Thermodynamic Analysis and Experimental Study of Masked Corrosion Protection of 304 Stainless Steel Processed with Nanosecond Pulsed Laser
Abstract
:1. Introduction
2. Experimental Materials and Methods
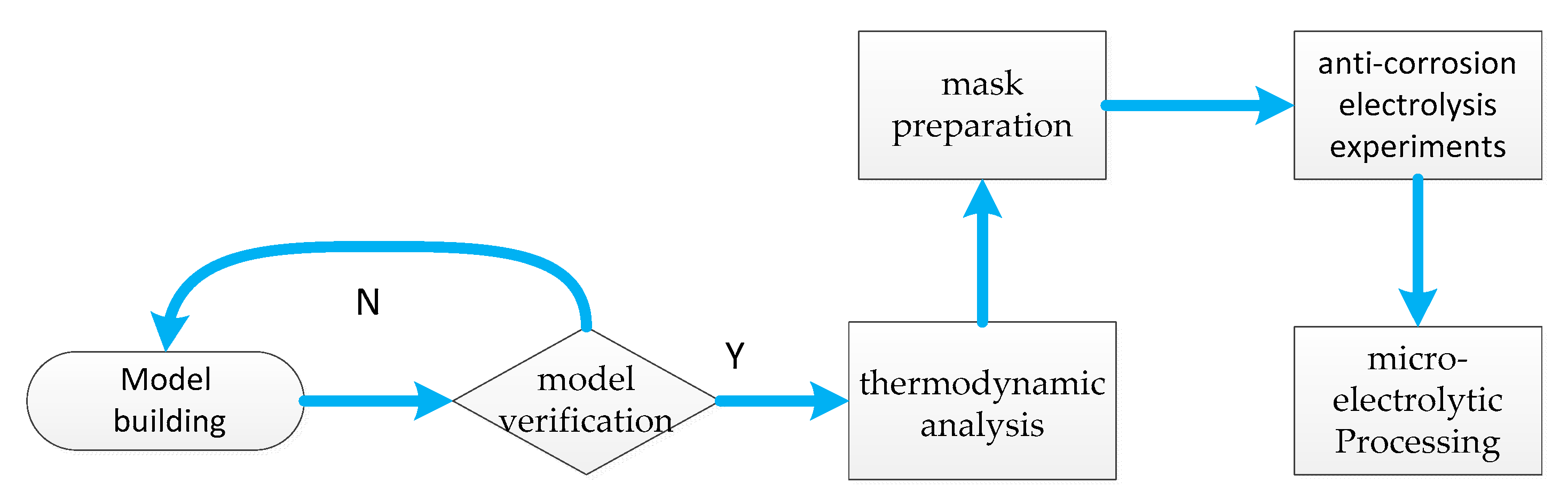
2.1. Experimental Materials and Workflow
2.2. The Laser Source and Micro Electrolytic Processing
2.3. Surface Morphology Characterization
2.4. Electrochemical Characterization
3. Three-Dimensional Thermodynamic Analysis Model for Nanosecond Pulsed Laser Processing
3.1. Numerical Simulation Models
3.2. Numerical Simulation Models
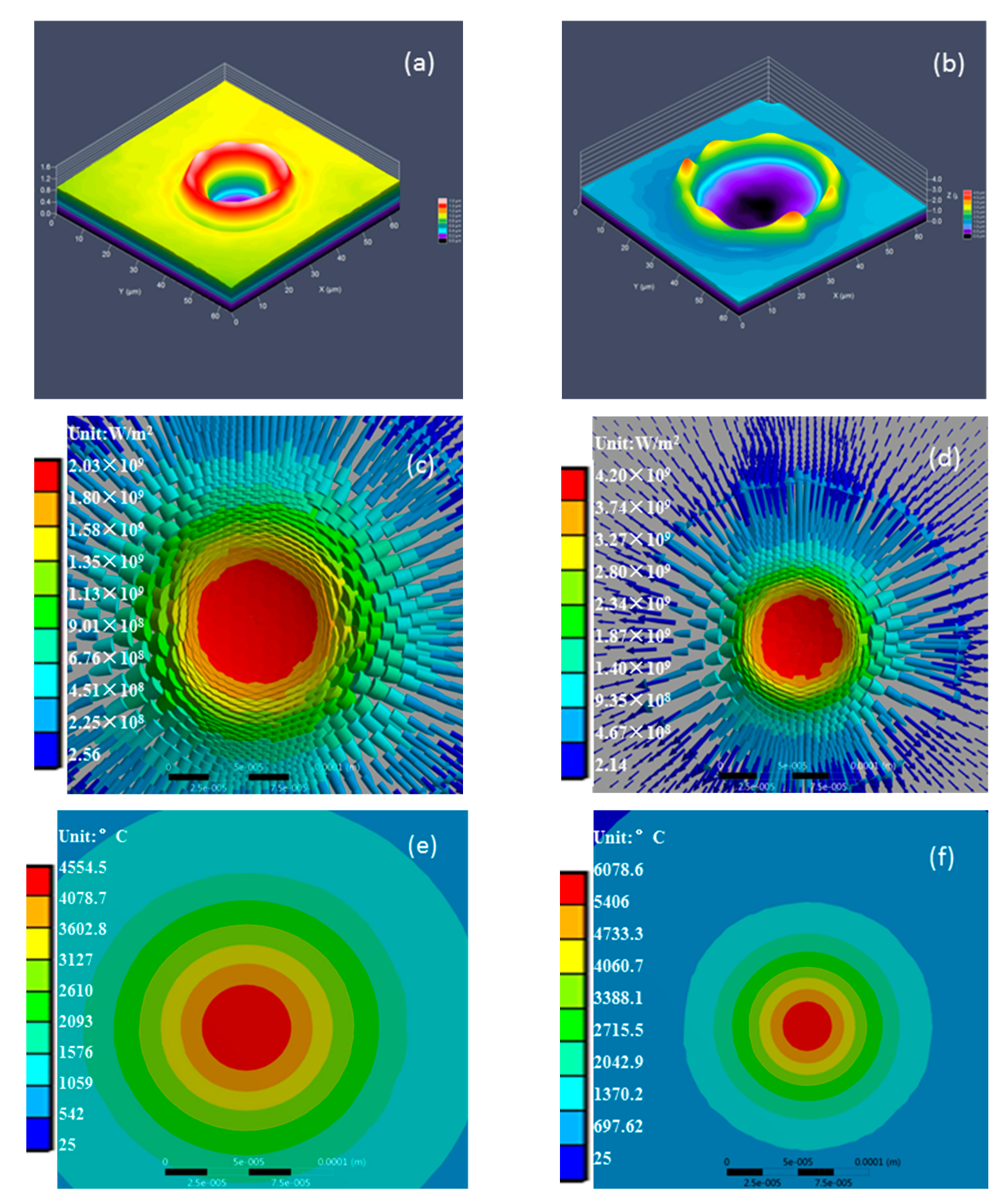
4. Thermodynamic Analysis of the Model
4.1. Orthogonal Simulation Experimental Design
4.2. Experimental Results and Discussion
5. Masked Anti-Corrosion Electrolysis Experiments
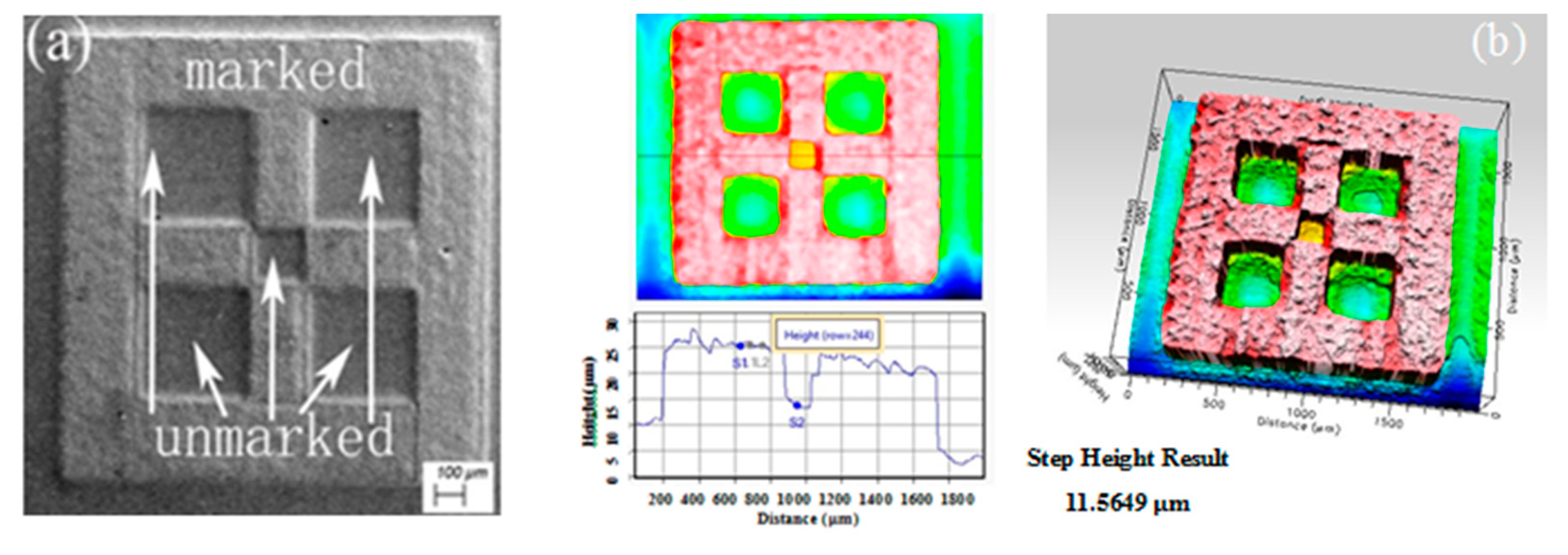
5.1. Mask Preparation and Corrosion-Resistance Testing
5.2. Micro Electrolytic Processing Experiments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raj, D.; Reddy, B.; Maity, S.R.; Pandey, K.M. Laser Beam Micromachining of Metals: A Review. Mater. Today Proc. 2019, 18, 98–103. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, X.; Yu, X.; Hu, A.; Vukelic, S.; Jung, M.B.G.; Joe, H.-E.; Yao, L.Y.; Shin, Y.C. Ultrafast Laser Applications in Manufacturing Processes: A State-of-the-Art Review. J. Manuf. Sci. Eng. 2020, 142, 031005. [Google Scholar] [CrossRef]
- Faisal, N.; Zindani, D.; Kumar, K.; Bhowmik, S. Laser micromachining of engineering materials—A review. Micro Nano Mach. Eng. Mater. 2019, 121–136. [Google Scholar] [CrossRef]
- Martan, J.; Prokešová, L.; Moskal, D.; Ferreira de Faria, B.C.; Honner, M.; Lang, V. Heat accumulation temperature measurement in ultrashort pulse laser micromachining. Int. J. Heat Mass Transf. 2021, 168, 120866. [Google Scholar] [CrossRef]
- Balachninaitė, O.; Tamulienė, V.; Eičas, L.; Vaičaitis, V. Laser micromachining of steel and copper using femtosecond laser pulses in GHz burst mode. Results Phys. 2021, 22, 103847. [Google Scholar] [CrossRef]
- Armbruster, O.; Naghilou, A.; Kitzler, M.; Kautek, W. Spot size and pulse number dependence of femtosecond laser ablation thresholds of silicon and stainless steel. Appl. Surf. Sci. 2017, 396, 1736–1740. [Google Scholar] [CrossRef]
- Yao, C.; Xu, S.; Ye, Y.; Jiang, Y.; Ding, R.; Gao, W.; Yunan, X. The influence of femtosecond laser repetition rates and pulse numbers on the formation of micro/nano structures on stainless steel. J. Alloys Compd. 2017, 722, 235–241. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The effect of bottom profile dimples on the femoral head on wear in metal-on-metal total hip arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef]
- Abd Ali, H.R.; Khalid, E.A.; Alwan, A.S.; Jaddoa, A.A. Effect of Fibre Laser Surface Treatment on Wear Resistance of Gray Cast Iron Astm A48. J. Mech. Eng. Res. Dev. 2021, 44, 141–149. [Google Scholar]
- Sedao, X.; Shugaev, M.V.; Wu, C.; Douillard, T.; Esnouf, C.; Maurice, C.; Reynaud, S.; Pigeon, F.; Garrelie, F.; Zhigilei, L.V.; et al. Growth Twinning and Generation of High-Frequency Surface Nanostructures in Ultrafast Laser-Induced Transient Melting and Resolidification. ACS Nano 2016, 10, 6995–7007. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhigilei, L.V. Nanocrystalline and Polyicosahedral Structure of a Nanospike Generated on Metal Surface Irradiated by a Single Femtosecond Laser Pulse. J. Phys. Chem. C 2016, 120, 4438–4447. [Google Scholar] [CrossRef] [Green Version]
- Shugaev, M.V.; He, M.; Levy, Y.; Mazzi, A.; Miotello, A.; Bulgakova, N.M.; Zhigilei, L.V. Laser-Induced Thermal Processes: Heat Transfer, Generation of Stresses, Melting and Solidification, Vaporization, and Phase Explosion. In Handbook of Laser Micro- and Nano-Engineering; Sugioka, K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–81. [Google Scholar]
- Harilal, S.S.; Freeman, J.R.; Diwakar, P.K.; Hassanein, A. Femtosecond Laser Ablation: Fundamentals and Applications. In Laser-Induced Breakdown Spectroscopy: Theory and Applications; Musazzi, S., Perini, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–166. [Google Scholar]
- Tangwarodomnukun, V.; Kringram, S.; Zhu, H.; Qi, H.; Rujisamphan, N. Fabrication of superhydrophobic surface on AISI316L stainless steel using a nanosecond pulse laser. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2022, 236, 680–693. [Google Scholar] [CrossRef]
- Tran, N.G.; Chun, D. Simple and fast surface modification of nanosecond-pulse laser-textured stainless steel for robust superhydrophobic surfaces. CIRP Ann. 2020, 69, 525–528. [Google Scholar] [CrossRef]
- Patil, D.; Aravindan, S.; Sarathi, R.; Rao, P.V. Fabrication of self-cleaning superhydrophobic silicone rubber insulator through laser texturing. Surf. Eng. 2021, 37, 308–317. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y. Influences of Pulse Frequency on Microstructure and Properties in Laser Cladding Layers. China Mech. Eng. 2021, 32, 2108. [Google Scholar]
- Dzienny, P.; Stepak, B.; Budnicki, A.; Antonczak, A. The influence of laser pulse duration on periodic surface structures on stainless steel. J. Laser Micro Nanoeng. 2020, 15, 1–4. [Google Scholar]
- Li, X.H.; Wang, S.M.; Xue, B.B. Technology of Electrochemical Micromachining Based on Surface Modification by Fiber Laser on Stainless Steel. Mater. Sci. Forum 2017, 909, 67–72. [Google Scholar] [CrossRef]
- Mekky, A.H. Computational Modelling for Specific Heat and Thermal Conductivity of Austenitic Stainless Steels Alloys at Solid Phase. Rev. Compos. Matriaux Av. 2020, 30, 23–27. [Google Scholar] [CrossRef]
- Banerjee, A.; Ogale, A.A.; Das, C.; Mitra, K.; Subramanian, C. Temperature distribution in different materials due to short pulse laser irradiation. Heat Transf. Eng. 2005, 26, 41–49. [Google Scholar] [CrossRef]
- Razavykia, A.; Brusa, E.; Delprete, C.; Yavari, R. An Overview of Additive Manufacturing Technologies-A Review to Technical Synthesis in Numerical Study of Selective Laser Melting. Materials 2020, 13, 3895. [Google Scholar] [CrossRef] [PubMed]
- Bäuerle, D. Laser Processing and Chemistry; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Rai, R.R.; Elmer, J.W.; Palmer, T.A.; DebRoy, T. Heat transfer and fluid flow during keyhole mode laser welding of tantalum, TiAlV, 304L stainless steel and vanadium. J. Phys. D Appl. Phys. 2007, 40, 5753. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Fuerschbach, P.W.; DebRoy, T. Heat transfer and fluid flow during laser spot welding of 304 stainless steel. J. Phys. D Appl. Phys. 2003, 36, 1388. [Google Scholar] [CrossRef]
- Yang, J.; Lian, J.; Dong, Q.; Guo, Z. Nanostructured films formed on the AISI 329 stainless steel by Nd-YAG pulsed laser irradiation. Appl. Surf. Sci. 2004, 229, 2–8. [Google Scholar] [CrossRef]
- Zeng, K.; Pal, D.; Stucker, B. A review of thermal analysis methods in laser sintering and selective laser melting. In 2012 International Solid Freeform Fabrication Symposium; University of Texas at Austin: Austin, TX, USA, 2012. [Google Scholar]
- Demos, S.G.; Negres, R.A.; Raman, R.N.; Shen, N.; Rubenchik, A.M.; Matthews, M.J. Mechanisms governing the interaction of metallic particles with nanosecond laser pulses. Opt. Express 2016, 24, 7792–7815. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Sharma, V.; Jain, V.K.; Ramkumar, J. Reducing overcut in electrochemical micromachining process by altering the energy of voltage pulse using sinusoidal and triangular waveform. Int. J. Mach. Tools Manuf. 2020, 151, 103526. [Google Scholar] [CrossRef]
- Volgin, V.M.; Lyubimov, V.V.; Kabanova, T.B.; Davydov, A.D. Theoretical analysis of micro/nano electrochemical machining with ultra-short voltage pulses. Electrochim. Acta 2021, 369, 137666. [Google Scholar] [CrossRef]
- Zhang, X.; Yocom, C.J.; Mao, B.; Liao, Y. Microstructure evolution during selective laser melting of metallic materials: A review. J. Laser Appl. 2019, 31, 31201. [Google Scholar] [CrossRef]
- Mroczkowska, K.M.; Dzienny, P.; Budnicki, A.; Antonczak, A.J. Corrosion Resistance of AISI 304 Stainless Steel Modified Both Femto- and Nanosecond Lasers. Coatings 2021, 11, 592. [Google Scholar] [CrossRef]




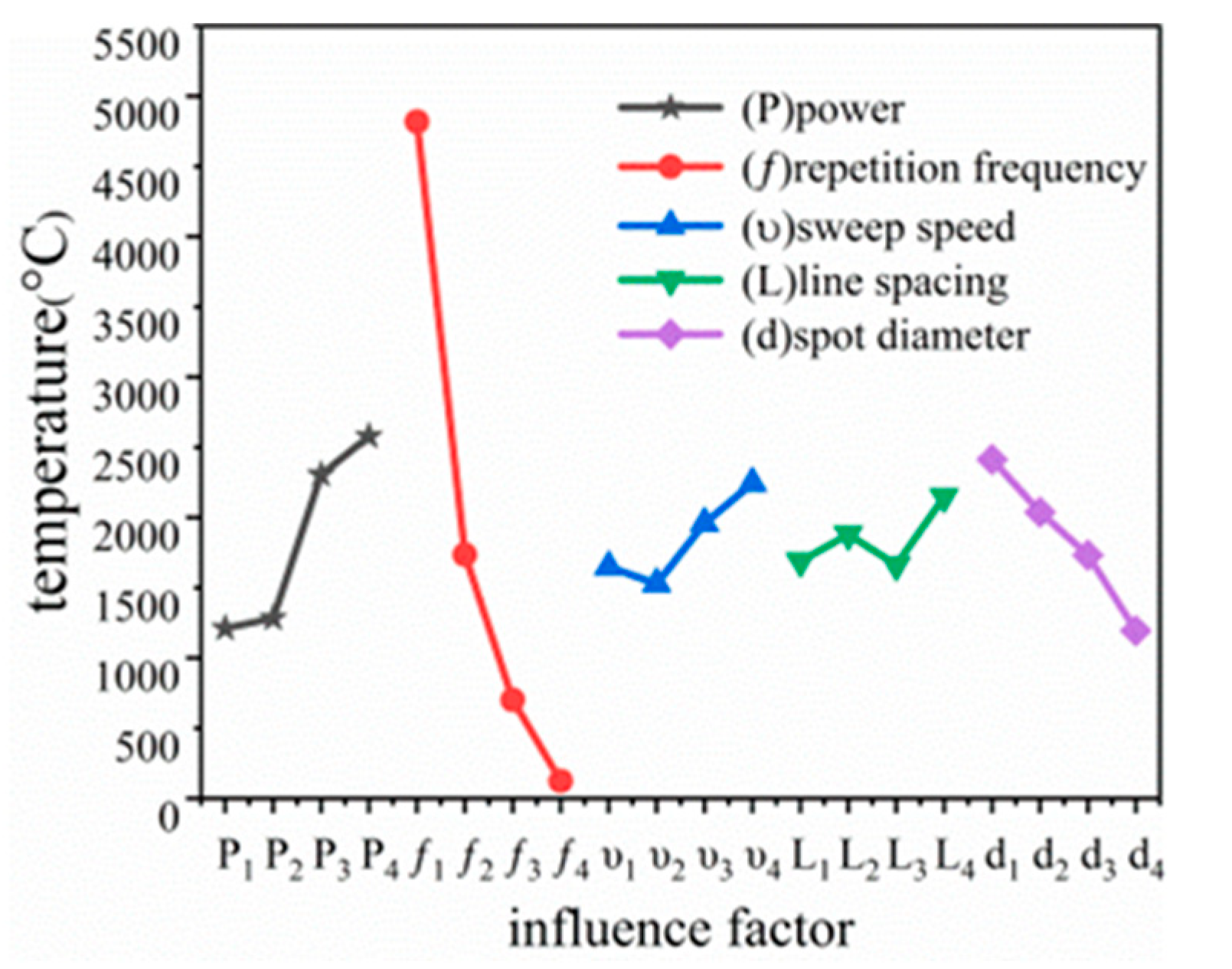

| Experimental Program | Power (W) | Repetition Frequency (kHz) | Scanning Speed (mm/s) | Line Spacing (μm) | Scanning Diameter (μm) | Maximum Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | 4 | 20 | 5 | 5 | 50 | 5853 |
| 2 | 4 | 40 | 10 | 10 | 60 | 2466 |
| 3 | 4 | 60 | 20 | 20 | 70 | 1337 |
| 4 | 4 | 80 | 40 | 40 | 80 | 993 |
| 5 | 5 | 20 | 10 | 20 | 80 | 4559 |
| 6 | 5 | 40 | 5 | 40 | 70 | 2619 |
| 7 | 5 | 60 | 40 | 5 | 60 | 2026 |
| 8 | 5 | 80 | 20 | 10 | 50 | 1733 |
| 9 | 6 | 20 | 20 | 40 | 60 | 7341 |
| 10 | 6 | 40 | 40 | 20 | 50 | 4430 |
| 11 | 6 | 60 | 5 | 10 | 80 | 1799 |
| 12 | 6 | 80 | 10 | 5 | 70 | 1448 |
| 13 | 7 | 20 | 40 | 10 | 70 | 7326 |
| 14 | 7 | 40 | 20 | 5 | 80 | 3227 |
| 15 | 7 | 60 | 10 | 40 | 50 | 3441 |
| 16 | 7 | 80 | 5 | 20 | 60 | 2124 |
| Polar difference (R) | 1367.5 | 4695.25 | 715.25 | 486 | 1219.75 | - |
| Test Program | Substance | 7 | 8 | 11 | 12 | 16 |
|---|---|---|---|---|---|---|
| Ecorr (mV vs. SCE) | −330 | −328 | −328 | −56 | −146 | −4 |
| Icorr (μA/cm2) | 0.48 | 0.40 | 3.86 × 10−4 | 7.06 × 10−3 | 0.81 | 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Tong, H.; Wang, D.; Li, X. Thermodynamic Analysis and Experimental Study of Masked Corrosion Protection of 304 Stainless Steel Processed with Nanosecond Pulsed Laser. Metals 2022, 12, 749. https://doi.org/10.3390/met12050749
Wang S, Tong H, Wang D, Li X. Thermodynamic Analysis and Experimental Study of Masked Corrosion Protection of 304 Stainless Steel Processed with Nanosecond Pulsed Laser. Metals. 2022; 12(5):749. https://doi.org/10.3390/met12050749
Chicago/Turabian StyleWang, Shuming, Han Tong, Dong Wang, and Xiaohai Li. 2022. "Thermodynamic Analysis and Experimental Study of Masked Corrosion Protection of 304 Stainless Steel Processed with Nanosecond Pulsed Laser" Metals 12, no. 5: 749. https://doi.org/10.3390/met12050749
APA StyleWang, S., Tong, H., Wang, D., & Li, X. (2022). Thermodynamic Analysis and Experimental Study of Masked Corrosion Protection of 304 Stainless Steel Processed with Nanosecond Pulsed Laser. Metals, 12(5), 749. https://doi.org/10.3390/met12050749





