Characterization of Galvanizing Flue Dust and Recycling Possibilities
Abstract
1. Introduction
2. Materials and Methods
Sample Preparation and Characterization
3. Results
3.1. Surface Analysis
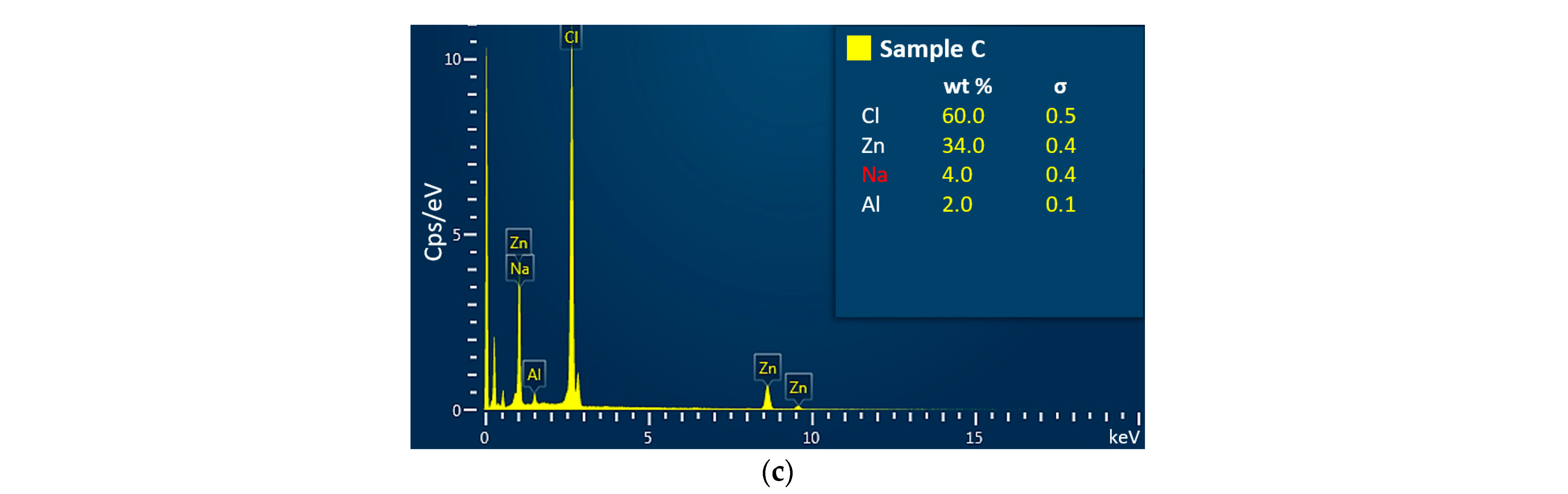
3.2. The Chemical Composition Analysis
3.3. The Phase Analysis
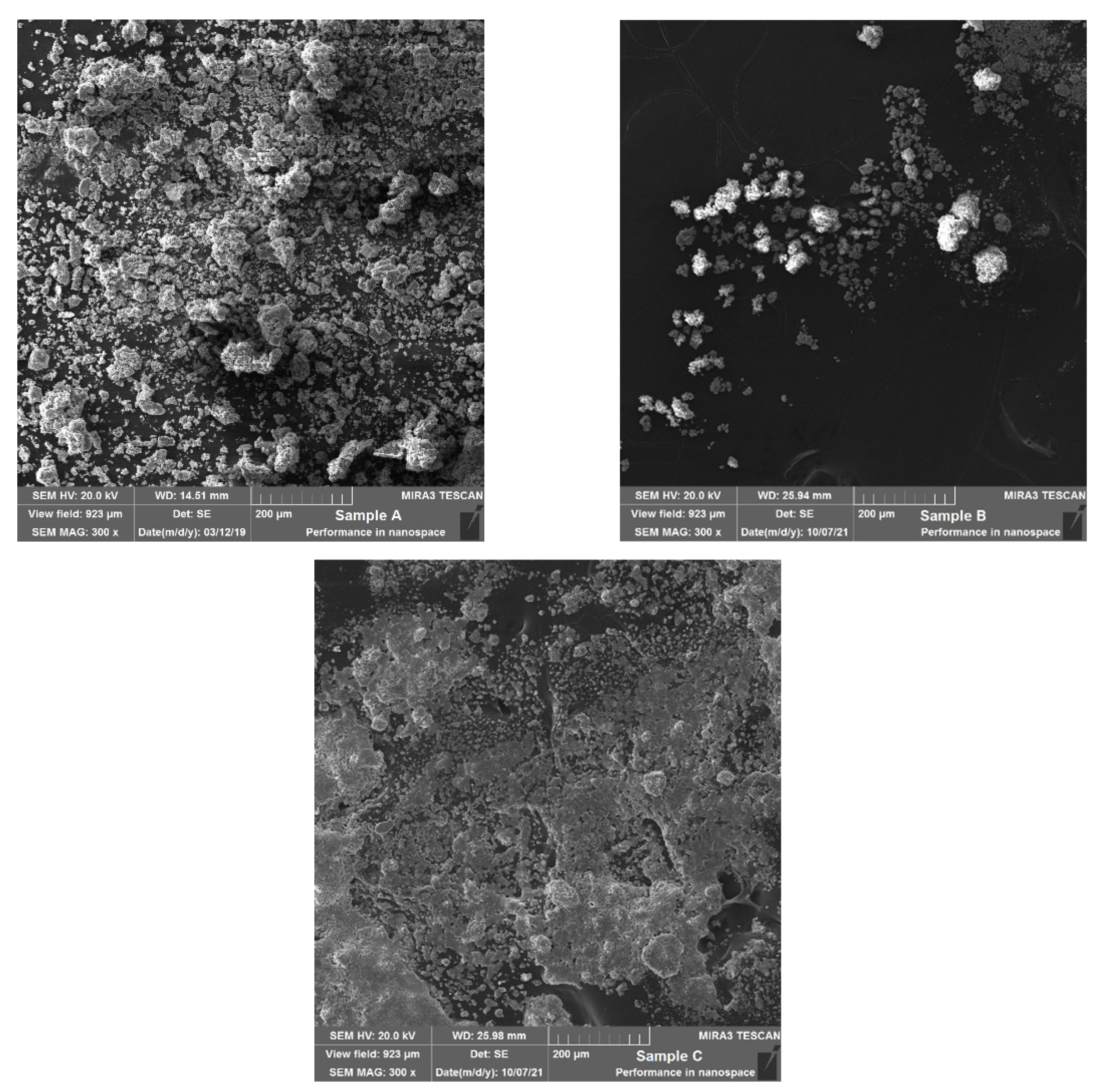
3.4. Morphology of Samples
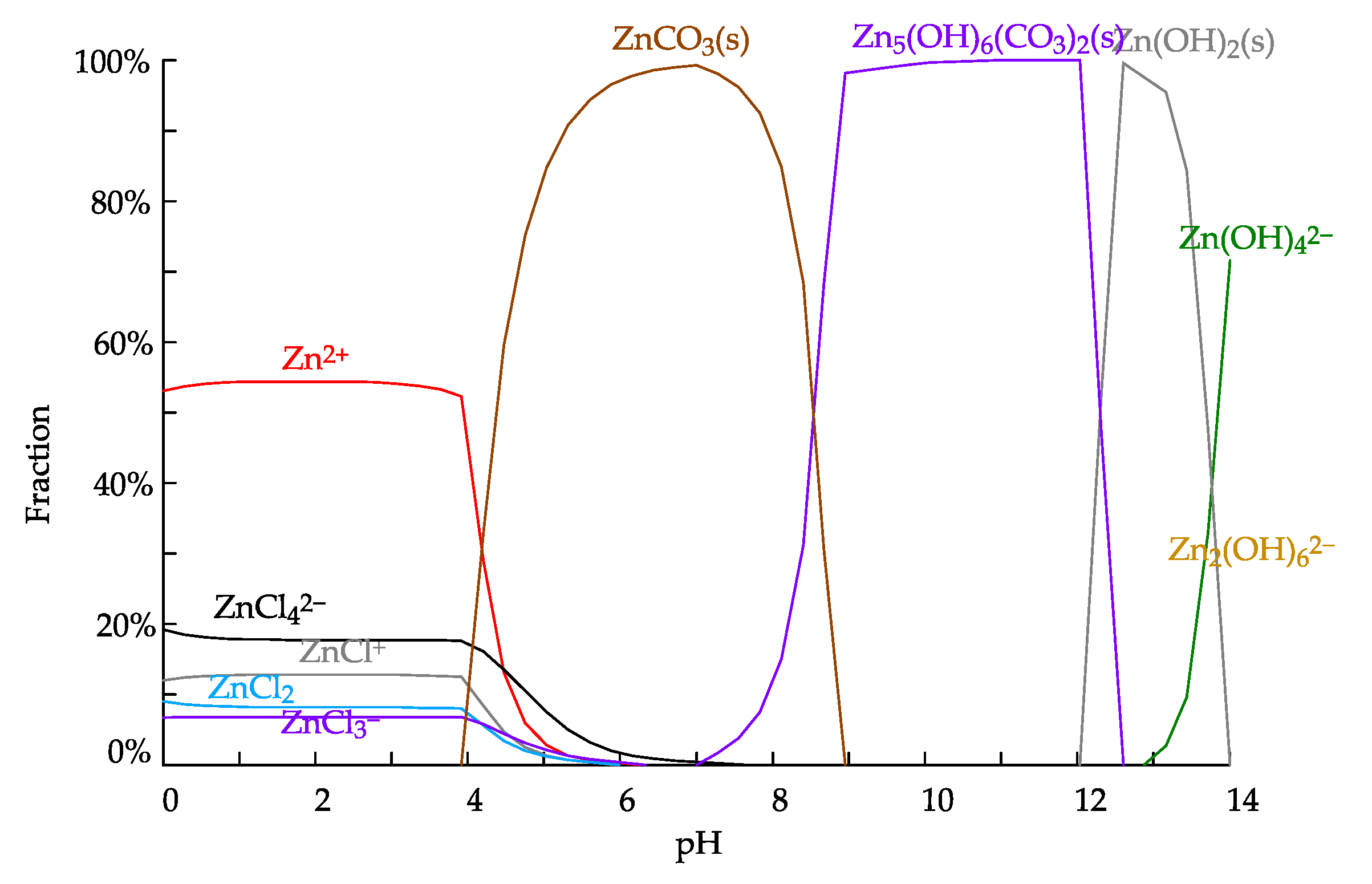
3.5. Leachability Testing
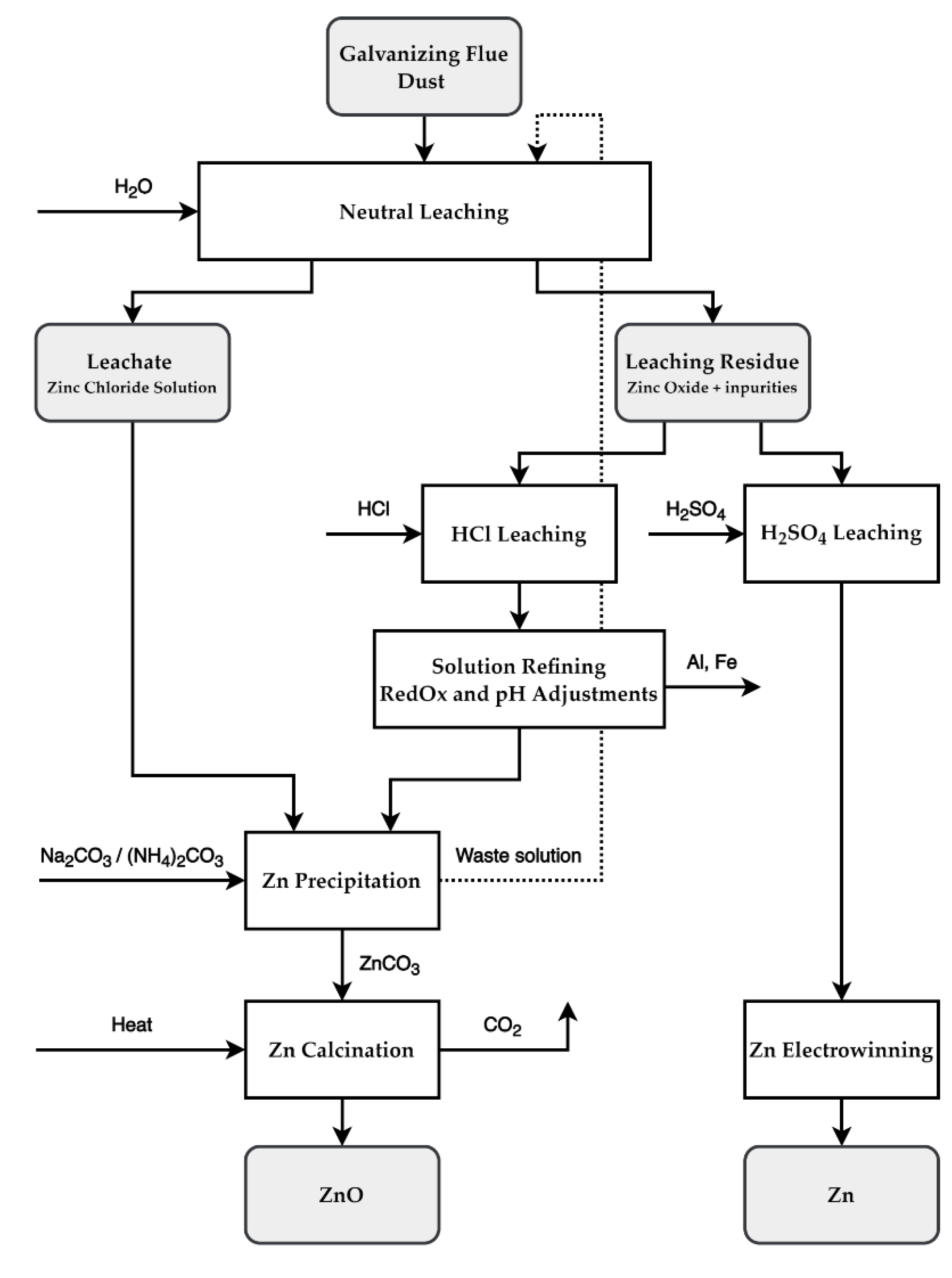
4. Recycling Possibilities
4.1. Proposed Two-Step Leaching Recycling Method
4.2. Proposed Solution Refining and Zinc Recovery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hot-Dip Galvanizing Summit—The Current Situation of Environmental Protection and Pollution Control Countermeasures in the Hot-Dip Galvanizing Industry Are Coming! 2020. Available online: https://news.metal.com/newscontent/101292420/[hot-dip-galvanizing-Summit]-the-current-situation-of-environmental-protection-and-pollution-control-countermeasures-in-the-hot-dip-galvanizing-industry-are-coming/ (accessed on 31 November 2021).
- Bellini, C.; Iacoviello, F.; Carlino, F.; Di Cocco, V. The influence of hot dip galvanizing process on intermetallic phases formation. Mat. Des. Process Comm. 2019, 1, e39. [Google Scholar] [CrossRef]
- Zinc—Statistics & Facts. 2020. Available online: https://www.statista.com/topics/2306/zinc/#dossierSummary__chapter4 (accessed on 14 November 2021).
- Mineral Commodity Summaries 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020.pdf (accessed on 21 October 2021).
- European Commission, CRM List 2020. 2020. Available online: https://rmis.jrc.ec.europa.eu/?page=crm-list-2020-e294f6 (accessed on 31 November 2021).
- Kuklik, V.; Kudlacek, J. Žárové zinkování; Tiskárny Havlíčův Brod, a.s.: Prague, Czech Republic, 2014; p. 208. ISBN 978-80-905298-2-3. [Google Scholar]
- Maass, P.; Peissker, P. Handbook of Hot-Dip Galvanization; WILEY-VCH Verlag GmbH: Hoboken, NJ, USA, 2011; p. 494. ISBN 9783527636891. [Google Scholar]
- Eriksson, H.; Hrinová, A. Príručka Žiarového Zinkovania; AČSZ: Ostrava, Czech Republic, 2009; p. 60. [Google Scholar]
- Trpcevska, J. Zinok, Jeho Aplikácia, Výroba a Recyklácia; Technical University of Košice: Košice, Slovakia, 2018; p. 117. ISBN 978-80-553-2997-0. [Google Scholar]
- Hazardous Waste Listings 2012. Available online: https://www.epa.gov/sites/default/files/2016-01/documents/hw_listref_sep2012.pdf (accessed on 30 November 2021).
- Rahman, L.; Saiful, Q.; Juliya, K.; Khadija, B.; Moriom, R.; Nahid, S.; Tahuran, N. Fabrication of zinc oxide from zinc dust and its characterization. IOSR J. Appl. Chem. 2017, 10, 21–26. [Google Scholar] [CrossRef]
- Bisol, F. Process for Treating Metallic Dust, Mostly Oxididised Waste, in Particular Galvanising Dust and/or Steelworks Smoke. EP0935005 A1, 24 April 2002. [Google Scholar]
- Barakat, M.A. Pyrometallurgical Processing of Zinc Ash and Flue Dust. JOM 2003, 55, 26–29. [Google Scholar] [CrossRef]
- Lemke, E.E. Air Pollution Control Measures for Hot Dip Galvanizing Kettels. J. Air Pollut. Control. Assoc. 1960, 10, 70–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pirosková, J. Spracovanie Vrchného Steru Vznikajúceho v Procese Mokrého Žiarového Zinkovania. Ph.D. Thesis, Technical University of Košice, Koš1ice, The Slovak Republic, 2015; p. 121. Available online: https://opac.crzp.sk/?fn=docviewChild0027CBF5 (accessed on 2 November 2021).
- McNeeney Stephen, P. Flux and Process for Hot Dip Galvanization, 2013. EP1974070 B1, 12 June 2013. [Google Scholar]
- Cook, T.H. Composition, testing, and control of hot dip galvanizing flux. Met. Finish. 2003, 101, 22–35. [Google Scholar] [CrossRef]
- Cook, T.H.; Finishing. How to Minimize Smoke in Galvanizing Plant. Available online: https://www.finishing.com/592/65.shtml (accessed on 24 November 2021).
- Trpcevska, J.; Pirošková, J.; Laubertová, M.; Hoľková, B. Vznik sterov v procese žiarového zinkovania a úloha hliníka. Konstrukce 2014, 13, 7–9. [Google Scholar]
- General Batch Galvanizing Procedures. Beyond Discovery. Available online: https://www.beyonddiscovery.org/corrosion-resistance-3/general-batch-galvanizing-procedures.html (accessed on 2 November 2021).
- Woods, R.M.; Cole, J.A. Galvanizing Handbook, Zaclon Incporated. Available online: https://www.zaclon.com/pdf/Zaclon%20Galvanizing%20Handbook.pdf (accessed on 24 November 2021).
- Singh, D.D.N. Pickling, Rinsing and Fluxing of Steels Before Galvanizing. Workshop on Hot Dip Galvanizing. HODGA 1997, 6–7, 61–84. [Google Scholar]
- Thorsen, G.; Grislingås, A.; Steintveit, G. Recovery of Zinc from Zinc Ash and Flue Dusts by Hydrometallurgical Processing. JOM 1981, 33, 24–29. [Google Scholar] [CrossRef]
- Popov, I.K.; Djokic, S.S.; Grgur, B.N. Fundamental Aspects of Electrometallurgy; Springer: New York, NY, USA, 2007; ISBN 0-306-47564-2. [Google Scholar]
- Oráč, D.; Klimko, J.; Klein, D.; Pirošková, J.; Liptai, P.; Vindt, T.; Miškufová, A. Hydrometallurgical Recycling of Copper Anode Furnace Dust for a Complete Recovery of Metal Values. Metals 2022, 12, 36. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Wang, X.; Forsberg, K.; Friedrich, B. Basic Sulfate Precipitation of Zirconium from Sulfuric Acid Leach Solution. Metals 2020, 10, 1099. [Google Scholar] [CrossRef]
- Chernyaev, A.; Wilson, B.P.; Lundström, M. Study on valuable metal incorporation in the Fe–Al precipitate during neutralization of LIB leach solution. Sci. Rep. 2021, 11, 23283. [Google Scholar] [CrossRef] [PubMed]




| Sample | Degasified in Vacuum 30 °C/24 h | Specific Surface Area (m2 g−1) | Density (g cm−3) |
|---|---|---|---|
| A—grey | ≈1% | 1.74 | 1.88 |
| B 1—brown | ≈0.3% | 0.28 | 1.72 |
| B 2—brown | 0.3 + 0.3% | 0.55 | 1.69 |
| C—light gray | ≈1.2% | 2.41 | 1.85 |
| Sample | Zn | Fe | Cu | Pb | Al | Si | Sn | Ca | Cl− | Residue |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 27.49 | 0.34 | <LoD | 0.07 | 1.03 | <LoD | <LoD | 0.17 | 23.40 | 47.5 |
| B | 26.9 | 0.32 | <LoD | 0.06 | 0.38 | <LoD | <LoD | 0.05 | 28.37 | 43.98 |
| C | 28.0 | 0.35 | <LoD | 0.07 | 0.97 | <LoD | <LoD | 0.08 | 42.56 | 29.01 |
| Sample | Concentration (mg.l−1) | |||||||||
| Al | As | Cd | Co | Cr | Cu | Hg | Mo | Ni | Pb | |
| A | 2.118 | <LoD | 0.5041 | 0.025 | <LoD | 1.76 | 0 | 0 | 0.7561 | 41.225 |
| B | 1.952 | <LoD | 0.5233 | 0.010 | <LoD | 1.05 | 0 | 0 | 0.6542 | 39.013 |
| C | 2.206 | <LoD | 0.5122 | 0.026 | <LoD | 1.43 | 0 | 0 | 0.8241 | 40.325 |
| Sample | Concentration (mg.l−1) | |||||||||
| Sb | Se | Sn | V | Zn | Cl− | F- | pH | |||
| A | 0 | 0 | <LoD | 0 | 80,145 | 43,024 | 1.52 | 50 | 5.872 | |
| B | 0 | 0 | <LoD | 0 | 72,750 | 37,871 | 1.23 | 48 | 5.682 | |
| C | 0 | 0 | <LoD | 0 | 87,780 | 47,123 | 1.01 | 43 | 5.914 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirošková, J.; Klimko, J.; Trpčevská, J.; Laubertová, M.; Plešingerová, B.; Liptai, P.; Vindt, T.; Oráč, D. Characterization of Galvanizing Flue Dust and Recycling Possibilities. Metals 2022, 12, 744. https://doi.org/10.3390/met12050744
Pirošková J, Klimko J, Trpčevská J, Laubertová M, Plešingerová B, Liptai P, Vindt T, Oráč D. Characterization of Galvanizing Flue Dust and Recycling Possibilities. Metals. 2022; 12(5):744. https://doi.org/10.3390/met12050744
Chicago/Turabian StylePirošková, Jana, Jakub Klimko, Jarmila Trpčevská, Martina Laubertová, Beatrice Plešingerová, Pavol Liptai, Tomáš Vindt, and Dušan Oráč. 2022. "Characterization of Galvanizing Flue Dust and Recycling Possibilities" Metals 12, no. 5: 744. https://doi.org/10.3390/met12050744
APA StylePirošková, J., Klimko, J., Trpčevská, J., Laubertová, M., Plešingerová, B., Liptai, P., Vindt, T., & Oráč, D. (2022). Characterization of Galvanizing Flue Dust and Recycling Possibilities. Metals, 12(5), 744. https://doi.org/10.3390/met12050744










