Influence of Cell Opening Methods on Electrolyte Removal during Processing in Lithium-Ion Battery Recycling
Abstract
:1. Introduction
2. Recycling of Spent LIBs
2.1. Design and Hazards of LIBs
2.2. Waste Management of EOL LIBs
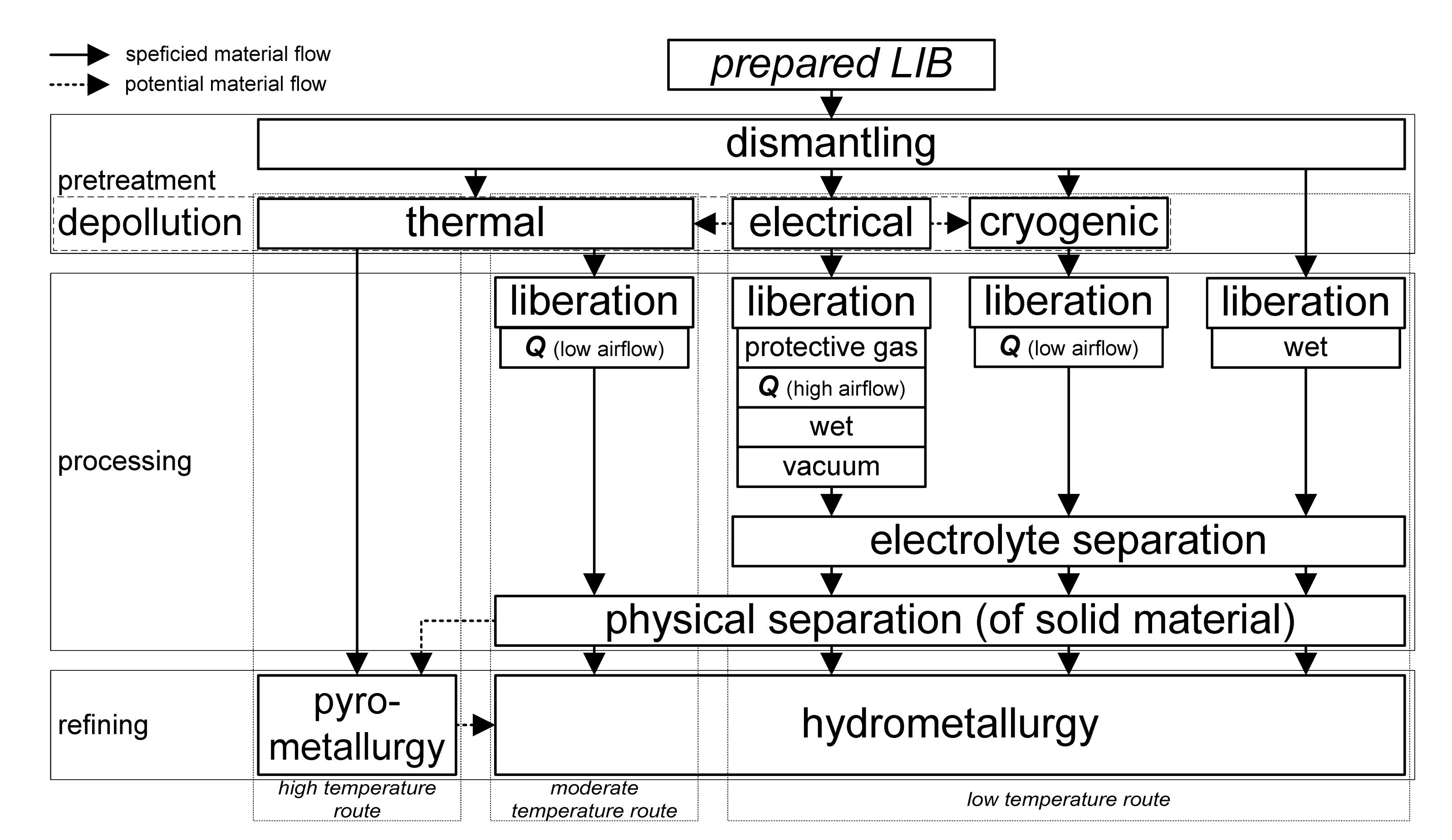
2.3. Processing of EOL LIBs
2.3.1. Mechanical Cell Opening
2.3.2. Process Medium for Mechanical Cell Opening
2.3.3. Handling of the Process Medium after Crushing
2.3.4. Electrolyte Removal
3. Materials and Methods
3.1. Materials
3.2. Test Equipment
3.3. Methods
4. Results and Discussion
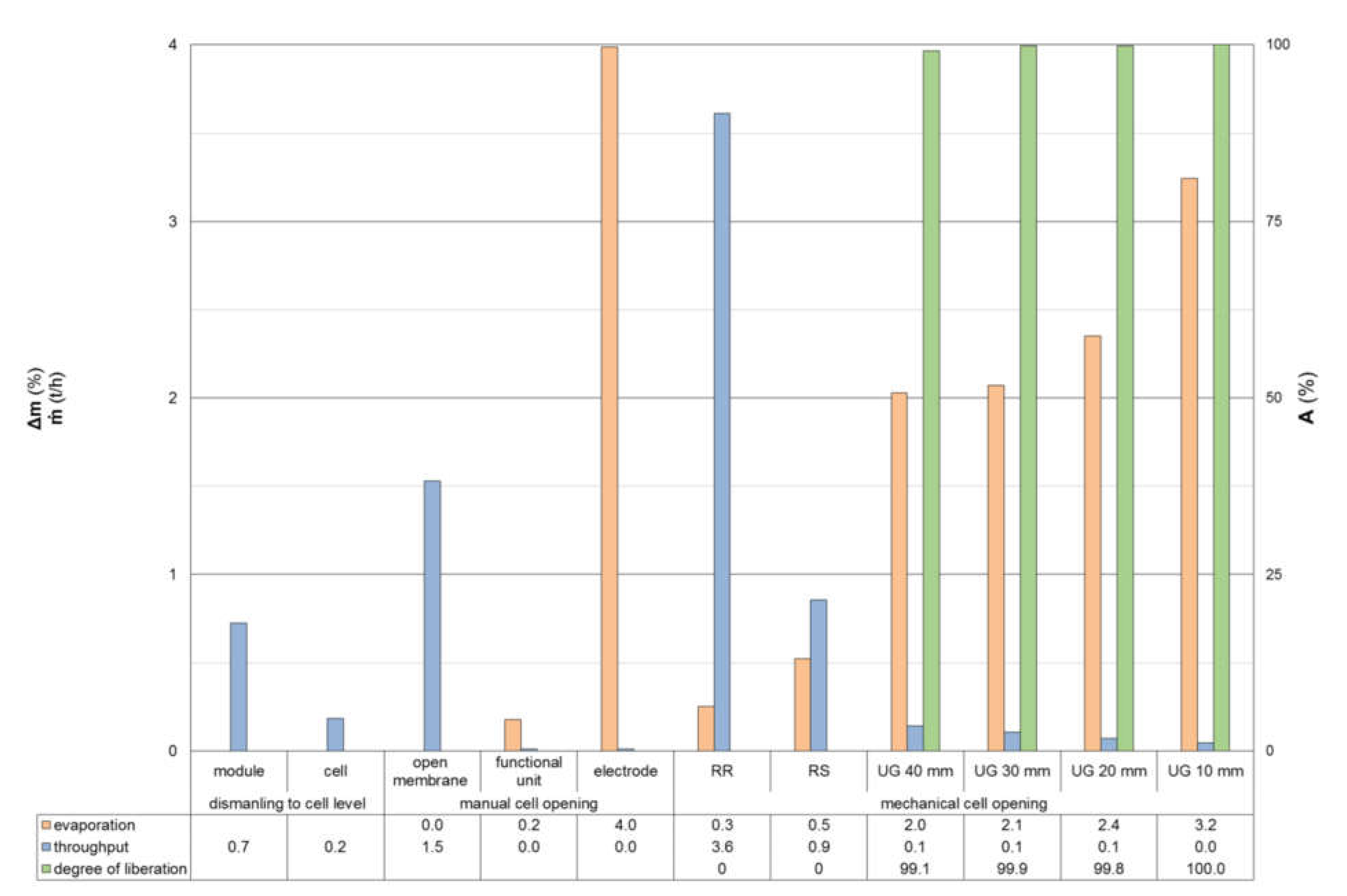
4.1. Manual and Mechanical Cell Opening
4.2. Liberation of Pretreated Materials
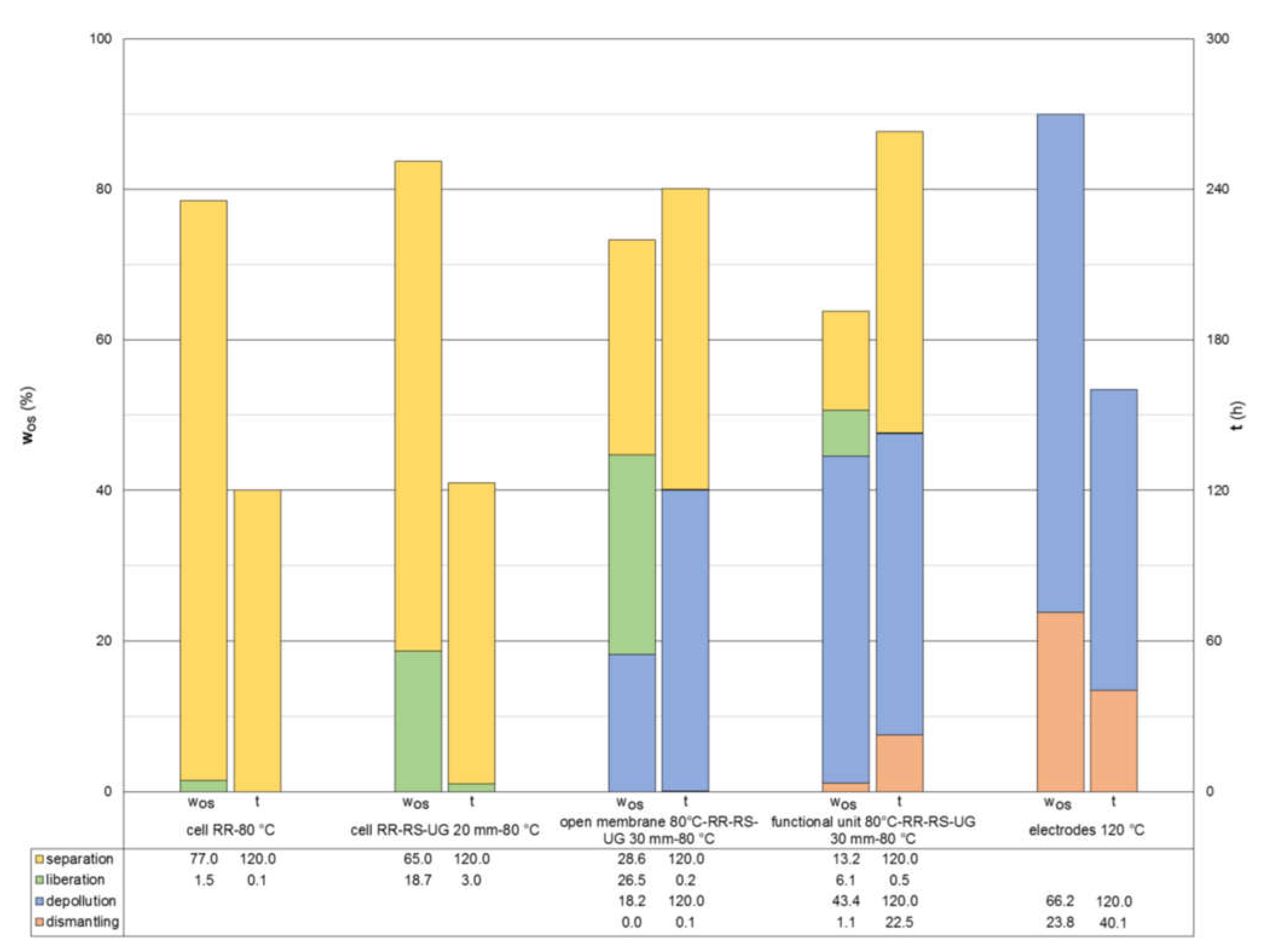
4.3. Thermal Drying of Crushing Products
4.4. Potential Process Chains
4.5. Recycling Efficiencies of the Process Routes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AM | Active materials |
| Al | Aluminium |
| CMC | Sodium Carboxymethyl Cellulose |
| Co | Cobalt |
| Cu | Copper |
| DEC | Diethyl carbonate |
| DMC | Dimethyl carbonate |
| EOL | End-of-life |
| EC | Ethylene carbonate |
| EMC | Ethyl methyl carbonate |
| HF | Hydrofluoric acid |
| Li | Lithium |
| LiPF6 | Lithium hexafluorophosphate |
| LIB(s) | Lithium-ion battery(ies) |
| MRR | Material recovery rates |
| Mn | Manganese |
| NMC | Lithium nickel manganese cobalt battery |
| NSD | Nail Penetration Safety Device |
| PC | Propylene carbonates |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| PVDF | Polyvinylidene fluoride |
| RE | Recycling efficiency |
| RR | Slow-rotating, two-shafts rotary shear with axial gap |
| RS | Slow-rotating, two-shafts rotary shear without axial gap |
| SBR | Styrene butadiene rubber |
| UG | One shaft rotary shear with outlet grid) final crushing |
References
- Babbitt, C.; Althaf, S.; Cruz Rios, F.; Bilec, M.M.; Graedel, T.E. The role of design in circular economy solutions for critical materials. Perspective 2021, 4, 353–362. [Google Scholar] [CrossRef]
- Hanisch, C.; Haselrieder, W.; Kwade, A. Recycling von Lithium-Ionen-Batterien–das Projekt LithoRec. In Recycling und Rohstoffe; Thomé-Kozmienski, K.J., Goldmann, D., Eds.; TK Verlag: Neuruppin, Germany, 2012; Volume 5, pp. 691–698. [Google Scholar]
- Vezzini, A. Manufacturers, Materials and Recycling Technologies. In Lithium-Ion Batteries Advances and Applications; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 529–551. [Google Scholar]
- Baars, J.; Domenech, T.; Bleischwitz, R.; Melin, H.E.; Heidrich, O. Circular economy strategies for electric vehicle batteries reduce reliance on raw materials. Nat. Sustain. 2021, 4, 71–79. [Google Scholar] [CrossRef]
- Rothermel, S.; Winter, M.; Nowak, S. Background. In Recycling of Lithium-Ion Batteries-The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: New York, NY, USA, 2018; pp. 1–31. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Perez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Werner, D.; Mütze, T.; Peuker, U.A. Influence of cell opening methods on organic solvent removal during pretreatment in lithium-ion battery recycling. Waste Manag. Res. 2021, 10, 316. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Met. Open Access Metall. J. 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; He, W.; Li, G. Recycling Technology and Principle of Spent Lithium-Ion Battery. In Recycling of Spent Lithium-Ion Batteries-Processing Methods and Environmental Impacts; An, L., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 1–26. [Google Scholar]
- Korthauer, R. Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer GmbH: Berlin, Germany, 2019. [Google Scholar]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety Issues and Mechanisms of Lithium-ion Battery Cell Upon Mechanical Abusive Loading: A Review. Energy Storage Mater. 2019, 24, 85–112. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Korthauer, R. Handbuch Lithium-Ionen-Batterien; Korthauer, R., Ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Wuschke, L. Mechanische Aufbereitung von Lithium-Ionen-Batteriezellen. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2018. [Google Scholar]
- Weyhe, R. Verbundprojekt ”Rückgewinnung der Rohstoffe aus Li-Ion Akkumulatoren“; Accurec GmbH: Mühlheim, Germany, 2008. [Google Scholar]
- Kwade, A.; Diekmann, J. Recycling of Lithium-Ion Batteries-The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Arnberger, A.; Gresslehner, K.-H.; Pomberger, R.; Curtis, A. Recycling von Lithium Ionen Batterien aus EVs & HEVs. In Proceedings of the DepoTech 2012, Leoben, Austria, 6 November 2012. [Google Scholar]
- Gaines, L.; Sullivan, J.; Burnham, A. Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling. In Proceedings of the 90th Annual Meeting of the Transportation Research Board, Washington, DC, USA, 23–27 January 2011. [Google Scholar]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutzc, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W. Targeting high value metals in lithium-ion battery recycling via shredding and size-based separation. Waste Manag. 2016, 51, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnberger, A.; Coskun, E.; Rutrecht, B. Recycling von Lithium-Ionen-Batterien. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; TK Verlag Karl Thomé-Kozmiensky: Neuruppin, Germany, 2018; Volume 11, pp. 583–599. [Google Scholar]
- Rahimzei, E. Begleit-und Wirkungsforschung Schaufenster Elektromobilität (BuW) Ergebnispapier Nr. 37-Sicherheit von Elektrofahrzeugen; Deutsches Dialog Institut GmbH: Frankfurt am Main, Germany, 2017. [Google Scholar]
- Elwert, T.; Römer, F.; Schneider, K.; Hua, Q.; Buchert, M. Recycling of Batteries from Electric Vehicles. In Behaviour of Lithium-Ion Batteries in Electric Vehicles-Battery Health, Performance, Safety, and Cost; Pistoia, G., Liaw, B., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Gama, M. European Li-Ion Battery Advanced Manufacturing for Electric Vehicles-ELIBAMA. Available online: https://cordis.europa.eu/project/id/285385 (accessed on 19 February 2022).
- Hanisch, C.; Diekmann, J.; Stieger, A.; Haselrieder, W.; Kwade, A. Recycling of Lithium-Ion Batteries. In Handbook of Clean Energy Systems; Yan, J., Cabeza, L.F., Sioshansi, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 5, pp. 2865–2888. [Google Scholar]
- Van Pels, W. Herausforderung hinsichtlich der Brandschutztechnologien in Recyclinganlagen insbesondere bei der Verarbeitung von Li-Ionen Traktionsbatterien. In Proceedings of the Recycling und Sekundärrohstoffe, Berlin, Germany, 2–3 March 2020; pp. 496–505. [Google Scholar]
- Wegener, K.; Andrew, S.; Raatz, A.; Dröder, K.; Herrmann, C. Disassembly of Electric Vehicle Batteries Using the Example of the Audi Q5 Hybrid System. In Proceedings of the Conference on Assembly Technologies and Systems, Dresden, Germany, 15–16 May 2014. [Google Scholar]
- Ritthoff, M. Recycling und Ressourcenschonung stoffliche Aspekte der Kreislaufwirtschaft. In NABU-DSD-Dialogforum Kreislaufwirtschaft; Wuppertal Institut: Berlin, Germany, 2016. [Google Scholar]
- Sojka, T.R. Sichere Aufbereitung von Lithium-basierten Batterien durch thermische Konditionierung. In Proceedings of the Recycling und Sekundärrohstoffe, Berlin, Germany, 2–3 March 2020; pp. 506–523. [Google Scholar]
- Tytgat, J. The Recycling Efficiency of Li-ion EV batteries according to the European Commission Regulation, and the relation with the End-of-Life Vehicles Directive recycling rate. In Proceedings of the International Battery, Hybrid and Fuel Cell Electric Vehicle Symposium, Barcelona, Spain, 17–20 November 2013. [Google Scholar]
- Pomberger, R. Über theoretische und reale Recyclingfähigkeit. Österr Wasser Und Abfallw 2020, 319, 12. [Google Scholar] [CrossRef]
- Martens, H.; Goldmann, G. Recyclingtechnik; Martens, H., Goldmann, G., Eds.; Springer Vieweg: Wiesbaden, Germany, 2016. [Google Scholar]
- Schubert, G. Comminution Equipment for Non-Brittle Waste and Scrap. Aufbereitungstechnik 2002, 43, 6–23. [Google Scholar]
- Schubert, H. Handbuch der Mechanischen Verfahrenstechnik; Schubert, H., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The Recycling of Spent Lithium-Ion Batteries: A Review of Current Processes and Technologies. Electrochem. Energy Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Nickel, W. Recyclinghandbuch: Strategien-Technologien-Produkte; Nickel, W., Ed.; VDI-Verlag: Düsseldorf, Germany, 1996. [Google Scholar]
- Marshall, J.; Gastol, D.; Sommerville, R.; Middleton, B.; Goodship, V.; Kendrick, E. Disassembly of Li Ion Cells-Characterization and Safety Considerations of a Recycling Scheme. Metals 2020, 10, 773. [Google Scholar] [CrossRef]
- Thompson, D.; Hyde, C.; Hartley, J.M.; Abbott, A.P.; Anderson, P.A.; Harper, G.D.J. To shred or not to shred: A comparative techno-economic assessment of lithium ion battery hydrometallurgical recycling retaining value and improving circularity in LIB supply chains. Resour. Conserv. Recycl. 2021, 175, 105741. [Google Scholar] [CrossRef]
- Gaines, L.; Dai, Q.; Vaughey, J.T.; Gillard, S. Direct Recycling R&D at the ReCell Center. Recycling 2021, 6, 31. [Google Scholar]
- Wuschke, L.; Jäckel, H.-G.; Leißner, L.; Peuker, U.A. Crushing of large Li-ion battery cells. Waste Manag. 2019, 85, 317–326. [Google Scholar] [CrossRef]
- Diekmann, J.; Sander, S.; Sellin, G.; Petermann, M.; Kwade, A. Crushing of Battery Modules and Cells. In Recycling of Lithium-Ion Batteries-The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: New York, NY, USA, 2018; pp. 127–138. [Google Scholar]
- Wuschke, L.; Jäckel, H.-G.; Peuker, U.A.; Gellner, M. Recycling of Li-ion batteries—A challenge. Recovery 2015, 4, 48–59. [Google Scholar]
- Weyhe, R. Recycling von Lithium-Ion-Batterien. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; TK Verlag Karl Thomé-Kozmiensky: Neuruppin, Germany, 2013; Volume 6, pp. 505–525. [Google Scholar]
- Pinegar, H.; Smith, Y.R. End-of-Life Lithium-Ion Battery Component Mechanical Liberation and Separation. J. Miner. Met. Mater. Soc. 2019, 71, 4447–4456. [Google Scholar] [CrossRef]
- Zhao, G. Resource Utilization and Harmless Treatment of Power Batteries. In Reuse and Recycling of Lithium-Ion Power Batteries; Zhao, G., Ed.; John Wiley & Sons: Singapore, 2017; pp. 335–378. [Google Scholar]
- Wuschke, L.; Jäckel, H.-G.; Gellner, M.; Peuker, U.A. Comminution of Li-Ion Traction Batteries. In Proceedings of the European Symposium on Comminution and Classification, Gothenborg, Sweden, 7–11 September 2015; p. 6. [Google Scholar]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Stehmann, F.; Bradtmöller, C.; Scholl, S. Separation of the Electrolyte—Thermal Drying. In Recycling of Lithium-Ion Batteries-The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: New York, NY, USA, 2018; pp. 139–153. [Google Scholar]
- Hu, Q.; Xu, L. An overview on Lithium-ion batteries recycling processes. J. Phys. Conf. Ser. 2021, 1885, 032031. [Google Scholar] [CrossRef]
- Friedrich, B.; Schwich, L. E-Mobility Batteries Recycling-How to Reach the Demanded Recycling Efficiency? Presented at the VDMA Webinar. Available online: https://www.researchgate.net/publication/352786897_E-Mobility_Batteries_Recycling_-_How_to_Reach_the_Demanded_Recycling_Efficiency (accessed on 19 February 2022).
- Valio, J. Critical Review on Lithium Ion Battery Recycling Technologies. Master’s Thesis, Aalto University, Helsinki, Finland, 2017. [Google Scholar]
- Woehrle, T.; Kern, R. Method for Performing Comminution of Battery Containing Lithium Hexafluorophosphate Used in e.g., Vehicle, Involves Using Environmental Fluid Containing Alkaline Earth Metal Surrounding the Battery for Realizing Comminution of Battery. Patent No. DE102011082187A1, 7 March 2013. [Google Scholar]
- Dunagan, D.J.; Ostrander, T.C. Systems for recycling volatile battery sources. Patent No. US 9.450,277 B2, 20 September 2016. [Google Scholar]
- Yang, H.; Zhuang, G.V.; Ross, P.N., Jr. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Hettesheimer, T.; Thielmann, A.; Neef, N.; Möller, K.-C.; Wolter, M.; Lorentz, V.; Gepp, M.; Wenger, M.; Prill, T.; Zausch, J.; et al. Entwicklungsperspektiven für Zellformate von Lithium-Ionen-Batterien in der Elektromobilität; Fraunhofer-Institut für System-und Innovationsforschung ISI: Karlsruhe, Germany, 2017. [Google Scholar]
- Schubert, G. Aufbereitung Metallischer Sekundärrohstoffe-Aufkommen Charakterisierung Zerkleinerung; Springer: Wien, Austria, 2012. [Google Scholar]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Weber, M.; Speckmann, F.-W. DeMoBat-Industrial Disassembly of Battery Modules and Electric Motors. Available online: https://www.ipa.fraunhofer.de/en/reference_projects/DeMoBat.html (accessed on 5 January 2021).
- Cerdas, F.; Gerbers, R.; Andrew, S.; Schmitt, J.; Dietrich, F.; Thiede, S.; Dröder, K.; Herrmann, C. Disassembly Planning and Assessment of Automation Potentials for Lithium-Ion Batteries. In Recycling of Lithium-Ion Batteries-The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: New York, NY, USA, 2018; pp. 83–97. [Google Scholar]
- Weyhe, R.; Friedrich, B. Demonstrationsanlage für ein Kostenneutrales, Ressourceneffizientes Processing Ausgedienter Li-Ionen Batterien aus der Elektromobilität-EcoBatRec-Abschlussbericht zum Verbundvorhaben; IME Metallurgische Prozesstechnik und Metallrecycling: Aachen, Germany, 2016. [Google Scholar]
- Sattler, K. Thermische Trennverfahren-Grundlagen, Auslegung, Apparate; WILEY-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Schnell, F. Ökonomik von Entsorgung und Recycling-Eine Analyse mit Fokus auf die Schweiz. In Proceedings of the Ewaste Forum, Zürich, Switzerland, 23 June 2017. [Google Scholar]
- Ekberg, C.; Petranikova, M. Lithium Batteries Recycling. In Lithium Process Chemistry-Resources, Extraction, Batteries and Recycling; Chagnes, A., Swiatowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–269. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Schwich, L.; Friedrich, B. Impact of process chain design on metal recycling efficiency-A critical view on the actual total weight-based target. In Proceedings of the International Conference for Battery Recycling, Lyon, France, 18–20 September 2019. [Google Scholar]
- Hagelüken, C. Cobalt for Li-Ion Batteries-Future demand expectations and actions for sustainable sourcing. In Proceedings of the World Resource Forum, Geneva, Switzerland, 24–25 October 2017. [Google Scholar]
- Krüger, S.; Hanisch, C.; Kwade, A.; Winter, M.; Nowak, S. Effect of impurities caused by a recycling process on the electrochemical performance of Li[Ni0.33Co0.33Mn0.33]O2. J. Electroanal. Chem. 2014, 726, 91–96. [Google Scholar] [CrossRef]
- Chen, X.; Cao, L.; Kang, D.; Li, J.; Li, S.; Wu, X. Hydrometallurgical Processes for Valuable Metals Recycling from Spent Lithium-Ion Batteries. In Recycling of Spent Lithium-Ion Batteries-Processing Methods and Environmental Impacts; An, L., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- Xiao, J.; Li, J.; Xu, Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J. Hazard. Mater. 2017, 338, 124–131. [Google Scholar] [CrossRef]


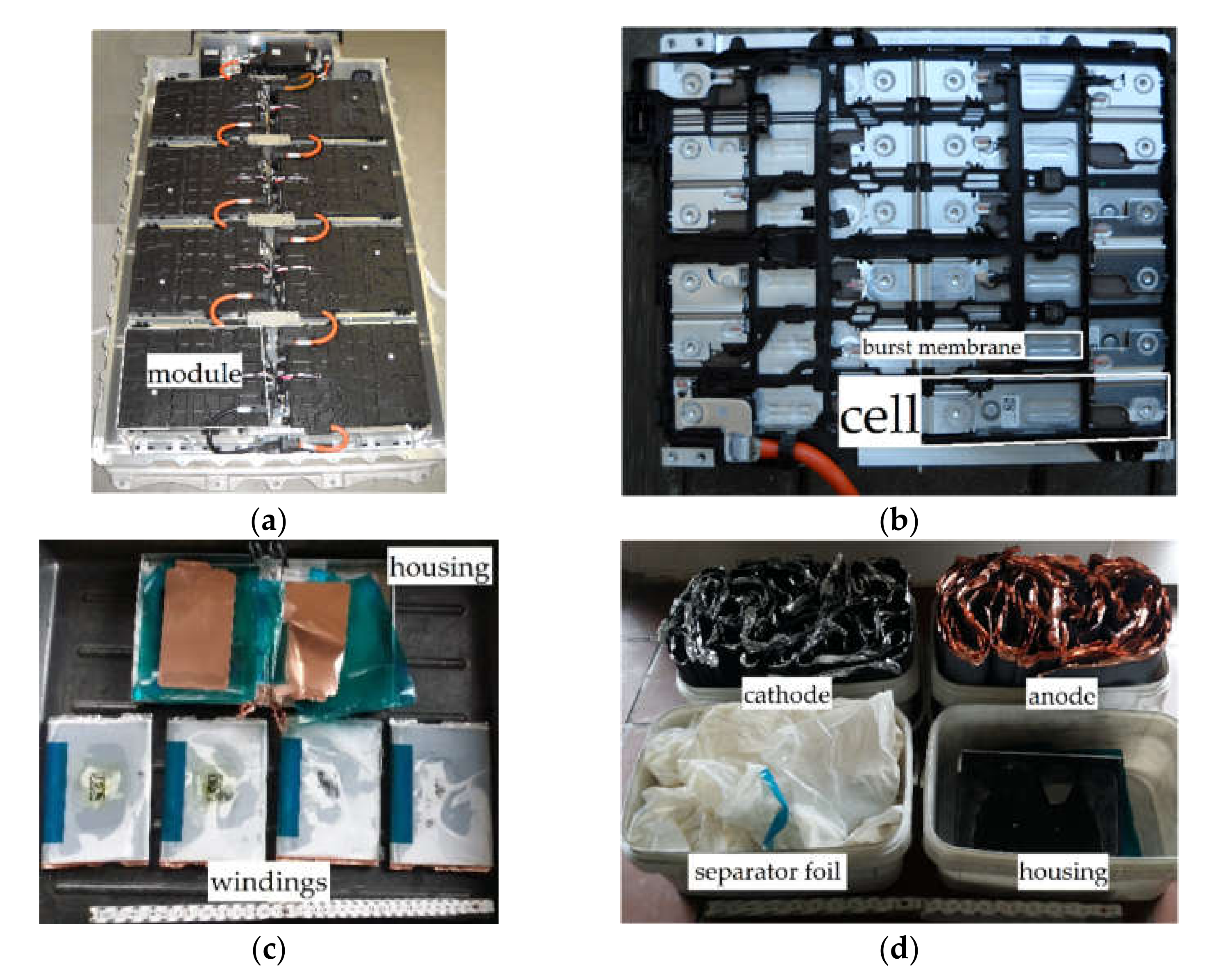


| Component | Function | Material | Mass Share w (%) |
|---|---|---|---|
| cathode | metal foil | aluminium | 3.0 |
| coating | NMC + PVDF + additives | 34.0 | |
| anode | metal foil | copper | 7.2 |
| coating | graphite + SBR + CMC + additives | 17.9 | |
| housing | NSD contact | copper | 0.9 |
| electrical contact | copper | 0.7 | |
| electrical contact | aluminium | 0.3 | |
| case | aluminium | 11.8 | |
| retainer | PP | 0.7 | |
| sleeve | PET | 0.3 | |
| NSD foil | PP | 0.1 | |
| foils | PP | 0.2 | |
| glue | unknown | 0.2 | |
| others | not specified | 0.5 | |
| separator | foil | PP/PE/PP | 1.9 |
| electrolyte | organic solvents | DMC | 16.8 |
| EMC | |||
| DEC | |||
| EC | |||
| conductive salt | LiPF6 | 2.6 | |
| additives | unknown | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, D.M.; Mütze, T.; Peuker, U.A. Influence of Cell Opening Methods on Electrolyte Removal during Processing in Lithium-Ion Battery Recycling. Metals 2022, 12, 663. https://doi.org/10.3390/met12040663
Werner DM, Mütze T, Peuker UA. Influence of Cell Opening Methods on Electrolyte Removal during Processing in Lithium-Ion Battery Recycling. Metals. 2022; 12(4):663. https://doi.org/10.3390/met12040663
Chicago/Turabian StyleWerner, Denis Manuel, Thomas Mütze, and Urs Alexander Peuker. 2022. "Influence of Cell Opening Methods on Electrolyte Removal during Processing in Lithium-Ion Battery Recycling" Metals 12, no. 4: 663. https://doi.org/10.3390/met12040663
APA StyleWerner, D. M., Mütze, T., & Peuker, U. A. (2022). Influence of Cell Opening Methods on Electrolyte Removal during Processing in Lithium-Ion Battery Recycling. Metals, 12(4), 663. https://doi.org/10.3390/met12040663







