Abstract
Vanadium extraction process demands low residual vanadium and carbon loss, and variations of dissolved elements in hot metal must be determined to achieve it. A three parts dynamic model that applies the concept of Gibbs’ free energy minimization at the slag–metal interface is proposed. Modeling simulation results shows good uniformity with plant experimental data, and the presented model can describe the vanadium extraction process in BOF qualitatively well. The effects of coolant addition and oxygen flow rate have been studied by modeling. The lack of coolant will reduce (FeO) content and elevate the molten bath temperature, which are harmful to deep vanadium removal with less carbon loss in semi-steel. The excessive oxygen flow rate has little effect on residual [V], and there is more carbon loss because of higher (FeO) content and molten bath temperature.
1. Introduction
Vanadium titanium magnetite (VTM) has the largest reserves among v-bearing resource mineral [1]. The most popular method to extract the widely used rare metal, vanadium, is oxygen blow smelting in Basic Oxygen Furnance from v-bearing hot metal [2]. Vanadium is removed from v-bearing hot metal to form vanadium-enriching slag.
However, vanadium extraction process demands low residual vanadium and high carbon content in semi-steel, which means temperature control plays an extremely important role to ensure the thermodynamics of selective oxidation [3,4]. Reasonable temperature strategy must determine the variations of dissolved elements in hot metal because the oxidizing reactions release a large amount of heat, which has a great impact on molten pool temperature and results in changes in the slag component. However, the extremely high temperature makes it very inconvenient for direct measuring and sampling. Thus, dynamic modeling calculation seems to be more effective and economic. In the recent past, there have been several studies on the dynamic modeling of the BOF vanadium extraction process. Huang et al. applied multi-phase coupling based on mass and energy balances to treat the blow progress [5]. Shukla et al. developed a model based on the way available oxygen is distributed based on respective Gibbs’ free energies of oxidation [6]. Pahlevani et al.’s kinetic model made arbitrary assumption that 20% of the available oxygen is utilized for decarburization and 70% is utilized for FeO generation [7]. As mentioned above, these models have completely ignored the thermodynamic aspects of refining reactions, and the equilibrium of systems is reached only when the total Gibbs’ free energy is minimized. In this paper, a model based on Gibbs’ free energy minimization has been presented that fully considers the physicochemical process during oxygen blowing. The variations of hot metal component were determined considering coolant addition and oxygen flow rate.
2. Model Formulation
2.1. Refining Zone Definitions
In the present model, the refining sites in BOF were divided into three parts, as shown in Figure 1.
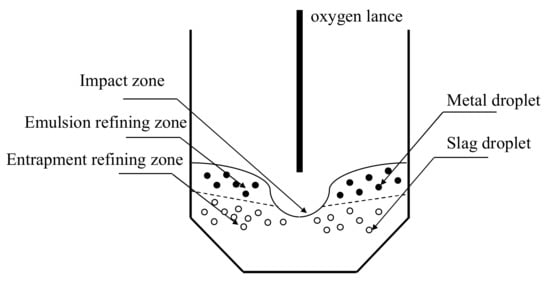
Figure 1.
Schemes of refining zones in molten bath.
—Site 1: Impact zone of oxygen flow.
—Site 2: Emulsion refining zone on the upper bath, where metal droplet was splashed into foaming slag.
—Site 3: Entrapment refining zone, where slag was drawn into molten iron in the lower bath.
2.2. Refining Mechanism of BOF Reactions
- a.
- Gaseous Oxygen O2 at the impact site reacts with a layer of metal, oxidizing all elements in it directly. The oxidation products are transferred to the slag phase, except for carbon oxides.
- b.
- Large numbers of metal droplets are generated at the impact zone, and they too splash into the slag zone in upper bath. Refining reactions of dissolved elements take place at the interface dictated by thermodynamics. Eventually, the droplets fall back to iron bath due to gravity. The two-phase reactions such as silicon take place directly with FeO:
2(FeO) + [Si] = (SiO2) + 2[Fe]
- c.
- A number of slag droplets are entrapped in the lower bath. The refining reactions also take place at the interface that is similar to emulsion refining zone.
—Phosphorus and sulphur removal will not be considered during smelting because no CaO will be added into molten bath during vanadium extraction process [8].
2.3. Assumptions of the Model
- a.
- According to the requirements of vanadium extraction process, the typical smelting time continues only about 6 min (360 s). The initial slag layer in bath is from addictive coolant before blowing and residual slag from previous heat. Slag compositions at this time are assumed from typical reported values from plant.
- b.
- Oxygen is partitioned among the elements based on their molar fraction available at the impact zone.
- c.
- Rates of refining reactions are assumed to be controlled only by mass transfer of FeO in the slag phase.
- d.
- Size distribution of metal droplets generated at the impact zone is heterogeneous. Sauter Mean Diameter is often used to describe it. A single size of metal droplets in emulsion is assumed for keeping the model simple. The size refers to Dogan’s [9].
- e.
- Residence time of the metal droplets is affected by initial conditions such as diameter and velocity. An average time of residence of metal droplets is assumed for keeping the model simple, which refers to Brooks’ and Sarkar’s studies [10,11].
- f.
- Necessary coolant for keeping reasonable thermodynamics [12] was added at 30th s, 90th s, and 150th s. The coolant consisted of FeO and Fe2O3, each 50%. Dissolving rate of addictive coolant depended on mass transfer of Fe2O3 to slag phase. Coolant meltage continued at 30 s equably.
2.4. Governing Equations
- a.
- Oxygen distribution at the impact zone
According to Assumption b, oxygen distribution at the impact zone is based on their molar fraction. The rate equations of each elements including Fe are as follows:
where m[i] stands for quantity of components in molten iron, kg. W[ipOq] stands for quantity of reaction product in slag, kg. x[i] stands for molar fraction. M[i] and M[ixOy] stand for molecular mass. RO is the mass of oxygen flow during per time-step, kg.
- b.
- Calculation of droplet generation rate
Metal droplets generation rate is calculated by empirical equation proposed by Subagyo et al. [13].
where RB is droplets generation rate, kg/s. FG is the top oxygen Flow Rate in Nm3/s. NB is constant defined as follows:
where ρg is gas density of top flow, kg/m3. ρm is density of molten iron, kg/m3. vg (m/s) is gas velocity related to the axial velocity at the point of impact, vj, as
and vj is expressed as a function of the dynamic lance height, using He et al.’s correlation [14].
vg = 0.44721vj
- c.
- Calculation slag–metal interface area
Area of slag–metal interface consists of two parts, as shown in Figure 1: metal droplets splashed to slag at upper bath and slag droplets entrapped into lower bath.
Slag–metal interface area of splashed metal droplets in emulsion refining zone is calculated following Assumption 4 and droplet generation rate RB.
where dm is average diameter of metal droplets, m.
Area of entrapped slag droplets into lower bath is calculated by empirical relationship proposed by F.Oersted.
where D is diameter of impact ring, m. ηs is viscosity of slag, Pa.s. σ is surface tension of slag, N/m. β is the angle between buoyancy force and interfacial tension.
- d.
- Oxygen distribution at the slag–metal interface
Refining reaction at the slag–metal interface plays an extremely important role during LD converter smelting. Thus, oxygen distribution at the slag–metal interface directly relates to the reasonability of present model. In a multi-component system, the equilibrium composition tends to be such that the Gibb’s free energy of the entire system is minimized [11]. Refining reactions at the slag–metal interface follow this thermodynamic principle. Let consider the following reactions:
and if ΔGC-FeO, ΔGSi-FeO, ΔGMn-FeO, ΔGTi-FeO, and ΔGV-FeO are the Gibbs’ free energy changes per mol atom of oxidized element for the above reactions in the same order as they are mentioned, then the actual Gibbs’ free energy changes ΔGT associated with these reactions can be calculated as follows:
where RC, RSi, RMn, RV, and RTi are the molar quantity of oxidized elements during per time step.
(FeO) + [C] = CO(g) + [Fe]
2(FeO) + [Si] = (SiO2) + 2[Fe]
2(FeO) + [Ti] = (TiO2) + 2[Fe]
(FeO) + [Mn] = (MnO) + [Fe]
3(FeO) + 2[V] = (V2O3) + 3[Fe]
2(FeO) + [Si] = (SiO2) + 2[Fe]
2(FeO) + [Ti] = (TiO2) + 2[Fe]
(FeO) + [Mn] = (MnO) + [Fe]
3(FeO) + 2[V] = (V2O3) + 3[Fe]
The restrictive steps of refining reactions are considered as mass transfer of FeO in slag and dissolved elements in metal; the equations are as follows:
where Asm is the area of slag–metal interface, m2. k is the mass transfer coefficient of components, m.s−1. Cb and C* are bulk concentration and interface concentration, mol/kg.
An equation set is obtained as follows, and RC, RSi, RMn, RV, and RTi are computed based on the constraint that ΔGT is minimized.
Thus, total quantity of each oxidized element during per time step is calculated based on Equations (1), (2) and (10), as follows:
3. Results and Discussion
Typical smelting time for one heat is about 360 s. As mentioned earlier, simulations were performed for input and process parameters shown in Table 1. Further parameters such as interaction coefficients of solute elements in metal and mass transfer coefficients in metal and slag are given in Table 2 and Table 3.

Table 1.
List of input parameters used for calculations.

Table 2.
Interaction coefficients of solute elements in metal.

Table 3.
Mass transfer coefficient in metal and slag.
3.1. Description of Vanadium Extraction Process
Reasonable convergence of modeling result is obtained with a time-step of 0.1 s. Variations of elements content in hot metal and components in slag based on modeling calculation are shown in Figure 2. The results show good uniformity with plant experimental data mentioned in previous study [12].
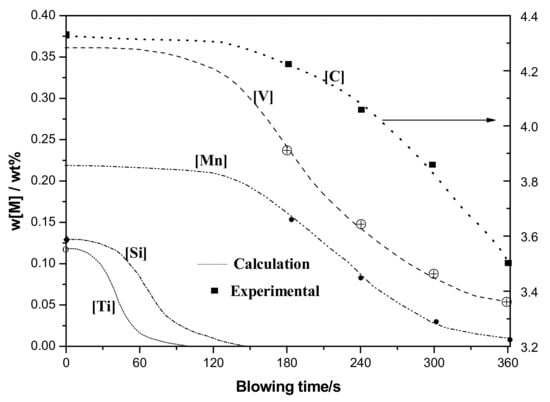
Figure 2.
Variations of element content in hot metal.
Titanium and silicon oxidation are the front part of the reaction sequence of vanadium extraction refining. These are in accord with thermodynamics prediction due to the higher affinity to oxygen. Contents of [Ti] and [Si] show a relatively low level at about 120 s, which determines that the oxidizing removals of [Ti] and [Si] have been done. Meanwhile, rapid oxidizing rates of [Ti] and [Si] appear till 20th second because not enough slag layers have been formed, and refining reaction remains low. Vanadium oxidation and manganese oxidation increase after about 120 s and decrease after 300 s, due to studied thermodynamics condition. Vanadium and manganese oxidation are prior to carbon when content is high and molten temperature remains low [15,16]. Carbon oxidation increases rapidly beginning at 120 s, which is similar to [V] and [Mn]. Thus, carbon oxidation plays an important role in vanadium removal; reasonable temperature strategy has been devised to guarantee the preferential oxidation of vanadium to carbon [15].
3.2. Effect of Coolant Addition
Coolant addition has great influence on molten temperature and (FeO) content in slag, which directly affect the thermodynamics condition. Two schemes on coolant addition have been carried out, as shown in Table 4. Coolant component is shown in Table 1 (50%FeO-50%Fe2O3). Modeling calculation results are shown in Figure 3.

Table 4.
Schemes of coolant addition.
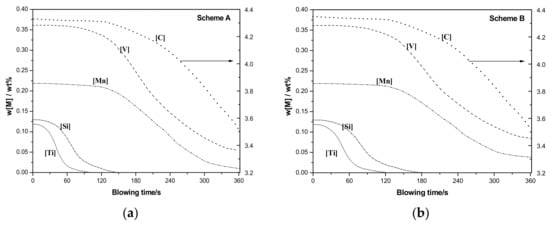
Figure 3.
Effect of coolant addition on vanadium extraction refining. (a) Scheme A (b) Scheme B.
Compared with Scheme A, not enough coolant has been added in Scheme B. This leads to lower (FeO) content in slag, which is unfavorable for refining reactions, and also leads to higher molten pool temperature that prevents the achievement of good thermodynamics to ensure [V] removal prior to [C] oxidation [15,16]. Thus, deep removal of [Ti] and [Si] have been delayed till about 180 s. Residual vanadium [V] have increased from 0.06 to 0.10%, and carbon content in semi-steel decreased from 3.5 to 3.3%.
3.3. Effect of Oxygen Flow Rate
Oxygen flow rate has effect on generation of metal-droplets in bath and (FeO) generation at impact zone. Vanadium extraction blowing with oxygen flow rate 24,000 Nm3/h and 26,000 Nm3/h has been modeled, and variations on element in hot metal are shown in Figure 4.
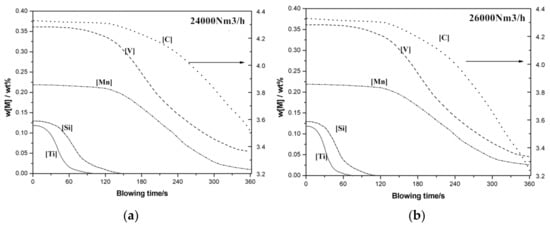
Figure 4.
Effect of oxygen flow rate on vanadium extraction refining. (a) 24,000 Nm3/h (b) 26,000 Nm3/h.
With the increase of oxygen flow rate, (FeO) content in slag increased with more oxygen supply, which is beneficial to refining reactions. Thus, deep removal of [Ti] and [Si] increased slightly. However, higher oxygen flow rate means more oxidizing reactions with more heat release in bath, which results in higher molten pool temperature. Therefore, residual vanadium [V] content changes very little, for the following reasons: advantage of higher (FeO) in refining reactions, and disadvantage of higher molten bath temperature in preferential oxidation of [V]. Carbon content in semi-steel decreased from 3.5 to 3.2%.
4. Conclusions
- (1)
- The three-part model, including the impact zone, emulsion zone, and entrapment zone, has been developed to model the vanadium extraction process in BOF. The concept of Gibbs’ free energy minimization at the slag–metal interface has been adopted to model the refining reactions.
- (2)
- Simulation results predicting variations of dissolved elements have shown good uniformity with plant experimental data. The present model describes the vanadium extraction process in BOF qualitatively well.
- (3)
- The effects of coolant addition and oxygen flow rate have been studied. The lack of coolant will reduce (FeO) content and elevate the molten bath temperature, harming deep vanadium removal, resulting in less carbon loss in semi-steel. Excessive oxygen flow rate has little affect on residual [V] but results in more carbon loss because of higher (FeO) content and molten bath temperature.
Author Contributions
Conceptualization, Z.Z. and C.L.; methodology, Z.Z. and C.L.; validation, Z.Z.; formal analysis, Z.Z. and C.L.; investigation, Z.Z. and C.L.; resources, C.L.; data curation, Z.Z.; writing—original draft preparation, Z.Z.; writing—review and editing, Z.Z. and C.L.; visualization, Z.Z.; supervision, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.H.; Zhang, W.; Xue, Z.L. An environment-friendly process featuring calcified roasting and precipitation purification to prepare vanadium pentoxide from the converter vanadium slag. Metals 2019, 9, 21. [Google Scholar] [CrossRef]
- Lidiane, M.A.; Heide, M.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C. Acute toxicity evaluation of non-innocent oxidovanadium(V) schiff base complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Hong, Y.; Peng, J.; Sun, Z.; Yu, Z.; Wang, A.; Wang, Y.; Liu, Y.-Y.; Xu, F.; Sun, L.-X. Transition metal oxodiperoxo complex modified metal-organic frameworks as catalysts for the selective oxidation of cyclohexane. Materials 2020, 13, 829. [Google Scholar] [CrossRef] [PubMed]
- Condruz, M.R.; Matache, G.; Paraschiv, A.; Badea, T.; Badilita, V. High temperature oxidation behavior of selective laser melting manufactured IN 625. Metals 2020, 10, 668. [Google Scholar] [CrossRef]
- Huang, Q.Y. Basic Research on the Related Technology of High Efficient Extraction of Vanadium from Hot Metal by BOF. Ph.D. Thesis, Chongqing University, Chongqing, China, 2012. [Google Scholar]
- Shukla, A.K.; Deo, B.; Millman, S.; Snoeijer, B.; Overbosch, A.; Kapilashrami, A. An insight into the mechanism and kinetics of reactions in BOF steel-making: Theory vs practice. Steel Res. Int. 2010, 81, 940–948. [Google Scholar] [CrossRef]
- Pahlevani, F.; Kitamura, S.; Shibata, H.; Maruoka, N. Simulation of Steel Refining Process in Converter. Steel Res. Int. 2010, 81, 617–622. [Google Scholar] [CrossRef]
- Taylor, P.R.; Shuey, S.A.; Vidal, E.E.; Gomez, J.C. Extractive metallurgy of vanadium-containing titaniferous magnetite ores: A review. Miner. Metall. Process. 2006, 23, 80–86. [Google Scholar] [CrossRef]
- Dogan, N.; Brooks, G.; Rhamdhani, M.A. Analysis of droplet generation in oxygen steel-making. ISIJ Int. 2009, 49, 24–28. [Google Scholar] [CrossRef][Green Version]
- Brooks, G.A.; Pan, Y.; Subagyo, H.; Coley, K.S. Modeling of trajectory and residence time of metal droplets in slag–metal-gas emulsions in oxygen steel-making. Metall. Mater. Trans. B 2005, 36, 525–535. [Google Scholar] [CrossRef]
- Sarkar, R.; Gupta, P.; Basu, S.; Ballal, N.B. Dynamic modeling of LD converter steelmaking: Reaction modeling using Gibbs’ free energy minimization. Met. Mater. Trans. A 2015, 46, 961–976. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Tang, P. Optimization on temperature strategy of BOF vanadium extraction to enhance vanadium yield with minimum carbon loss. Metals 2021, 11, 906. [Google Scholar] [CrossRef]
- Subagyo; Brooks, G.A.; Coley, K.S.; Irons, G.A. Generation of Droplets in Slag–metal Emulsions Through Top Gas Blowing. ISIJ Int. 2003, 43, 983–989. [Google Scholar] [CrossRef]
- He, Q.L.; Standish, N. A model study of droplet generation in the BOF steelmaking. ISIJ Int. 1990, 30, 305–309. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Tang, P.; Hou, Z.B.; Wen, G.H. Investigation of the end-point temperature control based on the critical temperature of vanadium oxidation during the vanadium extraction process in BOF. Trans. Indian Inst. Met. 2018, 71, 1957. [Google Scholar] [CrossRef]
- Zhou, C.G.; Li, J.; Wu, H. Dependence of Temperature and slag composition on dephosphorization at the first deslagging in BOF steelmaking process. High Temp. Mater. Process. 2016, 35, 433–440. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).