Effects of Carrier, Leveller, and Booster Concentrations on Zinc Plating from Alkaline Zincate Baths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hull Cell Studies
2.2. Zinc Coating Characterization
2.3. Electrochemical Experiments
2.4. Current Efficiency (CE) and Throwing Power (TP)
3. Results and Discussion
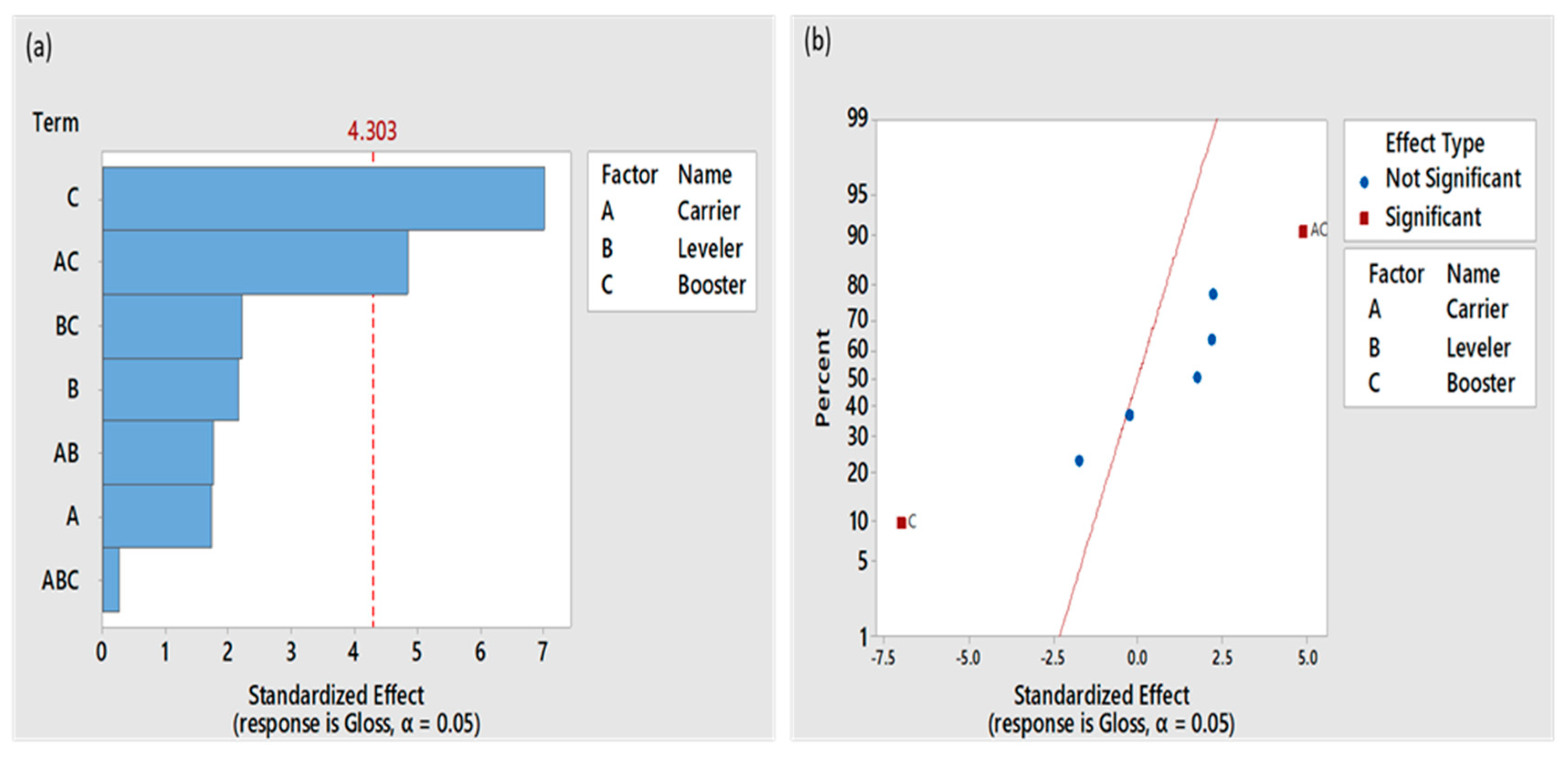
3.1. Deposit Appearance and Brightness
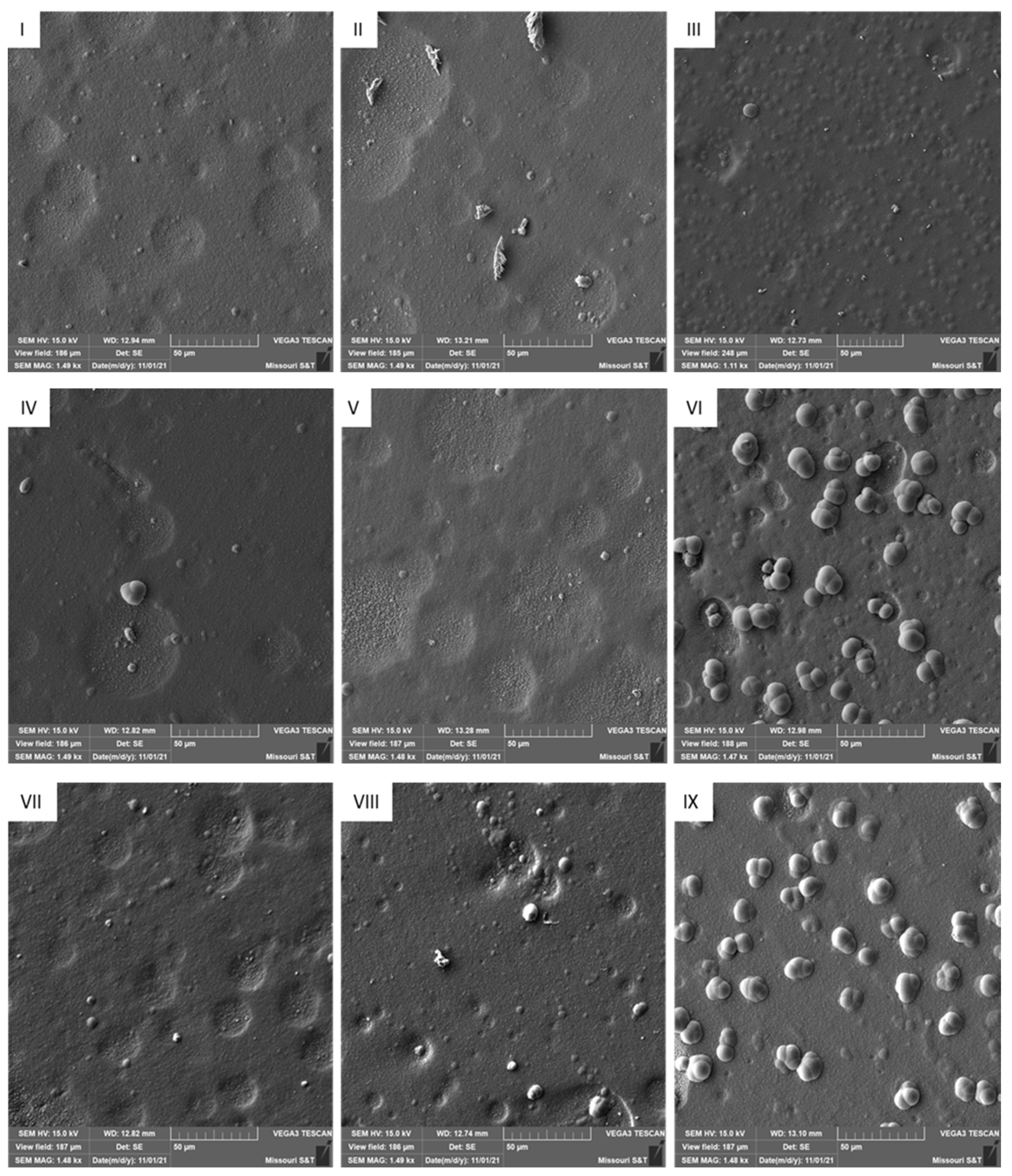
3.2. Deposit Morphology
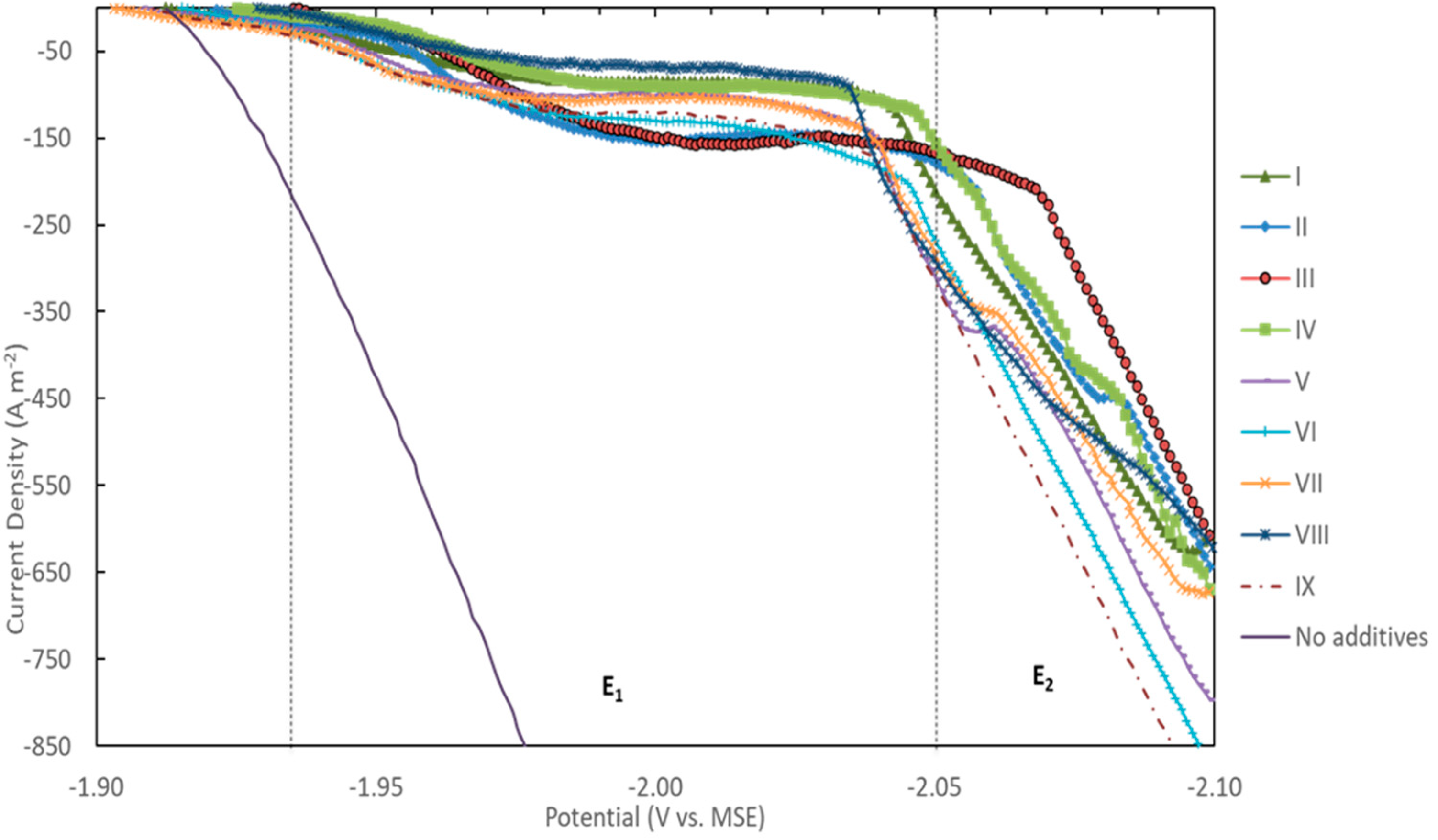
3.3. Electrochemical Studies
3.4. Throwing Power and Cathodic Current Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, W.J.; Goodwin, F.E. Hot dip coatings. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Dallin, G.W. Galvanizing-2015; Galvinfo Center—A Program of The International Zinc Association: Durham, NC, USA, 2015. [Google Scholar]
- Anchor Bolt and Construction Fastener Manufacturer. How Does Hot-Dip Galvanizing Differ from Electrogalvanizing? Available online: https://www.portlandbolt.com/technical/faqs/hot-dip-galvanizing-vs-electrogalvanizing-zinc-plating (accessed on 10 November 2021).
- Biddulph, C. Zinc electroplating. Prod. Finish. 2012, 1, 106. [Google Scholar]
- Vagramyan, T.; Leach, J.L.; Moon, J.R. The structures of zinc electrodeposits formed at low current densities. J. Mater. Sci. 1979, 14, 1170–1174. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, Z.Y.; Liu, S.J.; Shu, W.F.; He, Y.N. Effects of additives on zinc electrodeposition from alkaline zincate solution. Trans. Nonferrous Met. Soc. China 2017, 27, 1656–1664. [Google Scholar] [CrossRef]
- Muralidhara, H.B.; Naik, Y.A.; Sachin, H.P.; Achary, G.; Venkatesha, T.V. A study on brightening and corrosive resistance property of electrodeposited zinc in non-cyanide alkaline bath. Indian J. Chem. Technol. 2008, 15, 259–265. [Google Scholar]
- Schlesinger, M.; Paunovic, M. Modern Electroplating; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 423–452. [Google Scholar]
- Oniciu, L.; Mureşan, L. Some fundamental aspects of levelling and brightening in metal electrodeposition. J. Appl. Electrochem. 1991, 21, 565–574. [Google Scholar] [CrossRef]
- Tietcha, G.F.; Mears, L.L.; Dworschak, D.; Roth, M.; Kluppel, I.; Valtiner, M. Adsorption and diffusion moderated by polycationic polymers during electrodeposition of zinc. ACS Appl. Mater. Interfaces 2020, 12, 29928–29936. [Google Scholar] [CrossRef]
- Hope, G.A.; Brown, G.M.; Schweinsberg, D.P.; Shimizu, K.; Kobayashi, K. Observations of inclusions of polymeric additives in copper electrodeposits by transmission electron microscopy. J. Appl. Electrochem. 1995, 25, 890–894. [Google Scholar] [CrossRef]
- Geduld, H. Zincate or Alkaline Non-Cyanide Zinc Plating in “Zinc Plating”; ASM International Metals Park: Novelty, OH, USA, 1988; pp. 90–106. [Google Scholar]
- Ravindran, V.; Krishnan, R.M.; Muralidharan, V.S. Characteristics of an alkaline tartrate zinc plating bath. Met. Finish. 1998, 96, 10–15. [Google Scholar] [CrossRef]
- Crotty, D.; Bagnall, K. Analysis methods for zinc plating brighteners. Plat. Surf. Finish. 1988, 75, 52–56. [Google Scholar]
- Shanmugasigamani; Pushpavanam, M. Role of additives in bright zinc deposition from cyanide free alkaline baths. J. Appl. Electrochem. 2006, 36, 315–322. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Hu, C.C.; Lee, T.C. Effects of polyamines on the deposition behavior and morphology of zinc electroplated at high-current densities in alkaline cyanide-free baths. Surf. Coat. Technol. 2009, 203, 3111–3115. [Google Scholar] [CrossRef]
- Ortiz-Aparicio, J.L.; Meas, Y.; Trejo, G.; Ortega, R.; Chapman, T.W.; Chainet, E. Effects of organic additives on zinc electrodeposition from alkaline electrolytes. J. Appl. Electrochem. 2013, 43, 289–300. [Google Scholar] [CrossRef]
- Naik, Y.A.; Venkatesha, T.V. A new brightener for zinc plating from non-cyanide alkaline bath. Indian J. Eng. Mater. Sci. 2003, 10, 318–323. [Google Scholar]
- Naik, Y.A.; Venkatesha, T.V. A new condensation product for zinc plating from non-cyanide alkaline bath. Bull. Mater. Sci. 2005, 28, 495–501. [Google Scholar] [CrossRef]
- Chotirach, M.; Rattanawaleedirojn, P.; Boonyongmaneerat, Y.; Chanajaree, R.; Schmid, K.; Metzner, M.; Rodthongkum, N. Systematic investigation of brightener’s effects on alkaline non-cyanide zinc electroplating using HPLC and molecular modeling. Mater. Chem. Phys. 2022, 277, 125567. [Google Scholar] [CrossRef]
- Pary, P.; Bengoa, J.F.; Conconi, M.S.; Bruno, S.; Zapponi, M.; Egli, W.A. Influence of organic additives on the behaviour of zinc electroplating from alkaline cyanide-free electrolyte. Trans. IMF 2017, 95, 83–89. [Google Scholar] [CrossRef]
- Wanotayan, T.; Kantichaimongkol, P.; Chobaomsup, V.; Sattawitchayapit, S.; Schmid, K.; Metzner, M.; Chookajorn, T.; Boonyongmaneerat, Y. Effects of chemical compositions on plating characteristics of alkaline non-cyanide electrogalvanized coatings. Nanomaterials 2020, 10, 2101. [Google Scholar] [CrossRef]
- Scott, M.; Moats, M. Optimizing additive ratios in alkaline zincate electrodeposition. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: Cham, Switzerland, 2020; pp. 123–131. [Google Scholar]
- Scott, M. Effect of Carriers, Brighteners, and Levelers on Zinc Electrogalvanizing in Alkaline Zincate Solutions. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2020. [Google Scholar]
- Antony, J. Design of Experiments for Engineers and Scientists; Butterworth-Heinemann: Burlinton, UK; Oxford, MA, USA, 2003; pp. 60–65. [Google Scholar]
- Hunter, R.S. Methods of determining gloss. J. Res. Natl. Bur. Stand. 1937, 18, 958. [Google Scholar] [CrossRef]
- Gabe, D.R. Test cells for plating. Met. Finish. 2002, 100, 579–586. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Hu, C.C.; Lee, T.C. The synergistic effects of additives on improving the electroplating of zinc under high current densities. J. Electrochem. Soc. 2008, 155, D675–D681. [Google Scholar] [CrossRef]
- Blakeley, A. Improving Acid Zinc Coverage and Brightness. Available online: https://www.pfonline.com/articles/plating-clinic-improving-acid-zinc-coverage-and-brightness (accessed on 16 November 2021).
- De Carvalho, M.F.; Rubin, W.; Carlos, I.A. Study of the influence of the polyalcohol mannitol on zinc electrodeposition from an alkaline bath. J. Appl. Electrochem. 2010, 40, 1625–1632. [Google Scholar] [CrossRef]
- Gomes, A.; da Silva Pereira, M.I. Pulsed electrodeposition of Zn in the presence of surfactants. Electrochim. Acta 2006, 51, 1342–1350. [Google Scholar] [CrossRef]
- Casanova, T.; Soto, F.; Eyraud, M.; Crousier, J. Hydrogen absorption during zinc plating on steel. Corros. Sci. 1997, 39, 529–537. [Google Scholar] [CrossRef]
- Ouakki, M.; El Fazazi, A.; Cherkaoui, M. Electrochemical deposition of zinc on mild steel. Mediterr. J. Chem. 2019, 8, 30–41. [Google Scholar] [CrossRef]
- Gamburg, Y.D.; Zangari, G. Theory and Practice of Metal Electrodeposition; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 12–13. [Google Scholar]
- Ibrahim, M.A.M. Improving the throwing power of acidic zinc sulfate electroplating baths. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2000, 75, 745–755. [Google Scholar] [CrossRef]
- Sekar, R.; Jagadesh, K.K.; Ramesh Bapu, G.N.K. Role of amino acids on electrodeposition and characterisation of zinc from alkaline zincate solutions. Trans. IMF 2015, 93, 133–138. [Google Scholar] [CrossRef]
- Bapu, G.R.; Devaraj, G.; Ayyapparaj, J. Studies on non-cyanide alkaline zinc electrolytes. J. Solid State Electrochem. 1998, 3, 48–51. [Google Scholar] [CrossRef]
- Kavitha, B.; Santhosh, P.; Renukadevi, M.; Kalpana, A.; Shakkthivel, P.; Vasudevan, T. Role of organic additives on zinc plating. Surf. Coat. Technol. 2006, 201, 3438–3442. [Google Scholar] [CrossRef]
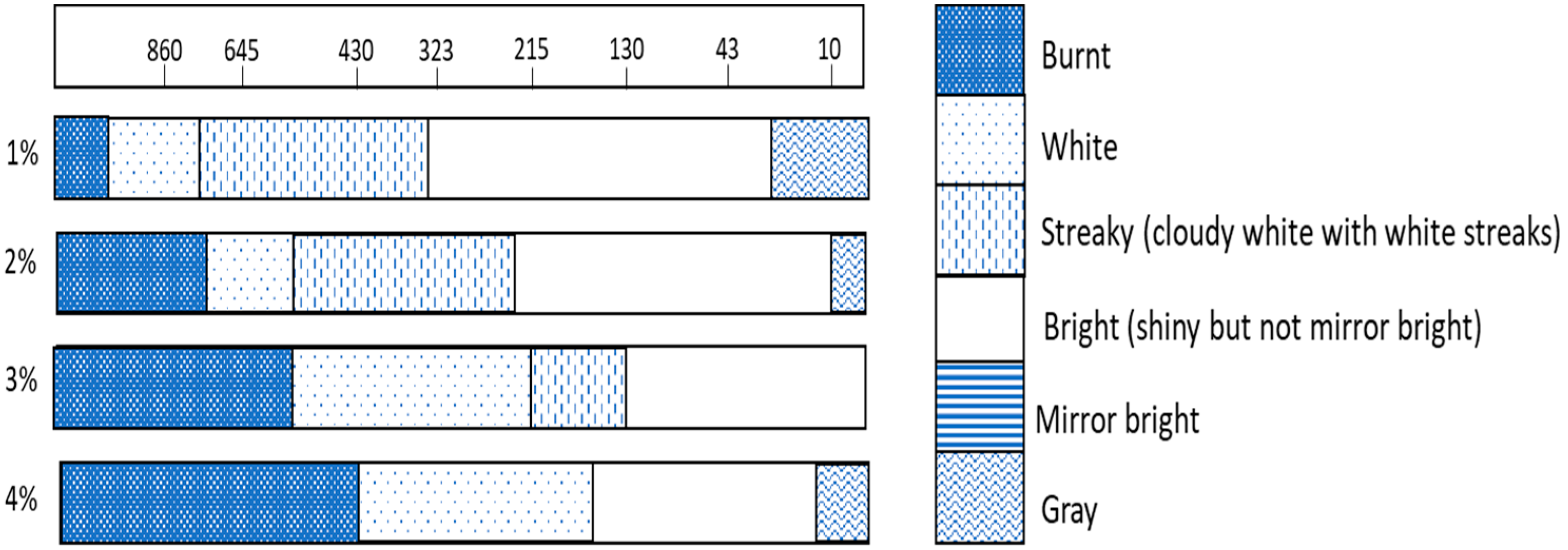
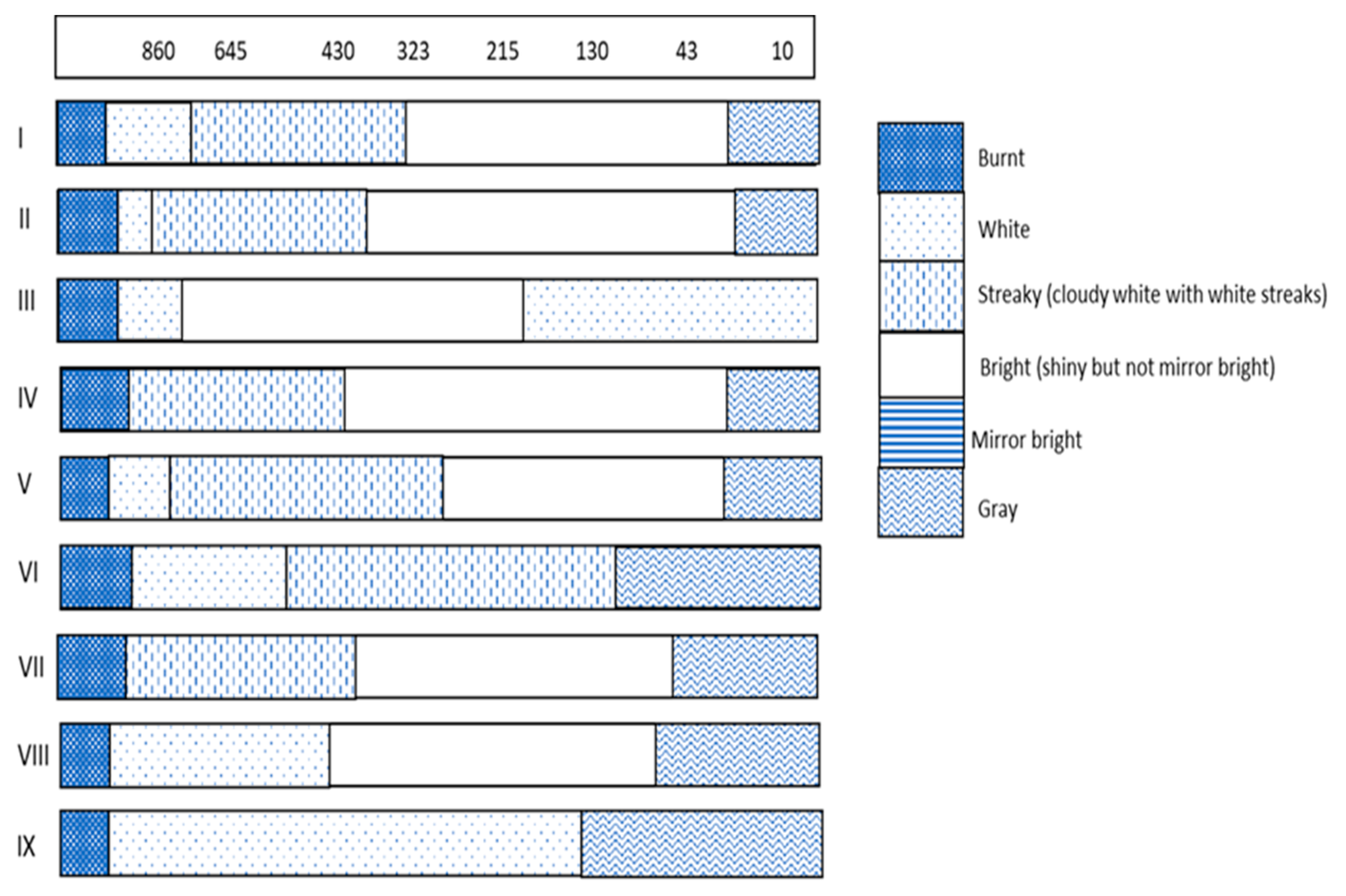

| Bath | Carrier (mL/L) | Leveller (mL/L) | Booster (mL/L) |
|---|---|---|---|
| I | 6.25 | 2.50 | 1.25 |
| II | 9.38 | 3.75 | 0.63 |
| III | 3.13 | 3.75 | 0.63 |
| IV | 9.38 | 3.75 | 1.88 |
| V | 9.38 | 1.25 | 1.88 |
| VI | 3.13 | 1.25 | 1.88 |
| VII | 9.38 | 1.25 | 0.63 |
| VIII | 3.13 | 1.25 | 0.63 |
| IX | 3.13 | 3.75 | 1.88 |
| Bath | Gloss |
|---|---|
| I | 71 |
| II | 70 |
| III | 85 |
| IV | 70 |
| V | 62 |
| VI | 35 |
| VII | 75 |
| VIII | 80 |
| IX | 55 |
| Bath | Throwing Power (%) | Current Efficiency (%) |
|---|---|---|
| I | 23 | 99 |
| II | 24 | 99 |
| III | 18 | 99 |
| IV | 30 | 99 |
| V | 37 | 98 |
| VI | 29 | 98 |
| VII | 32 | 98 |
| VIII | 30 | 99 |
| IX | 21 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.J.; Moats, M. Effects of Carrier, Leveller, and Booster Concentrations on Zinc Plating from Alkaline Zincate Baths. Metals 2022, 12, 621. https://doi.org/10.3390/met12040621
Mohammed AJ, Moats M. Effects of Carrier, Leveller, and Booster Concentrations on Zinc Plating from Alkaline Zincate Baths. Metals. 2022; 12(4):621. https://doi.org/10.3390/met12040621
Chicago/Turabian StyleMohammed, Abdul Jalil, and Michael Moats. 2022. "Effects of Carrier, Leveller, and Booster Concentrations on Zinc Plating from Alkaline Zincate Baths" Metals 12, no. 4: 621. https://doi.org/10.3390/met12040621
APA StyleMohammed, A. J., & Moats, M. (2022). Effects of Carrier, Leveller, and Booster Concentrations on Zinc Plating from Alkaline Zincate Baths. Metals, 12(4), 621. https://doi.org/10.3390/met12040621






