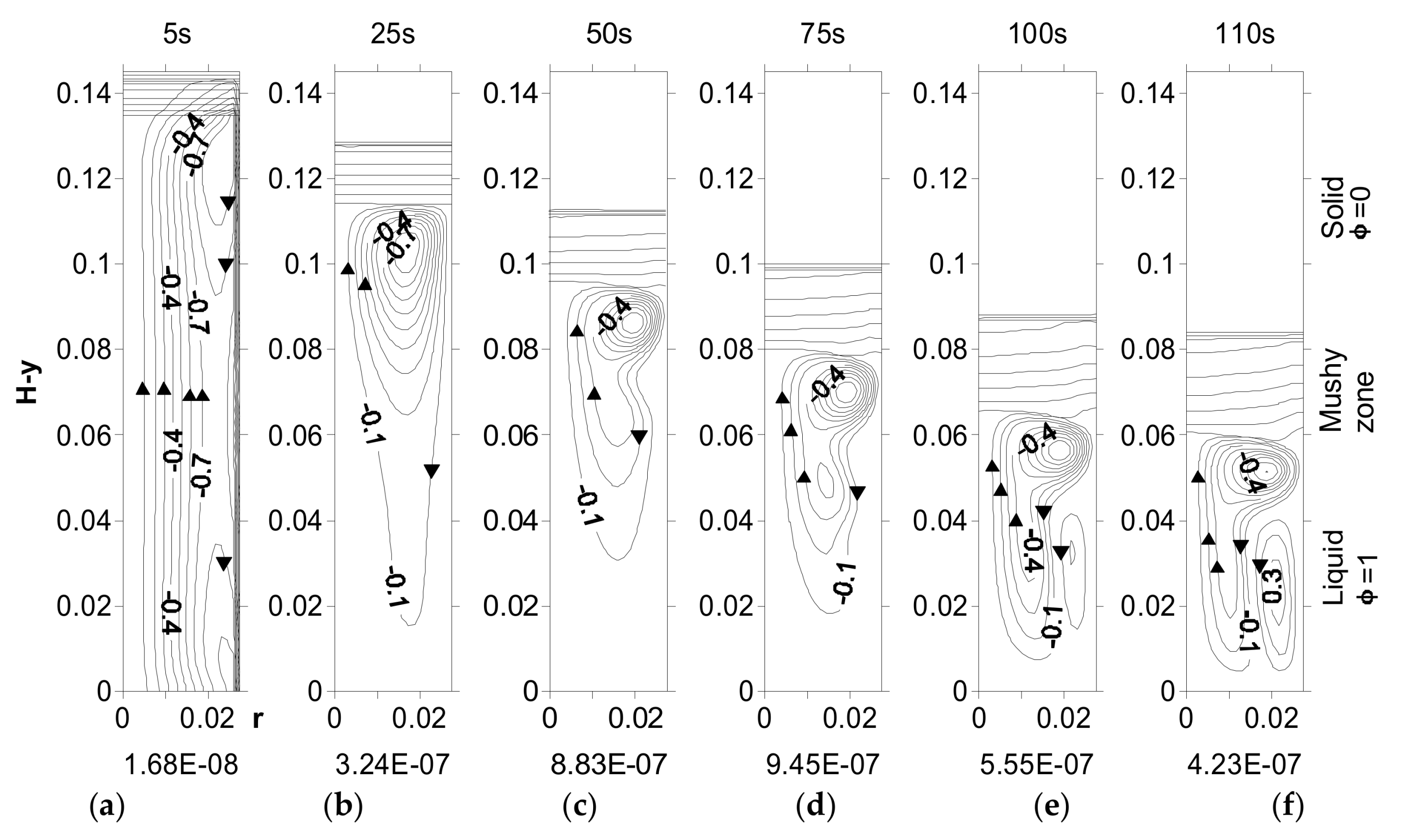
4.4. Effect of Undercooling on Fluid Flow—Upward Solidification
4.5. Effect of Undercooling on Fluid Flow—Downward Solidification
5. Effect of Undercooling on G
5.1. Effect of Undercooling on R
5.2. Effect of Undercooling on the Estimation of λ1
6. Summary and Conclusions
- The undercooling has been successfully quantified in numerical simulations and has a significant impact on the selection of the primary arm spacing during solidification, especially in modes that involve development of significant fluid flow during the process arising from notable density gradients in liquid from solute redistribution. The effect of undercooling, ΔT has a significant effect on the velocity of the fluid flow in the computing domain only in the downward solidification and there is no significant effect in the upward solidification mode. There are three distinct regions in the downward solidification mode because of significant changes in the flow velocity arising from gravity-assisted density gradients in the liquid in these regions.
- Simulations with the effect of undercooling were found to increase solidification time and be far more agreeable with the experiment results for solidification times than those without the inclusion of the undercooling term.
- The effect of ΔT is pronounced on the distribution of the temperature gradient of the liquid at mushy zone/liquid interface, G, during both upward and downward solidification modes.
- The effect of ΔT is pronounced on the distribution of the velocity of the mushy zone/liquid interface, R, during both upward and downward solidification modes. The effect of ΔT is more pronounced at higher values of R and reduces with decreasing R because ΔT is high at higher values of R as a result of increased heat extraction during initial stages of solidification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekulic, D.P.; Galenko, P.K.; Krivilyov, M.D.; Walker, L.; Gao, F. Dendritic growth in Al-Si alloys during brazing. Part 2: Computational modeling. Int. J. Heat Mass Transf. 2005, 48, 2385–2396. [Google Scholar] [CrossRef]
- Factsage, 5th ed.; Computherm LLC.: Madison, WI, USA, 2001.
- Gunduz, M.; Hunt, J.D. The measurement of solid-liquid surface energies in the Al-Cu, Al-Si and Pb-Sn systems. Acta Metall. 1985, 33, 1651–1672. [Google Scholar] [CrossRef]
- JMatPro, 4th ed.; Sente Software Ltd.: Guildford, UK, 2017.
- Ho, C.-J.; Viskanta, R. Heat transfer during inward melting in a horizontal tube. Int. J. Heat Mass Transf. 1984, 27, 705–716. [Google Scholar] [CrossRef]
- Yao, L.S. Natural convection effects in the continuous casting of a horizontal cylinder. Int. J. Heat Mass Transf. 1984, 27, 697–704. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification Processing; McGraw-Hill Book Co.: New York, NY, USA, 1974. [Google Scholar]
- Gong, T.; Chen, Y.; Li, S.; Cao, Y.; Li, D.; Chen, X.Q.; Reinhart, G.; Nguyen-Thi, H. Revisiting dynamics and models of microsegregation during polycystalline solidification of binary alloy. J. Mater. Sci. Technol. 2021, 74, 155–167. [Google Scholar] [CrossRef]
- Bennon, W.D.; Incropera, F.P. A continuum model for momentum, heat and species transport in binary solid—Liquid phase change systems—I. Model formulation. Int. J. Heat Mass Transf. 1987, 30, 2161–2170. [Google Scholar] [CrossRef]
- Felicelli, S.D.; Heinrich, J.C.; Poirier, D.R. Simulation of freckles during vertical solidification of binary alloys. Metall. Trans. B Process Metall. 1991, 22, 847–859. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, J.C.; Poirier, D.R. The effect of volume change during directional solidification of binary alloys. Model. Simul. Mater. Sci. Eng. 2004, 12, 881–899. [Google Scholar] [CrossRef]
- McBride, E.; Heinrich, J.C.; Poirier, D.R. Numerical simulation of incompressible flow driven by density variations during phase change. Int. J. Numer. Methods Fluids 1999, 31, 787–800. [Google Scholar] [CrossRef]
- Santos, R.G.; Melo, M.L.N.M. Permeability of interdendritic channels. Mater. Sci. Eng. A 2005, 391, 151–158. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. Fixed grid numerical modeling methodology for convection—Diffusion mushy region phase—Change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Peres, M.D.; Siqueira, C.A.; Garcia, A. Macrostructural and microstructural development in Al-Si alloys directionally solidified under unsteady-state conditions. J. Alloys Compd. 2004, 381, 168–181. [Google Scholar] [CrossRef]
- Poirier, D.R. Permeability for flow of interdendritic liquid in columnar-dendritic alloys. Metall. Trans. B Process Metall. 1987, 18B, 245–255. [Google Scholar] [CrossRef]
- Yuan, L.; Lee, P.D. Dendritic Solidification under natural and forced convection in binary alloys: 2D versus 3D simulatiom. Model. Simul. Mater. Sci. Eng. 2010, 18, 55008. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Dutta, P. Effect of shrinkage induced flow on binary alloy dendrite growth: An equivalent undercooling model. Int. Commun. Heat Mass Transf. 2014, 57, 216–220. [Google Scholar] [CrossRef]
- Rappaz, M.; Boettinger, W.J. On dendritic solidification of multicomponent alloys with unequal liquid diffusion coefficients. Acta Mater. 1999, 47, 3205–3219. [Google Scholar] [CrossRef]
- Bouchard, D.; Kirkaldy, J.S. Prediction of dendrite arm spacings in unsteady- and steady-state heat flow of unidirectionally solidified binary alloys. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1997, 28B, 651–663. [Google Scholar] [CrossRef]
- Hunt, J.D. Solidification and casting of metals. In Proceedings of the International Conference on Solidification and Casting of Metals; The Metals Society: London, UK, 1979; pp. 3–9. [Google Scholar]
- Felicelli, S.D.; Heinrich, J.C.; Poirier, D.R. Numerical models for dendritic solidification of binary alloys. Numer. Heat Transf. 1993, 23, 461–481. [Google Scholar] [CrossRef]
- Trivedi, R. Interdendritic spacing: Part II. A comparison of theory and experiment. Metall. Trans. A Phys. Metall. Mater. Sci. 1984, 15, 977–982. [Google Scholar] [CrossRef]
- Burden, M.H.; Hunt, J.D. Cellular and dendritic growth. I. J. Cryst. Growth 1974, 22, 99–108. [Google Scholar] [CrossRef]
- Burden, M.H.; Hunt, J.D. Cellular and dendritic growth. II. J. Cryst. Growth 1974, 22, 109–116. [Google Scholar] [CrossRef]
- Carman, P.C. Fluid flow through granular beds. Trans. Inst. Chem. Engs. 1937, 15, 150–156. [Google Scholar] [CrossRef]
- Carman, P.C. The determination of the specific surface of powders I. J. Soc. Chem. Ind. 1938, 57, 225–234. [Google Scholar]
- Asai, S.; Muchi, I. Thearoetical analysis and model experiments of the formation mechanism of channel—Type segregation. Trans. Iron Steel Inst. Jpn. 1978, 18, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, P.; Moreaub, R.; Camela, D.; Bolcatob, R. A simple analysis of the effect of convection on the structure of the mushy zone in the case of horizontal Bridgman solidification. Comparison with experimental results. J. Cryst. Growth 1998, 183, 690–704. [Google Scholar] [CrossRef]
- Spinelli, J.E.; Peres, M.D.; Garcia, A. Thermosolutal convective effects on dendritic array spacings in downward transient directional solidification of Al-Si alloys. J. Alloys Compd. 2005, 403, 228–238. [Google Scholar] [CrossRef]
- Magnusson, T.; Arnberg, L. Density and solidification shrinkage of hypoeutectic aluminum-silicon alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 2605–2613. [Google Scholar] [CrossRef]
- Wang, H. Solidification Simulation of Binary Al-Si Alloys: Prediction of Primary Dendrite Arm Spacing with Macro-Scale Simulations (~1 mm Length Scale). Ph.D. Thesis, Department of Mechanical Engineering, McMaster University, Hamilton, ON, Canada, 2009. [Google Scholar]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; McGraaw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Zhao, Y.; Qin, R.; Chen, D.; Wan, X.; Li, Y.; Ma, M. A three dimensional cellular automata model for dendrite growth in Non-Equilibrium solidification of binary alloy. Steel Res. Int. 2015, 86, 1490–1497. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 4th ed.; Trans Tech Publication: Zurich, Switzerland, 1998. [Google Scholar]
























| Properties | Al-3wt%Si | Al-5wt%Si | Al-7wt%Si |
|---|---|---|---|
| Ks | 228.08 − 0.061055 × T | 226.01 − 0.077488 × T | 223.93 − 0.093920 × T |
| Kl | 85.476 | 84.568 | 83.661 |
| Cps | 887.23 + 0.50227 × T | 883.54 + 0.50227 × T | 879.85 + 0.50227 × T |
| Cpl | 1168.9 | 1163.7 | 1158.6 |
| ρs | 2627.9 | 2621.6 | 2614.5 |
| ρl | 2415.0 | 2422.8 | 2430.6 |
| L | 4.05 × 105 | 4.25 × 105 | 4.45 × 105 |
| Steps | Time | T | CS (New Layer) | CL | Figure |
|---|---|---|---|---|---|
| Step 1 | 0 to t1 | Tini to Tliq | 0 | Co | Figure 4b,c |
| Step 2 | t2 | Tliq to (Tliq − ΔT) | kCL1(≅kCo) | Figure 4d | |
| Step 3 | (t2 + Δt) | (Tliq − ΔT) (no change) | kCL2 | Figure 4e | |
| Step 4 | t* | (Tliq − ΔT) (no change) | kCL* | Figure 4f | |
| Step 5 | t3 | T3 < (Tliq − ΔT) | kCL3 | Figure 4g | |
| Step 6 | tn | Teut | Ceut | Ceut | Figure 4h |
| Step 7 | tfinal | Teut | Ceut | Ceut | Figure 4i |
| Simulation Identification | Type | Co | ΔT |
|---|---|---|---|
| SA | Upward Solidification | Al-3wt%Si | 0 |
| SB | |||
| S1 | Upward Solidification | Al-3wt%Si | 0 |
| S2 | Equation (1) | ||
| S3 | Al-7wt%Si | 0 | |
| S4 | Equation (1) | ||
| S5 | Downward Solidification | Al-5wt%Si | 0 |
| S6 | Equation (1) | ||
| S7 | Al-7wt%Si | 0 | |
| S8 | Equation (1) |
| Time (s) | Maximum Velocity (mm/s) | Flow due to Shrinkage | Flow due to Natural Convection | Height of the Mushy Zone (mm) |
|---|---|---|---|---|
| 5 | 1.22 × 10−4 | Dominant flow from bottom to top | Small circular flow limited to mushy zone/liquid interface around r = 27.5 mm | 13 |
| 25 | 4.1 × 10−3 | Small effect of shrinkage in liquid. Large effect of shrinkage in mushy zone. | Dominates the liquid. Small effect in mushy zone. | 20 |
| 50 | 7.97 × 10−3 | Strengthens in liquid. | 23 | |
| 75 | 8.73 × 10−3 | Strengthens further in liquid. | 24 | |
| 100 | 7.38 × 10−3 | Weakens in liquid. | 28 | |
| 110 | 6.9 × 10−3 | Further weakens in liquid. | 31 |
| Time (s) | Maximum Velocity (m s−1) | |
|---|---|---|
| S6 (with ΔT) | S5 (without ΔT) | |
| 5 | 1.22 × 10−4 | 1.55 × 10−4 |
| 25 | 4.1 × 10−3 | 2.43 × 10−3 |
| 50 | 7.97 × 10−3 | 5.93 × 10−3 |
| 75 | 8.73 × 10−3 | 6.68 × 10−3 |
| 100 | 7.38 × 10−3 | 5.73 × 10−3 |
| Simulation | ΔΤ | Interface Location y (mm) | Solidification Time (s) | Maximum Fluid Flow Velocity (mm.s−1) | Variation in Maximum Velocity | G (K.mm−1) |
|---|---|---|---|---|---|---|
| S5 (Al-5wt%Si) | 0 | 8(A) | 2 | 0.16 | none | 0.6 |
| 22(B) | 11 | 0.37 | 131% | 0.3 | ||
| 36(C) | 25 | 2.43 | 557% | 0.24 | ||
| 85(D) | 97 | 5.95 | 145% | 0.03 | ||
| S6 (Al-5wt%Si) | Equation (3) | 8(A) | 3.2 | 0.16 | none | 0.9 |
| 22(B) | 14.4 | 0.47 | 194% | 0.49 | ||
| 36(C) | 30.3 | 5.68 | 1109% | 0.55 | ||
| 85(D) | 108.9 | 6.95 | 22% | 0.16 | ||
| S7 (Al-7wt%Si) | 0 | 8(A) | 3.6 | 0.07 | none | 0.58 |
| 22(B) | 13 | 0.68 | 871% | 0.34 | ||
| 36(C) | 27 | 3.03 | 346% | 0.31 | ||
| 85(D) | 95.6 | 7.65 | 152% | 0.02 | ||
| S8 (Al-7wt%Si) | Equation (3) | 8(A) | 5.4 | 0.07 | none | 0.9 |
| 22(B) | 16.8 | 0.99 | 1314% | 0.6 | ||
| 36(C) | 32.8 | 10 | 910% | 0.77 | ||
| 85(D) | 107.3 | 8.35 | −17% | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Hamed, M.S.; Shankar, S. Solidification Simulation of Al-Si Alloys with Dendrite Tip Undercooling. Metals 2022, 12, 608. https://doi.org/10.3390/met12040608
Wang H, Hamed MS, Shankar S. Solidification Simulation of Al-Si Alloys with Dendrite Tip Undercooling. Metals. 2022; 12(4):608. https://doi.org/10.3390/met12040608
Chicago/Turabian StyleWang, Hongda, Mohamed S. Hamed, and Sumanth Shankar. 2022. "Solidification Simulation of Al-Si Alloys with Dendrite Tip Undercooling" Metals 12, no. 4: 608. https://doi.org/10.3390/met12040608
APA StyleWang, H., Hamed, M. S., & Shankar, S. (2022). Solidification Simulation of Al-Si Alloys with Dendrite Tip Undercooling. Metals, 12(4), 608. https://doi.org/10.3390/met12040608






