The Laser Deposited Nickel-Aluminum Bronze Coatings on SUS630 Stainless Steel and Its Corrosion Resistance in 3.5 wt.% NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Forming Composite Structures
2.3. X-ray Diffraction
2.4. Scanning Electron Microscopy (SEM)
2.5. Tensile Property
2.6. Corrosion Resistance Tests
2.6.1. Electrochemical Measurements
2.6.2. Electrochemical Impedance Spectroscopy
2.6.3. Corrosion Behavior
3. Results and Discussion
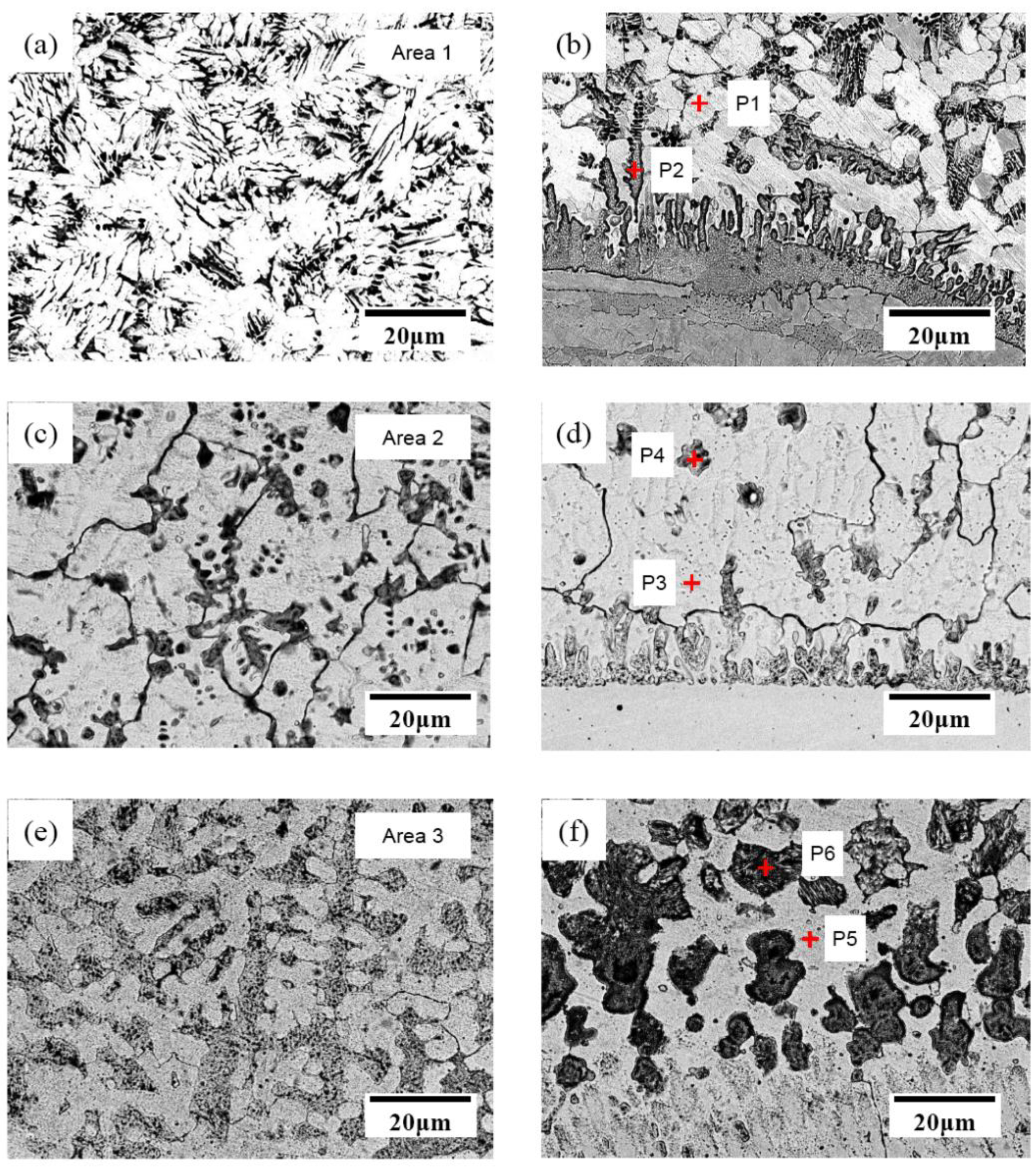
3.1. Depositing Microstructure and Composition
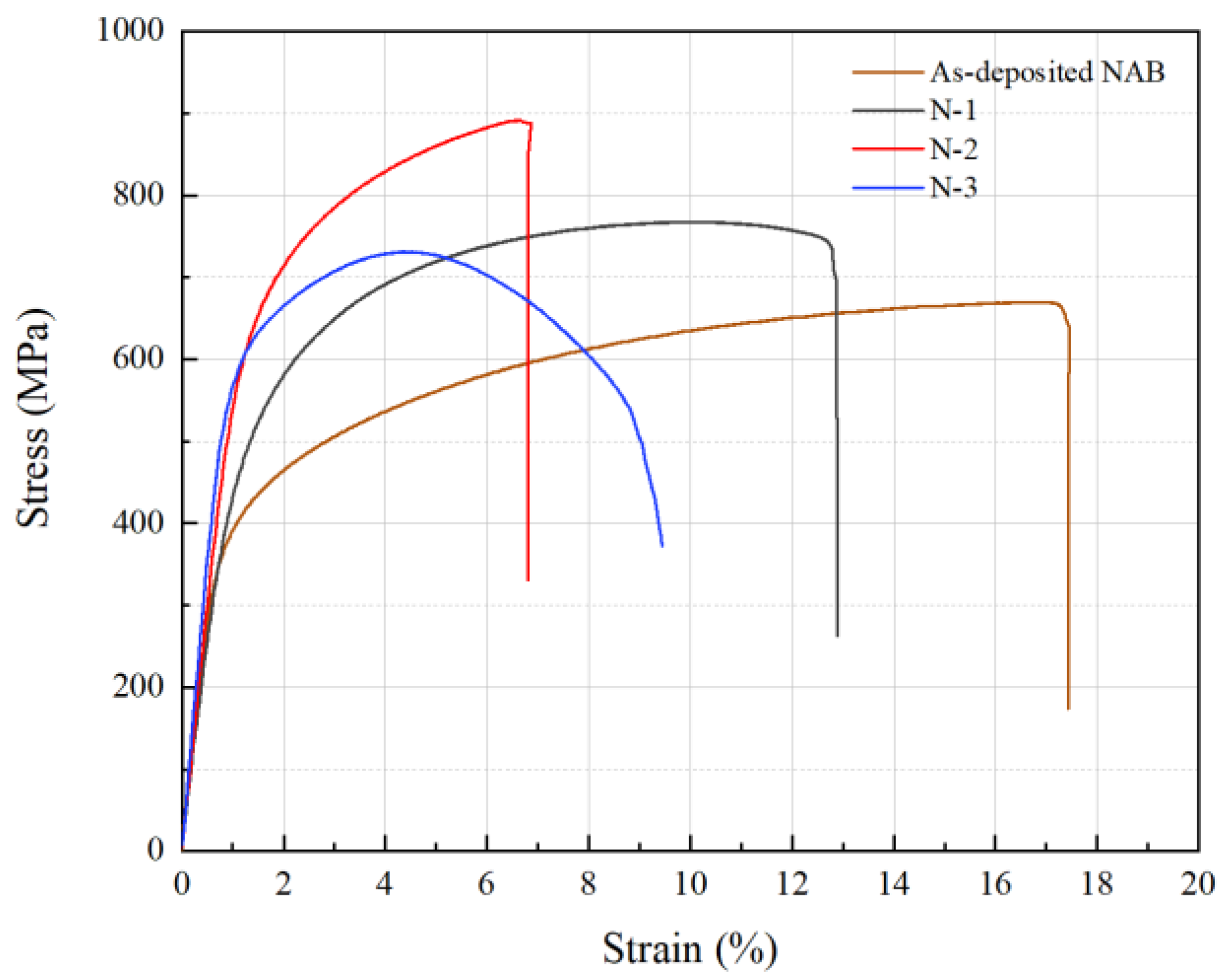
3.2. Physical-Mechanical Property
3.3. Corrosion Behavior in 3.5 wt.% NaCl Solution
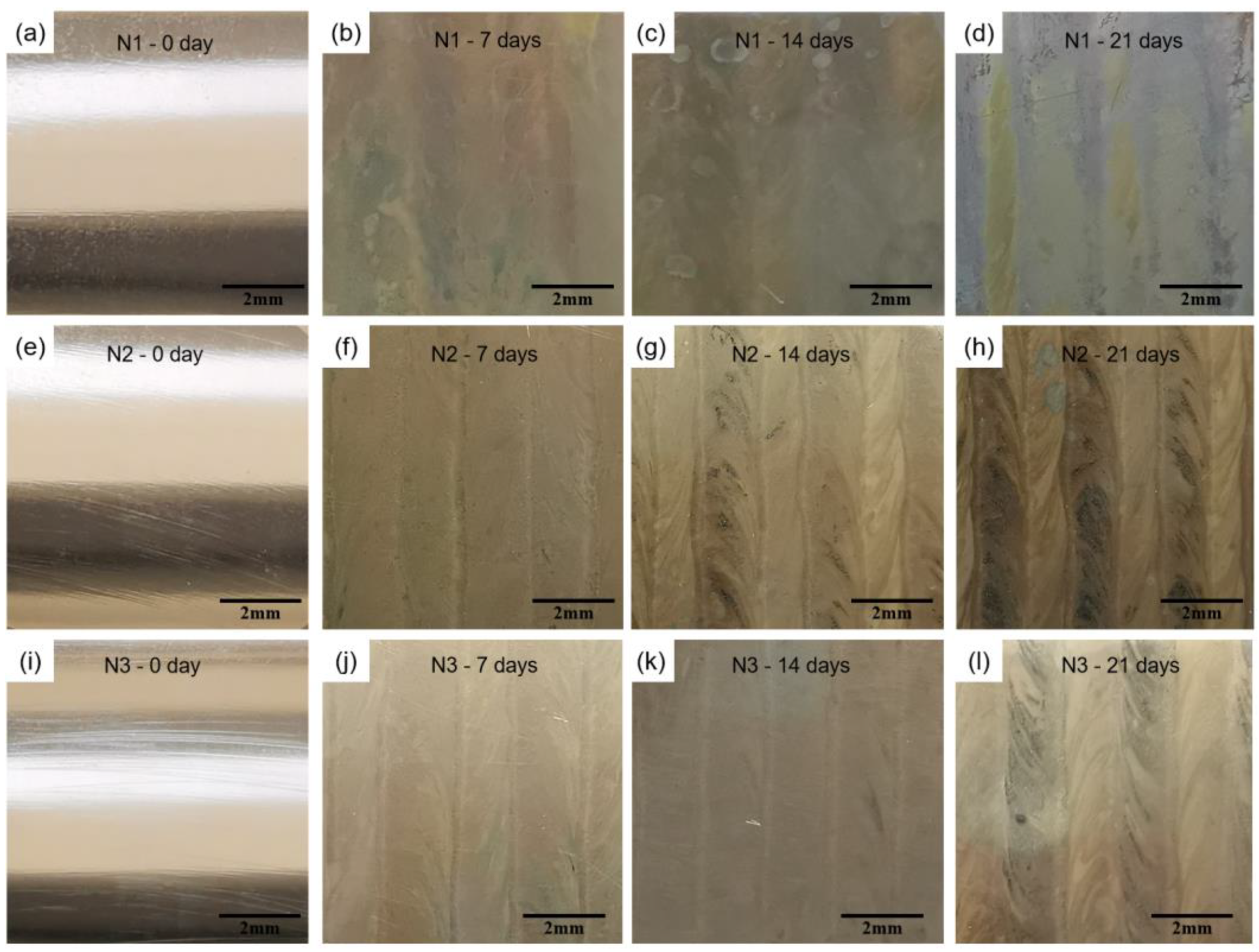
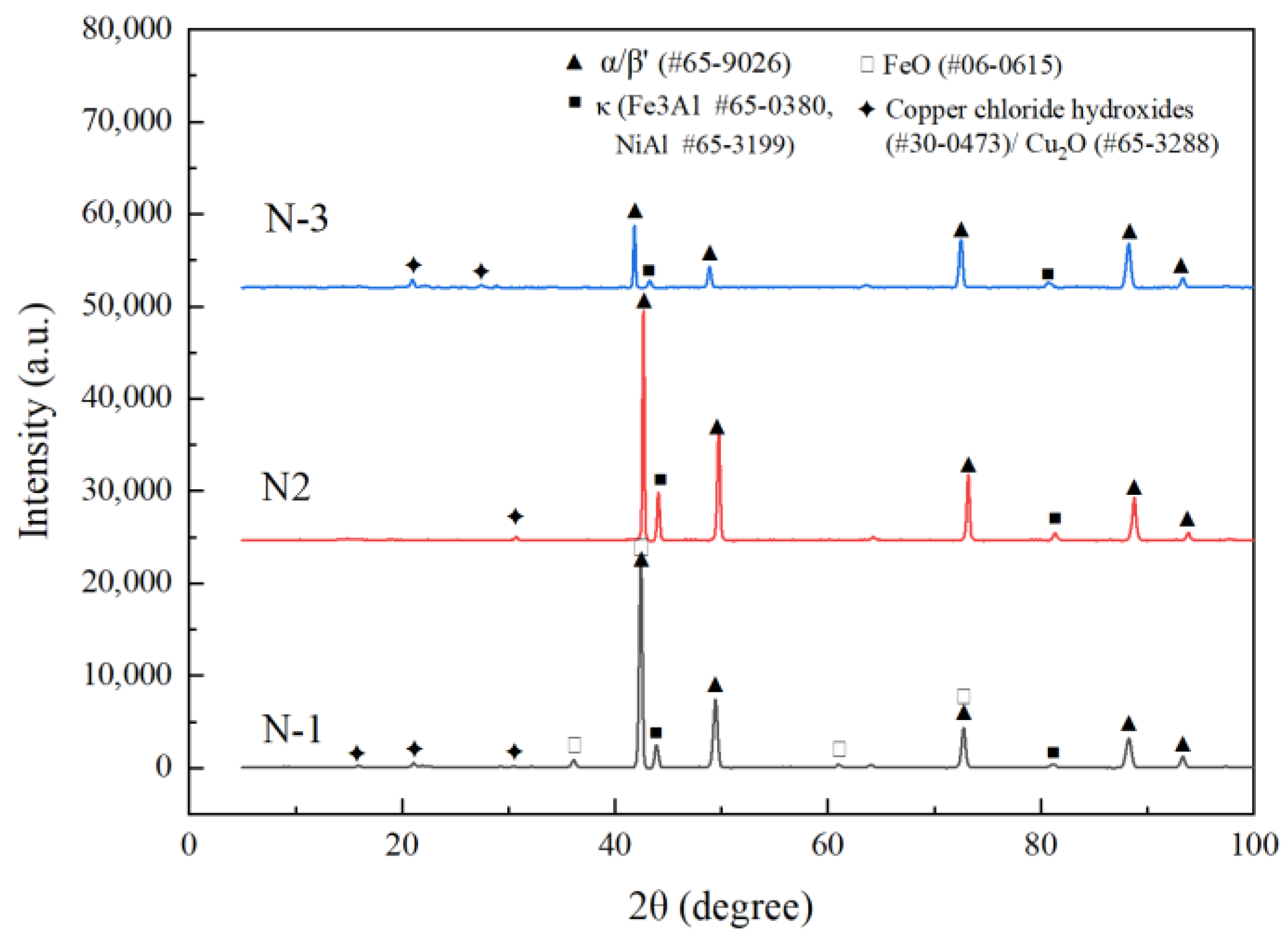
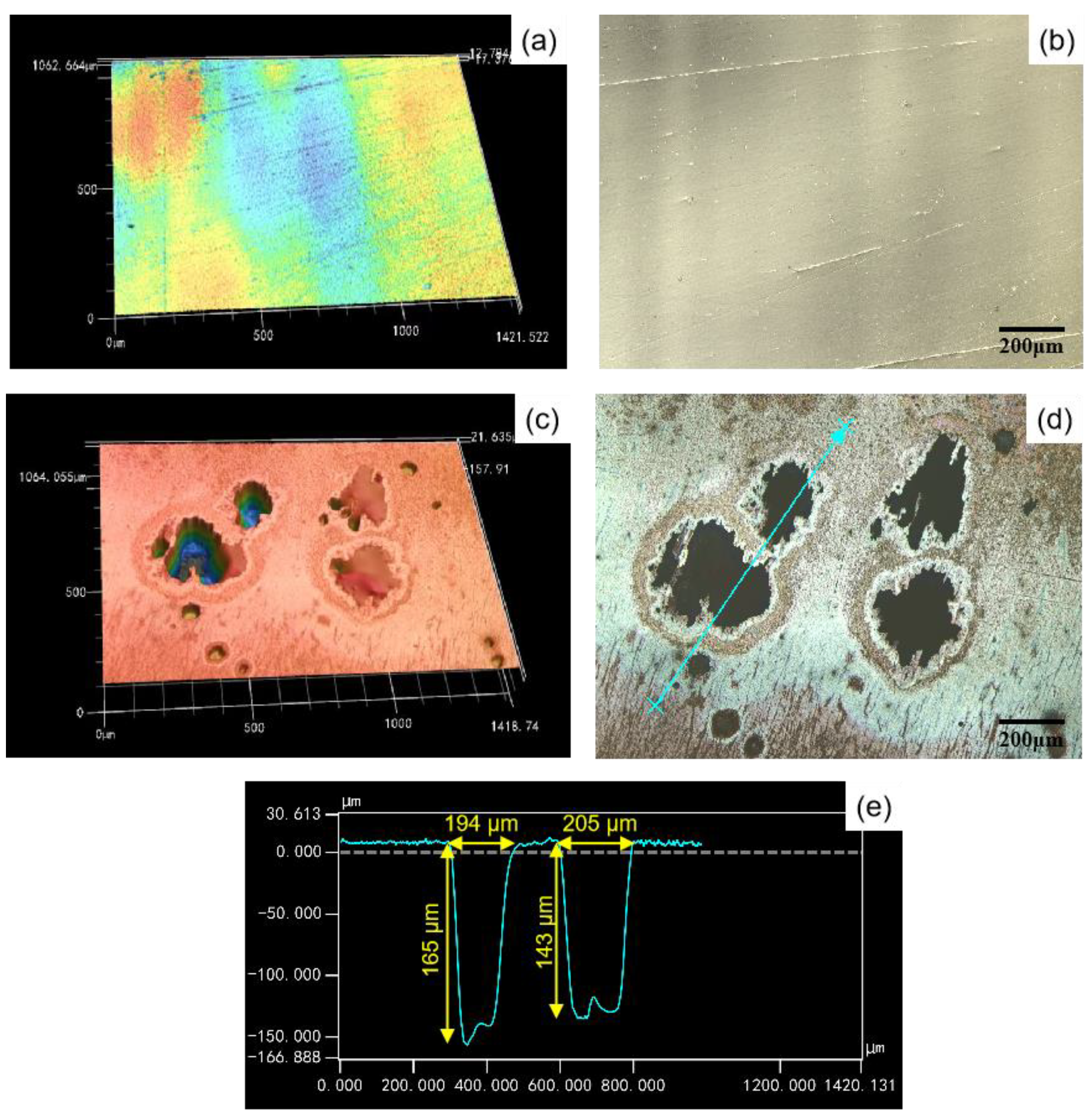
3.3.1. Morphology and Composition
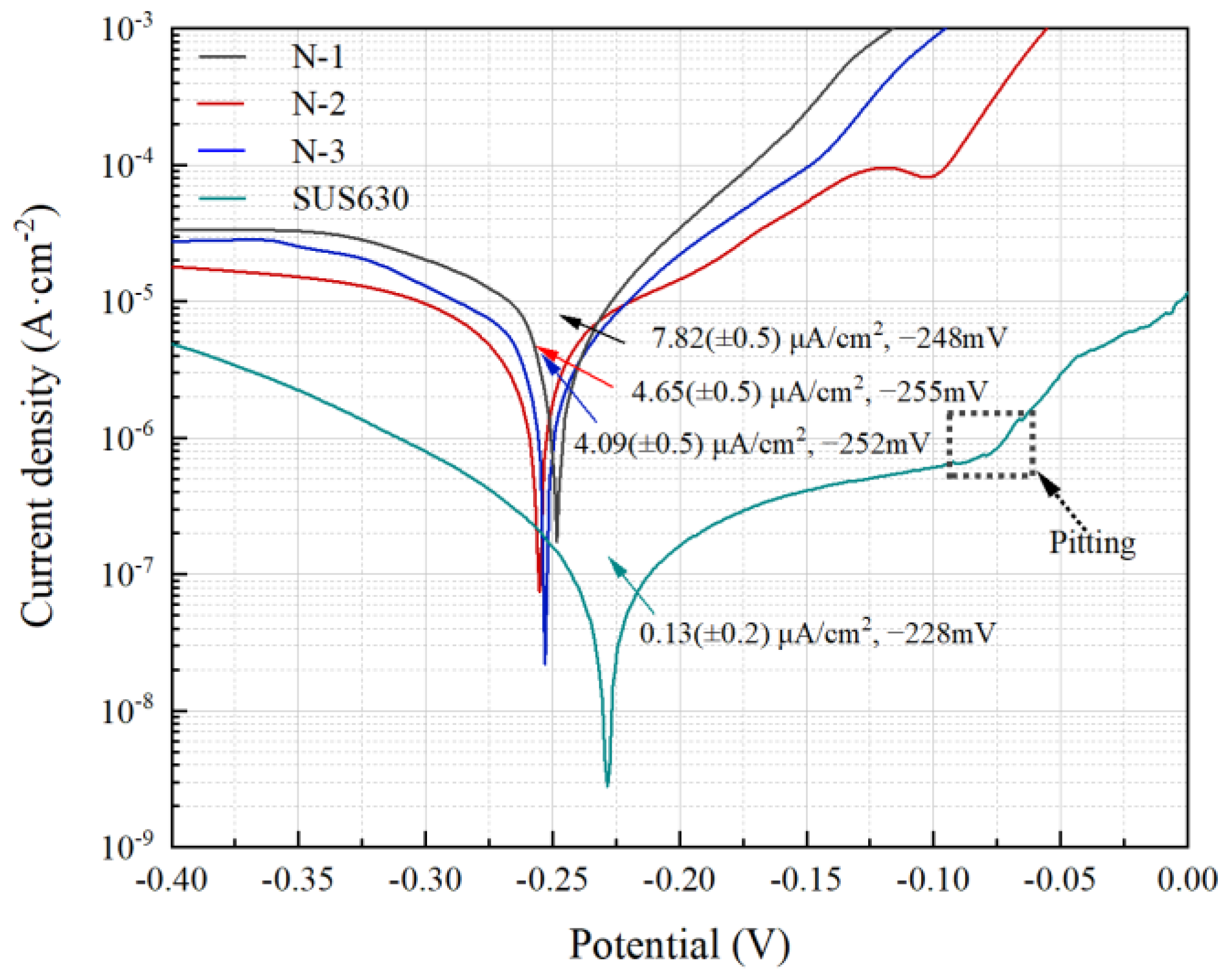
3.3.2. Polarization Measurements
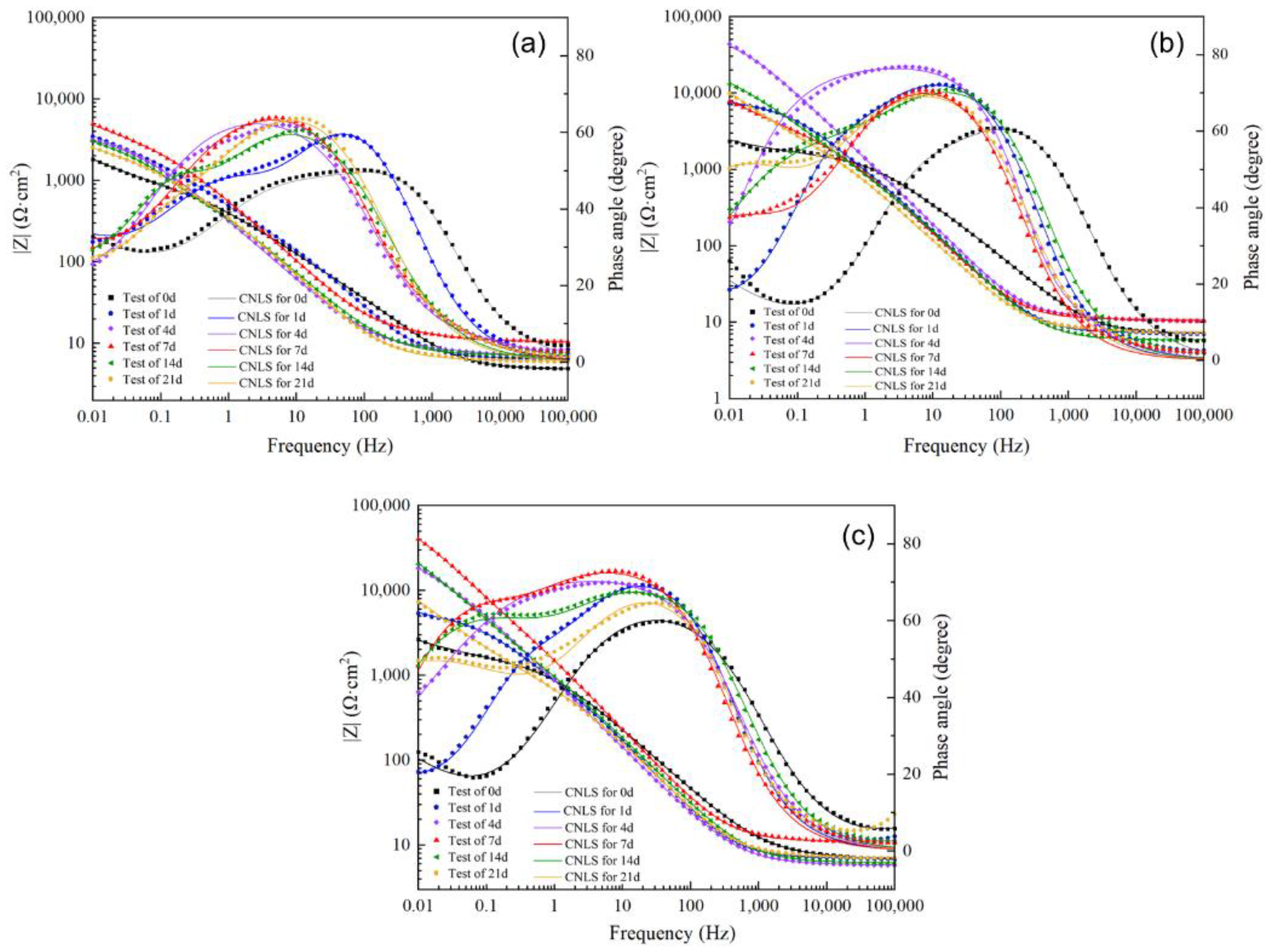
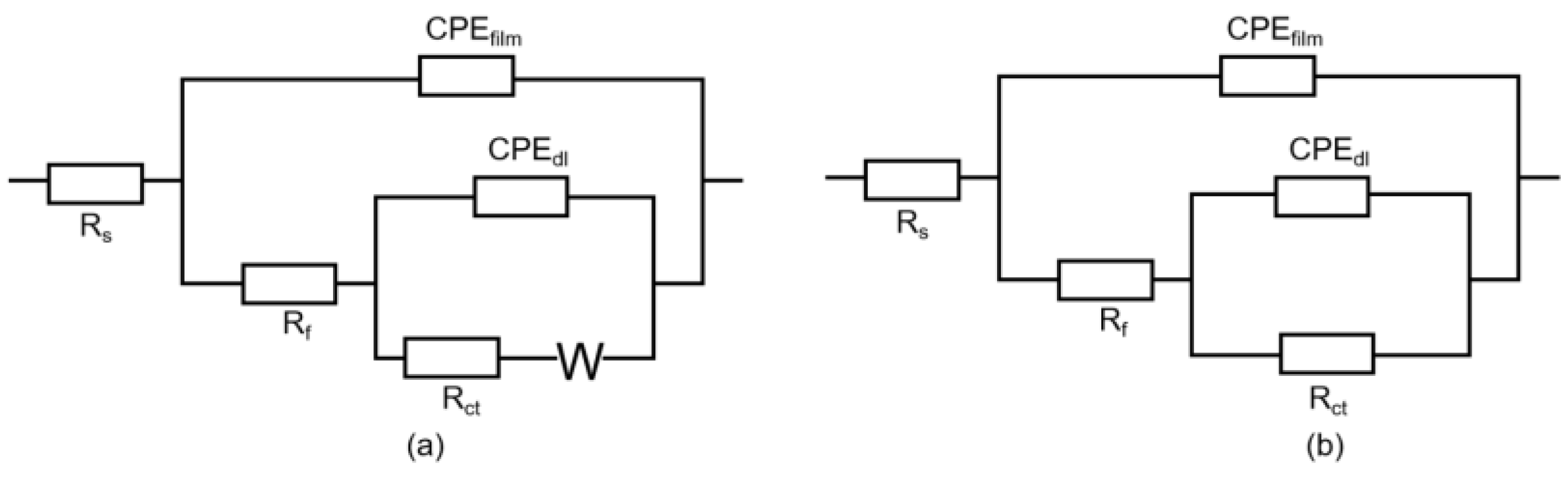
3.3.3. Electrochemical Impedance Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hashem, A.; Riad, W. The role of microstructure of nickel–aluminium–bronze alloy on its cavitation corrosion behavior in natural seawater. Mater. Charact. 2002, 48, 37–41. [Google Scholar] [CrossRef]
- Anantapong, J.; Uthaisangsuk, V.; Suranuntchai, S.; Manonukul, A. Effect of hot working on microstructure evolution of as-cast Nickel Aluminum Bronze alloy. Mater. Des. 2014, 60, 233–243. [Google Scholar] [CrossRef]
- Öztürk, S.; Sünbül, S.E.; Metoğlu, A.; Kürşat, İ.Ç. Improvement of microstructure, tribology and corrosion characteristics of nickel-aluminum bronze by P/M method. Tribol. Int. 2020, 151, 106519. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakrabarti, A.; Nagesha, A.; Saravanan, T.; Arunmuthu, K.; Sandhya, R.; Philip, J.; Mathew, M.D.; Jayakumar, T. Influence of Casting defects on S–N fatigue behavior of Ni-Al bronze. Metall. Mater. Trans. A 2015, 46, 708–725. [Google Scholar] [CrossRef]
- Barik, R.C.; Wharton, J.A.; Wood, R.J.K.; Tan, K.S.; Stokes, K.R. Erosion and erosion–corrosion performance of cast and thermally sprayed nickel–aluminum bronze. Wear 2005, 259, 230–242. [Google Scholar] [CrossRef]
- Hyatt, C.V.; Magee, K.H.; Betancourt, T. The effect of heat input on the microstructure and properties of nickel aluminum bronze laser clad with a consumable of composition Cu-9.0Al-4.6Ni-3.9Fe-1.2Mn. Metall. Mater. Trans. A 1998, 29, 1677–1690. [Google Scholar] [CrossRef]
- Qin, Z.; Luo, Q.; Zhang, Q.; Wu, Z.; Liu, L.; Shen, B.; Hu, W. Improving corrosion resistance of nickel-aluminum bronzes by surface modification with chromium ion implantation. Surf. Coat. Technol. 2018, 334, 402–409. [Google Scholar] [CrossRef]
- Luo, Q.; Wu, Z.; Qin, Z.; Liu, L.; Hu, W. Surface modification of nickel-aluminum bronze alloy with gradient Ni-Cu solid solution coating via thermal diffusion. Surf. Coat. Technol. 2017, 309, 106–113. [Google Scholar] [CrossRef]
- Osório, W.R.; Peixoto, L.C.; Moutinho, D.J.; Gomes, L.G.; Ferreira, I.L.; Garcia, A. Corrosion resistance of directionally solidified Al–6Cu–1Si and Al–8Cu–3Si alloys castings. Mater. Des. 2011, 32, 3832–3837. [Google Scholar] [CrossRef]
- Osório, W.R.; Freitas, E.S.; Garcia, A. EIS and potentiodynamic polarization studies on immiscible monotectic Al–In alloys. Electrochim. Acta 2013, 102, 436–445. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Niu, Y.; Gesmundo, F.; Fu, G.Y.; Douglass, D.L. Sulfidation of three two-phase Cu-Cr alloys in H2-H2S mixtures at 400–600 °C. Oxid. Met. 1998, 50, 327–354. [Google Scholar] [CrossRef]
- Fonlupt, S.; Bayle, B.; Delafosse, D.; Heuze, J.L. Role of second phases in the stress corrosion cracking of a nickel–aluminum bronze in saline water. Corros. Sci. 2005, 47, 2792–2806. [Google Scholar] [CrossRef]
- Tian, R.Z.; Wang, Z.T. Handbook of Copper Alloy and Its Processing, 2nd ed.; Central South University Press: Changsha, China, 2007; p. 11. [Google Scholar]
- Lee, M.K.; Hong, S.M.; Kim, G.H.; Kim, K.H.; Kim, W.W. Structural properties in flame quenched Cu9Al4.5Ni4.5Fe alloy. Met. Mater. Int. 2004, 10, 313–319. [Google Scholar] [CrossRef]
- Yang, F.; Kang, H.; Guo, E.; Li, R.; Chen, Z.; Zeng, Y.; Wang, T. The role of nickel in mechanical performance and corrosion behaviour of nickel-aluminum bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 139, 333–345. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Mishra, R.; Balasubramaniam, R. Effect of nanocrystalline grain size on the electrochemical and corrosion behavior of nickel. Corros. Sci. 2004, 46, 3019–3029. [Google Scholar] [CrossRef]
- López, F.H.E.; Tiburcio, C.G.; Zambrano-Robledo, P.; Cabral, J.A.; Calderon, F.A. Electrochemical study of 17-4 and 17-7 PH stainless steels used in the aeronautical industry. In Proceedings of the Symposium of Aeronautical and Aerospace Processes, Materials and Industrial Applications, Cancún, Mexico, 14–19 August 2016; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Raja, K.S.; Rao, K.P. Pitting behavior of type 17-4 PH stainless steel weldments. Corrosion 1995, 51, 586–592. [Google Scholar] [CrossRef]
- Ni, D.R.; Xiao, B.L.; Ma, Z.Y.; Qiao, Y.; Zheng, Y. Corrosion properties of friction–stir processed cast NiAl bronze. Corros. Sci. 2010, 52, 1610–1617. [Google Scholar] [CrossRef]
- Sabbaghzadeh, B.; Parvizi, R.; Davoodi, A.; Moayed, M.H. Corrosion evaluation of multi-pass welded nickel–aluminum bronze alloy in 3.5% sodium chloride solution: A restorative application of gas tungsten arc welding process. Mater. Des. 2014, 58, 346–356. [Google Scholar] [CrossRef]
- Amegroud, H.; Guenbour, A.; Bellaouchou, A.; El Aoufir, Y.; Lgaz, H.; Chung, I.-M.; Alrashdi, A.A. A comprehensive investigation of the electrochemical behavior of nickel-aluminum bronze alloy in alkaline solution: The effect of film formation potential. Colloids Surf. A 2021, 614, 126126. [Google Scholar] [CrossRef]
- Luo, Q.; Qin, Z.; Wu, Z.; Shen, B.; Liu, L.; Hu, W. The corrosion behavior of Ni-Cu gradient layer on the nickel aluminum-bronze (NAB) alloy. Corros. Sci. 2018, 138, 8–19. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O. Surface characterization and corrosion behavior of 70/30 Cu–Ni alloy in pristine and sulfide-containing simulated seawater. Corros. Sci. 2007, 49, 1276–1304. [Google Scholar] [CrossRef]
- Rao, B.V.A.; Kumar, K.C. Effect of hydrodynamic conditions on corrosion inhibition of Cu-Ni (90/10) alloy in seawater and sulphide containing seawater using 1,2,3-benzotriazole. J. Mater. Sci. Technol. 2014, 30, 65–76. [Google Scholar]
- Kear, G.; Barker, B.D.; Stokes, K.R.; Walsh, F.C. Anodic characteristics of copper, wrought CuNi10, and CuAl10Ni5Fe4 in seawater: Part 2—Polarizations below 500 mV at rotating disc electrodes. Corrosion 2009, 65, 95–104. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Fathi, A.M. Effect of Ni content on the corrosion behavior of Cu–Ni alloys in neutral chloride solutions. Electrochim. Acta 2005, 50, 3603–3608. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, R.; Qin, Z.; Wu, Z.; Wang, L.; Liu, L.; Lu, W. Evolution of the corrosion product film on nickel-aluminum bronze and its corrosion behavior in 3.5 wt % NaCl solution. Materials 2019, 12, 209. [Google Scholar] [CrossRef] [Green Version]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS investigation of the corrosion behavior of steel bars embedded into modified concretes with eggshell contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Proença, C.S.; Serrano, B.; Correia, J.; Araújo, M.E.M. Evaluation of tannins as potential green corrosion inhibitors of aluminium alloy used in aeronautical industry. Metals 2022, 12, 508. [Google Scholar] [CrossRef]
- Vida, T.A.; Freitas, E.S.; Cheung, N.; Garcia, A.; Osório, W.R. Electrochemical Corrosion Behavior of as-cast Zn-rich Zn-Mg Alloys in a 0.06M NaCl Solution. Int. J. Electrochem. Sci. 2017, 12, 5264–5283. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. Electrochemical behavior and compressive strength of Al-Cu/xCu composites in NaCl solution. J. Solid State Electrochem. 2021, 25, 2455. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Liu, L.; Wang, F. Study on the mechanism of hydrostatic pressure promoting electrochemical corrosion of pure iron in 3.5% NaCl solution. Acta Mater. 2021, 203, 116467. [Google Scholar] [CrossRef]
- Hurtado, M.R.F.; Sumodjo, P.T.A.; Benedetti, A.V. Electrochemical studies with a Cu–5wt.%Ni alloy in 0.5 M H2SO4. Electrochim. Acta 2003, 48, 2791–2798. [Google Scholar] [CrossRef]





| Material Name | Element (wt.%) | ||||
|---|---|---|---|---|---|
| Cu | Al | Ni | Fe | Cr | |
| NAB | 81.1 | 10.8 | 4.1 | 4.0 | - |
| KF-6 | - | 5.0 | 95.0 | - | - |
| KF-306 | - | - | 80.0 | - | 20.0 |
| Specimen Group | Substrate | Intermediate Layer | Outer Layer | Dosage of Layer 1 (Intermediate Powder) (g/cm2) | Dosage of Layer 2 (Outer Powder) (g/cm2) |
|---|---|---|---|---|---|
| N-1 | SUS630 | - | NAB | - | 2.9 |
| N-2 | SUS630 | KF-6 | NAB | 1.7 | 2.9 |
| N-3 | SUS630 | KF-306 | NAB | 1.7 | 2.9 |
| Position | Chemical Composition (in wt.%) | ||||
|---|---|---|---|---|---|
| Cu | Al | Ni | Fe | Cr | |
| P1 | 84.6 | 7.1 | 4.9 | 3.4 | - |
| P2 | 21.9 | 5.6 | 5.5 | 56.1 | 10.9 |
| P3 | 79.7 | 8.7 | 8.6 | 3 | - |
| P4 | 52.9 | 11.3 | 25.8 | 10.0 | - |
| P5 | 77.2 | 9.5 | 8.8 | 3.3 | 1.2 |
| P6 | 58.6 | 14.7 | 17.6 | 5.9 | 3.2 |
| Area1 | 75.2 | 9.8 | 3.8 | 11.2 | 0 |
| Area2 | 73.2 | 10.4 | 12.4 | 4 | 0 |
| Area3 | 72.3 | 10.9 | 10.8 | 4.1 | 1.9 |
| Sample | σb (MPa) | Elongation (%) |
|---|---|---|
| As-deposited NAB | 669 ± 10.4 | 17.46 ± 1.2 |
| N-1 | 767.80 ± 7.3 | 12.89 ± 1.0 |
| N-2 | 891.54 ± 8.5 | 6.87 ± 0.5 |
| N-3 | 731.05 ± 6.7 | 9.44 ± 0.6 |
| Sample | icorr (A/cm2) | Ecorr (mV) | ba (mV dec−1) | bc (mV dec−1) | Rp (Ω cm2) |
|---|---|---|---|---|---|
| N-1 | 7.82 × 10−6 | −248 | 61.02 | −223.10 | 2810 |
| N-2 | 4.65 × 10−6 | −255 | 82.23 | −296.03 | 5275 |
| N-3 | 4.09 × 10−6 | −252 | 79.60 | −216.98 | 4865 |
| Samples | Immersion Period (Day) | Rs (Ω cm2) | Ceff-film (F·cm−2) | n1 | Rfilm (Ω cm2) | Ceff-ct (F·cm−2) | n2 | Rct (Ω cm2) | W | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| N-1 | 0 | 4.80 | 1.71 × 10−4 | 0.77 | 75.26 | 4.87 × 10−4 | 0.58 | 870 | 0.00272 | 2.87 × 10−4 |
| 1 | 6.51 | 1.82 × 10−4 | 0.83 | 291 | 4.61 × 10−4 | 0.70 | 2035 | 0.00154 | 4.94 × 10−4 | |
| 4 | 6.81 | 2.16 × 10−4 | 0.75 | 2 | 5.51 × 10−4 | 0.75 | 3049 | 0.00346 | 1.09 × 10−3 | |
| 7 | 10.51 | 3.65 × 10−4 | 0.73 | 16 | 6.20 × 10−5 | 0.90 | 3332 | 0.00127 | 3.80 × 10−4 | |
| 14 | 7.14 | 5.89 × 10−4 | 0.76 | 951 | 7.05 × 10−4 | 0.91 | 1805 | 0.00284 | 7.91 × 10−4 | |
| 21 | 6.19 | 5.31 × 10−4 | 0.80 | 843 | 8.58 × 10−4 | 0.73 | 1444 | 0.00334 | 9.99 × 10−4 | |
| N-2 | 0 | 7.05 | 7.74 × 10−5 | 0.80 | 508 | 6.97 × 10−5 | 0.77 | 995 | 0.0034 | 1.23 × 10−3 |
| 1 | 7.27 | 1.77 × 10−4 | 0.86 | 2766 | 1.41 × 10−4 | 0.87 | 3906 | 0.00199 | 7.43 × 10−4 | |
| 4 | 10.36 | 1.26 × 10−4 | 0.82 | 8 | 2.41 × 10−5 | 0.87 | 60,650 | - | 5.22 × 10−4 | |
| 7 | 10.88 | 2.06 × 10−4 | 0.85 | 2766 | 6.23 × 10−4 | 0.57 | 39,570 | - | 1.17 × 10−3 | |
| 14 | 5.90 | 1.88 × 10−4 | 0.85 | 1380 | 2.08 × 10−4 | 0.60 | 30,420 | - | 7.56 × 10−4 | |
| 21 | 6.23 | 3.35 × 10−4 | 0.83 | 2196 | 5.89 × 10−4 | 0.68 | 51,470 | - | 1.07 × 10−3 | |
| N-3 | 0 | 5.47 | 4.15 × 10−6 | 0.83 | 2 | 1.87 × 10−4 | 0.75 | 1522 | 0.00283 | 4.60 × 10−4 |
| 1 | 6.96 | 1.72 × 10−4 | 0.85 | 1460 | 2.21 × 10−4 | 0.81 | 3146 | 0.00217 | 4.26 × 10−4 | |
| 4 | 5.86 | 2.57 × 10−4 | 0.80 | 15,730 | 2.18 × 10−4 | 0.56 | 32,130 | - | 3.70 × 10−4 | |
| 7 | 11.09 | 1.33 × 10−4 | 0.84 | 9812 | 6.53 × 10−5 | 0.76 | 80,120 | - | 8.12 × 10−4 | |
| 14 | 6.17 | 1.89 × 10−4 | 0.81 | 2344 | 1.45 × 10−4 | 0.69 | 59,770 | - | 3.48 × 10−4 | |
| 21 | 7.13 | 2.06 × 10−4 | 0.82 | 938.3 | 6.05 × 10−4 | 0.60 | 85,100 | - | 2.27 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, K.; Yao, J.; Yuan, Y.; Du, B. The Laser Deposited Nickel-Aluminum Bronze Coatings on SUS630 Stainless Steel and Its Corrosion Resistance in 3.5 wt.% NaCl Solution. Metals 2022, 12, 781. https://doi.org/10.3390/met12050781
Zhao L, Li K, Yao J, Yuan Y, Du B. The Laser Deposited Nickel-Aluminum Bronze Coatings on SUS630 Stainless Steel and Its Corrosion Resistance in 3.5 wt.% NaCl Solution. Metals. 2022; 12(5):781. https://doi.org/10.3390/met12050781
Chicago/Turabian StyleZhao, Lu, Kailun Li, Jun Yao, Yixiang Yuan, and Baorui Du. 2022. "The Laser Deposited Nickel-Aluminum Bronze Coatings on SUS630 Stainless Steel and Its Corrosion Resistance in 3.5 wt.% NaCl Solution" Metals 12, no. 5: 781. https://doi.org/10.3390/met12050781
APA StyleZhao, L., Li, K., Yao, J., Yuan, Y., & Du, B. (2022). The Laser Deposited Nickel-Aluminum Bronze Coatings on SUS630 Stainless Steel and Its Corrosion Resistance in 3.5 wt.% NaCl Solution. Metals, 12(5), 781. https://doi.org/10.3390/met12050781





