Kinetic Highlights of the Reduction of Silver Tungstate by Mg + C Combined Reducer
Abstract
:1. Introduction
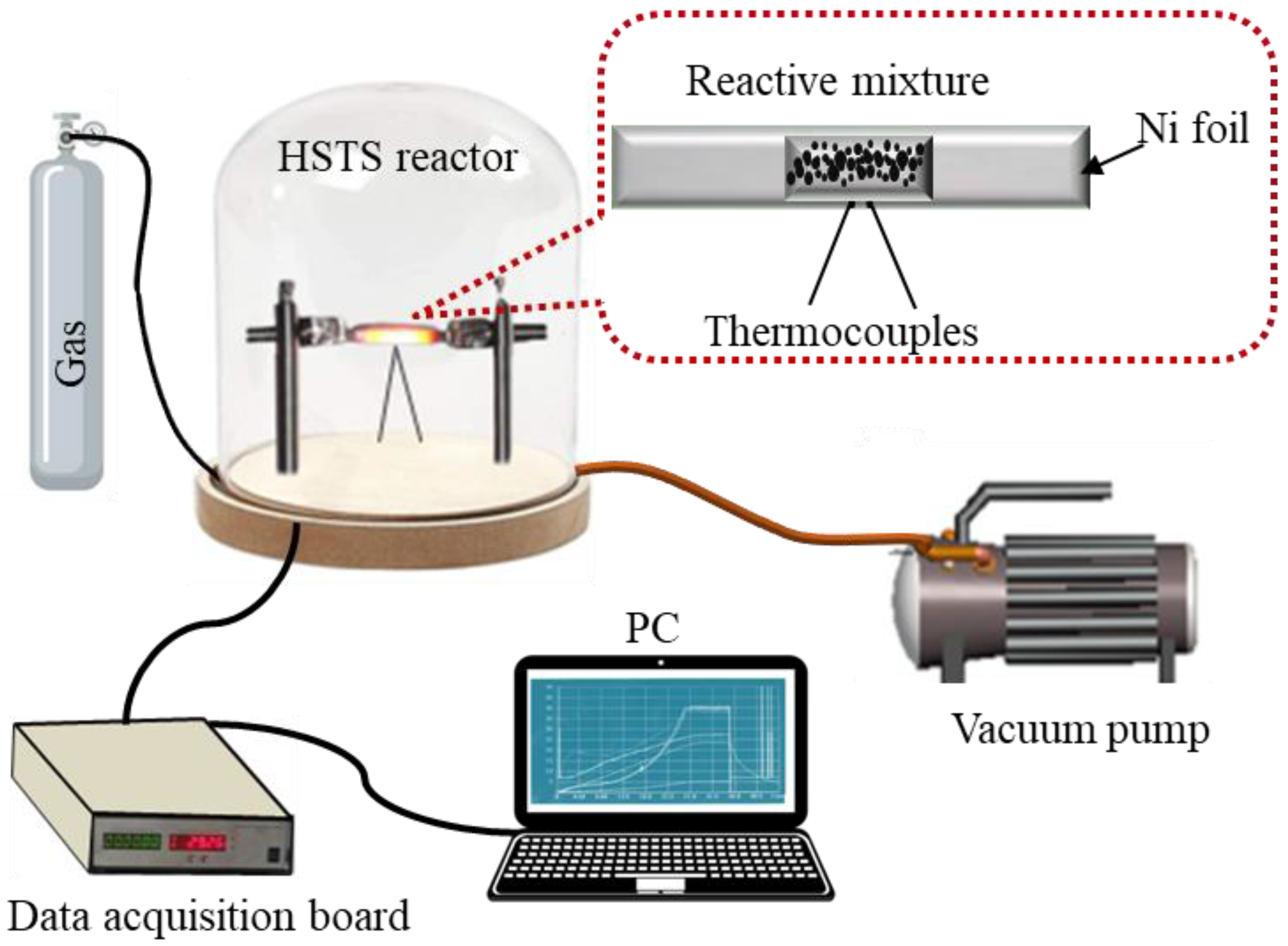
2. Materials and Methods
3. Results and Discussion
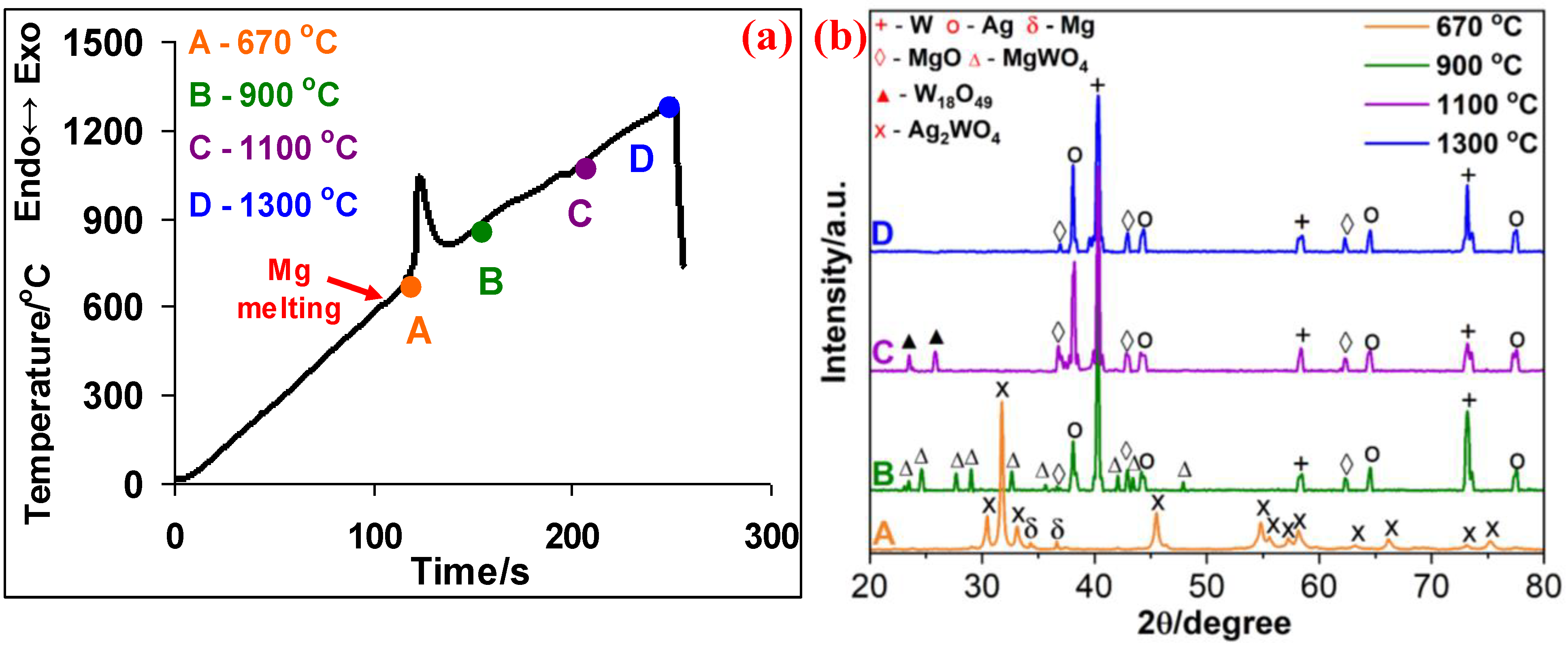
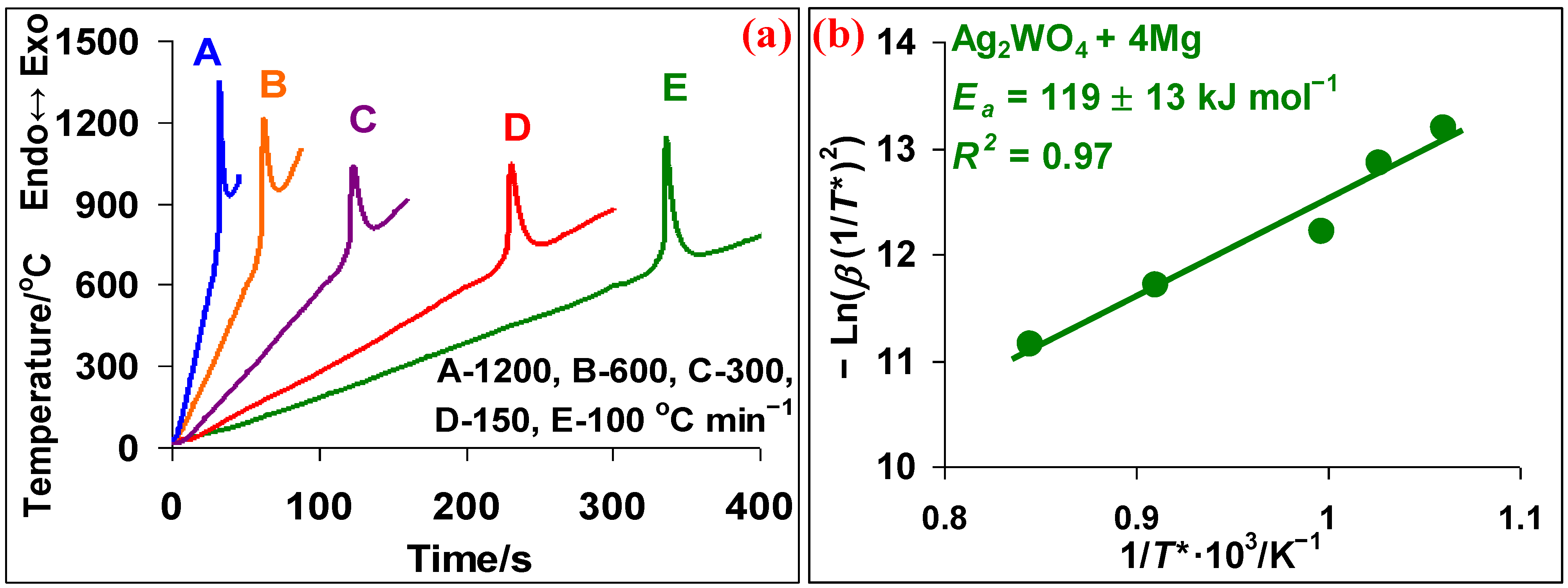
3.1. Ag2WO4–Mg System
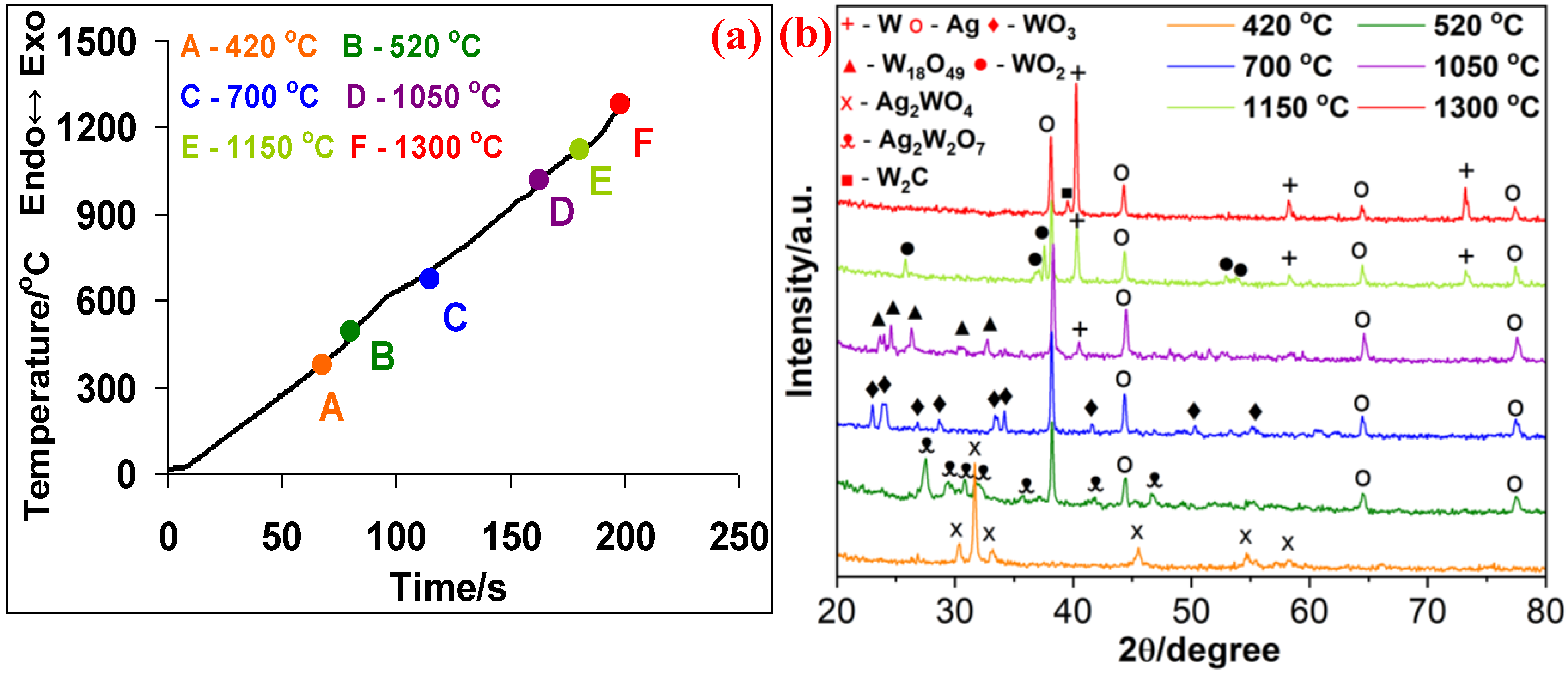
3.2. Ag2WO4–C System
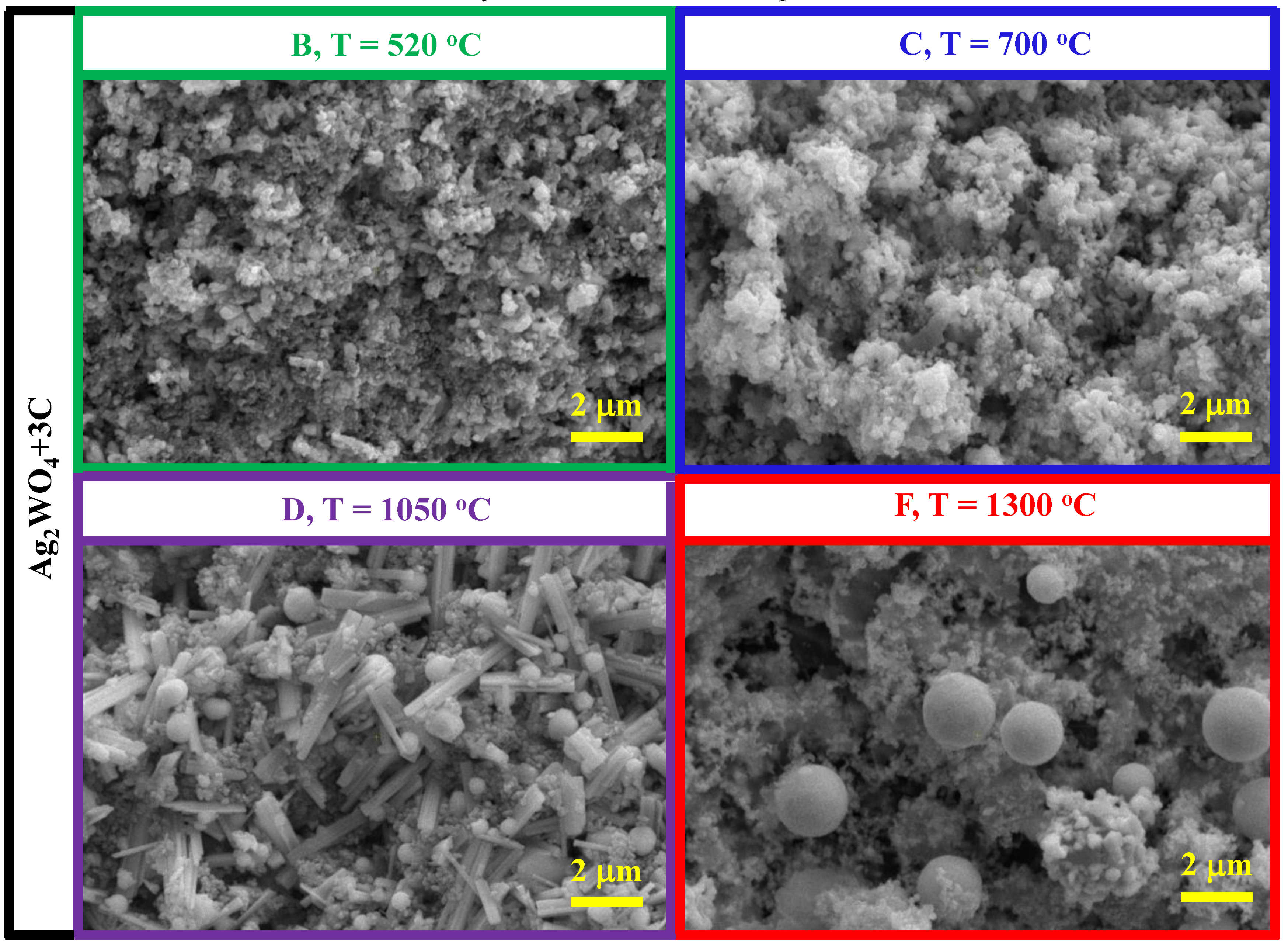
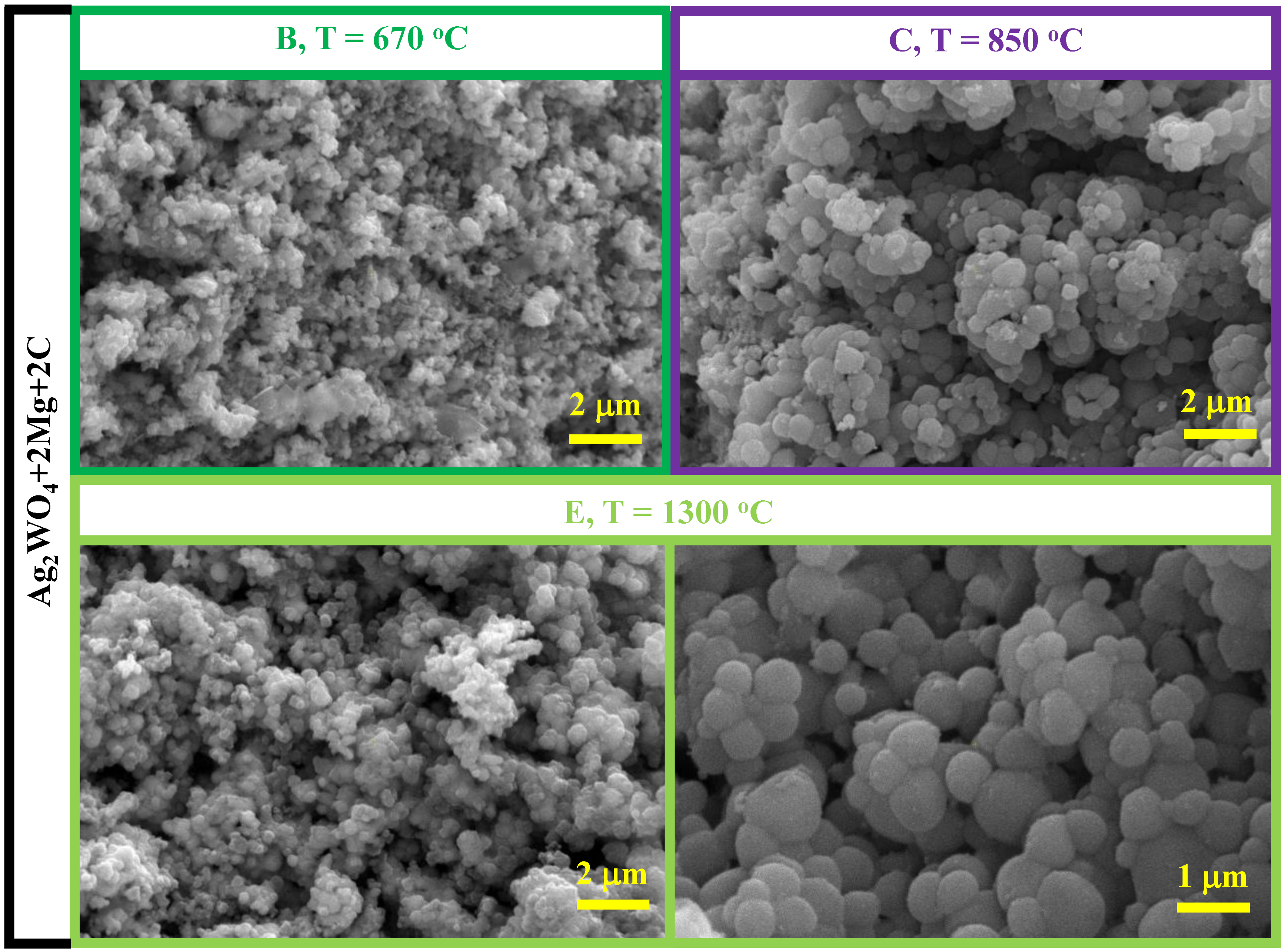
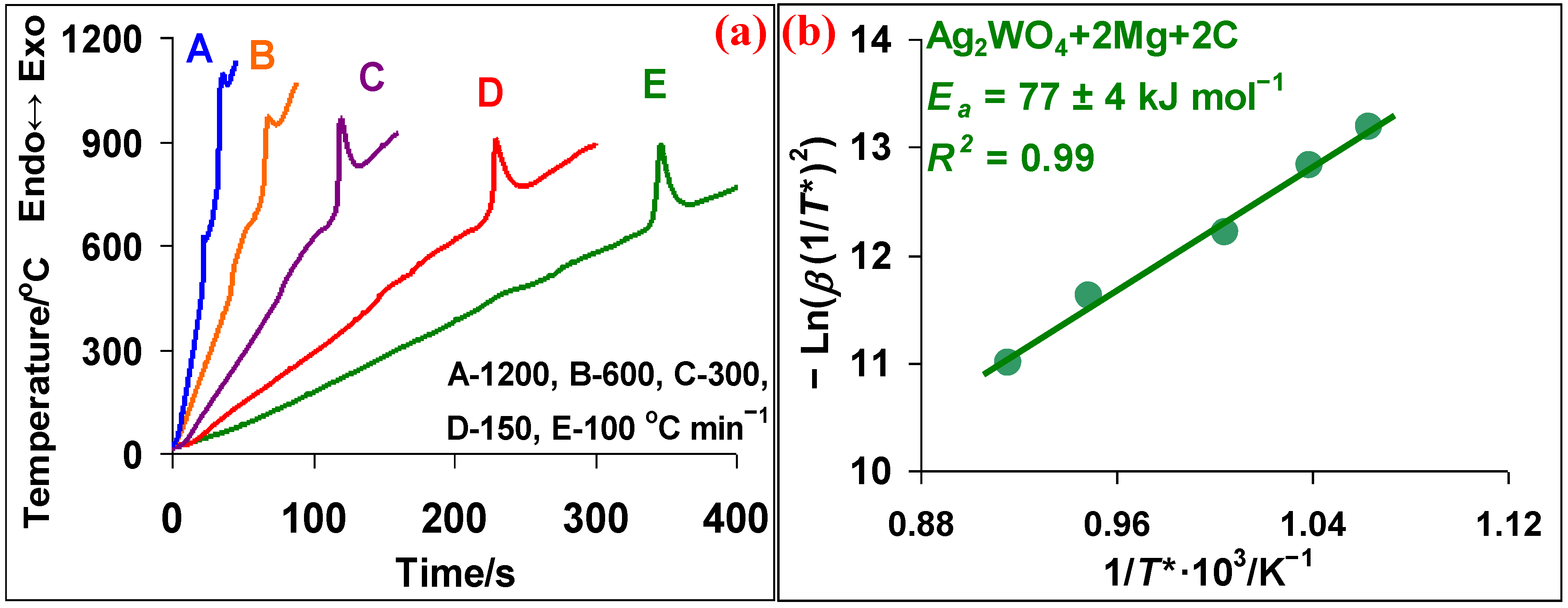
3.3. Ag2WO4–Mg–C System
4. Conclusions
- In the Ag2WO4-Mg system, in contrast to low heating rates, β = 5−20 °C min−1, there is no interaction before the melting point of Mg. The magnesiothermic reduction of both metals occurs simultaneously and a complete reduction is confirmed only at 1300 °C.
- In the Ag2WO4-C system, silver is reduced first, followed by a stepwise reduction of tungsten in accordance with this scheme: Ag2WO4 → WO3 → W18O49 → WO2 → W. These intermediate products are completely different by their microstructures. At 520 °C, Ag2W2O7 is detected: the reduction of silver tungstate begins without its preliminary decomposition.
- The reduction in the Ag2WO4-Mg-C system starts with a weaker reducing agent: carbon. The reduction temperatures are commensurate with the temperatures of binary Ag2WO4 + 4Mg and Ag2WO4 + 3C interactions. The product of the complete reduction reaction is a powder with a round granular homogeneous microstructure with a particle size of 300−700 nm.
- The effective activation energy value calculated by the Kissinger method for the Ag2WO4 + 4Mg reaction is 119 ± 13 kJ mol−1, and for the Ag2WO4 + 2Mg + 2C reaction—77 ± 4 kJ mol−1. Thus, the addition of carbon facilitates the reduction reaction with decreasing the effective activation energy value.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, F.; Silva, A.; Filho, F.; Gomes, U. Solid state sintering of a W-25 wt% Ag powder prepared by high energy milling. Int. J. Refract. Met. Hard Mater. 2008, 26, 318–323. [Google Scholar] [CrossRef]
- Saiz, E.; Tomsia, A.P. Atomic dynamics and Marangoni films during liquid-metal spreading. Nat. Mater. 2004, 3, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Findik, F.; Uzun, H. Microstructure, hardness and electrical properties of silver-based refractory contact materials. Mater. Des. 2003, 24, 489–492. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Kidalov, S.V.; Shakhov, F.M. High thermal conductivity composites consisting of diamond filler with tungsten coating and copper (silver) matrix. J. Mater. Sci. 2010, 46, 1424–1438. [Google Scholar] [CrossRef]
- Roberts, A.; Garboczi, E. Elastic properties of a tungsten–silver composite by reconstruction and computation. J. Mech. Phys. Solids 1999, 47, 2029–2055. [Google Scholar] [CrossRef]
- Leung, C.H.; Wingert, P.C. Microstructure effects on dynamic welding of Ag/W contacts. IEEE Trans. Compon. Hybrids Manuf. Technol. 1988, 11, 64–67. [Google Scholar] [CrossRef]
- Costa, F.; Silva, A.; Filho, F.; Gomes, U.; Vieira, F. Synthesis of a nanocrystalline composite W–25 wt.% Ag powder by high energy milling. Powder Technol. 2008, 188, 30–33. [Google Scholar] [CrossRef]
- Caceres, P.G. Effect of microstructure on the abrasive wear properties of infiltrated tungsten alloys. Mater. Charact. 2002, 49, 1–9. [Google Scholar] [CrossRef]
- Stone, D.; Liu, J.; Singh, D.; Muratore, C.; Voevodin, A.; Mishra, S.; Rebholz, C.; Geb, Q.; Aouadia, S. Layered atomic structures of double oxides for low shear strength at high temperatures. Scr. Mater. 2010, 62, 735–738. [Google Scholar] [CrossRef]
- Kaidatzis, A.; Psycharis, V.; Niarchos, D. Sputtered tungsten-silver and tungsten-aluminium thin films for non-volatile magnetic memories applications. Microelectron. Eng. 2016, 159, 6–8. [Google Scholar] [CrossRef]
- Leung, C.H.; Wingert, P.C.; Kim, H.J. Comparison of reignition properties of several Ag/W, Ag/WC and Ag/Mo electrical contact materials. IEEE Trans. Compon. Hybrids Manuf. Technol. 1986, 9, 86–91. [Google Scholar] [CrossRef]
- German, R.M.; Hens, K.F.; Johnson, J.L. Powder metallurgy processing of thermal management materials for microelectronic applications. Int. J. Powder Metall. 1994, 30, 205–216. [Google Scholar]
- Vettivel, S.C.; Selvakumar, N.; Leema, N.; Lenin, A.H. Electrical resistivity, wear map and modeling of extruded tungsten reinforced copper composite. Mater. Des. 2014, 56, 791–806. [Google Scholar] [CrossRef]
- Kecskes, L.J.; Klot, B.R.; Cho, K.C.; Dowding, R.J.; Trexler, M.D. Densification and structural change of mechanically alloyed W-Cu composites. Metall. Mater. Trans. A 2001, 32, 2885–2893. [Google Scholar] [CrossRef]
- Walkden, P.; Albiston, J.N.; Sale, F.R. Reduction of tungstates for production of silver-tungsten and silver-tungsten-nickel electrical contacts. Powder Metall. 1985, 28, 36–42. [Google Scholar] [CrossRef]
- Azhar, S.; Es-Saheb, M. Homogenous silver-tungsten composite production for electrical contacts. Res. J. Appl. Sci. Eng. Technol. 2015, 9, 549–560. [Google Scholar] [CrossRef]
- Peikrishvili, A.; Godibadze, B.; Chagelishvili, E.; Tsiklauri, M.; Dgebuadze, A. Hot explosive consolidation of nanostructured tungsten-silver precursors. Eur. Chem. Bull. 2015, 4, 37–42. [Google Scholar]
- Leżański, J.; Madej, M. The influence of production process parameters on the properties W-Ag, Mo-Ag composites. Mater. Sci. Forum 2007, 534, 1513–1516. [Google Scholar] [CrossRef]
- Zakaryan, M.K. Reduction of silver tungstate in combustion mode and synthesis of W-Ag pseudoalloy. Arm. Chem. J. 2020, 73, 300–310. [Google Scholar]
- Sridhar, S.; Sichen, D.U.; Seetharaman, S. Investigation of the kinetics of reduction of nickel tungstate by hydrogen. Metall. Mater. Trans. B 1994, 25, 391–396. [Google Scholar] [CrossRef]
- Zakaryan, M.; Aydinyan, S.; Kharatyan, S. Combustion synthesis and consolidation of Ni-W nanocomposite material. Ceram. Mod. Technol. 2019, 1, 67–74. [Google Scholar] [CrossRef]
- Aydinyan, S.V.; Kirakosyan, H.V.; Zakaryan, M.K.; Abovyan, L.S.; Kharatyan, S.L.; Peikrishvili, A.; Mamniashvili, G.; Godibadze, B.; Chagelishvili, E.S.; Lesuer, D.R.; et al. Fabrication of Cu-W nanocomposites by integration of self-propagating high-temperature synthesis and hot explosive consolidation technologies. Eurasian Chem.-Technol. J. 2018, 20, 301–309. [Google Scholar] [CrossRef]
- Zakaryan, M.; Kirakosyan, H.; Aydinyan, S.; Kharatyan, S. Combustion synthesis of W-Cu composite powders from oxide precursors with various proportions of metals. Int. J. Refract. Met. Hard Mater. 2017, 64, 176–183. [Google Scholar] [CrossRef]
- Aydinyan, S.; Kharatyan, S.; Hussainova, I. SHS-derived powders by reactions’ coupling as primary products for subsequent consolidation. Materials 2021, 14, 5117. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, T.; Kirakosyan, H.; Aydinyan, S.; Liu, L.; Kharatyan, S.; Hussainova, I. Mo-Cu pseudoalloys by combustion synthesis and spark plasma sintering. J. Mater. Sci. 2018, 53, 16598–16608. [Google Scholar] [CrossRef]
- Aydinyan, S.V.; Kirakosyan, H.V.; Kharatyan, S.L. Cu-Mo composite powders obtained by combustion-coreduction process. Int. J. Refract. Met. Hard Mater. 2016, 54, 455–463. [Google Scholar] [CrossRef]
- Kirakosyan, H.V.; Aydinyan, S.V.; Kharatyan, S.L. W-Cu composite powders obtained by joint reduction of oxides in combustion mode. Int. J. SHS 2016, 25, 215–223. [Google Scholar] [CrossRef]
- Nazaretyan, K.; Kirakosyan, H.; Zakaryan, M.; Abovyan, L.; Volobujeva, O.; Aydinyan, S. The interaction pathway in the mechano-ultrasonically assisted and carbon-nanotubes augmented nickel-aluminum system. Metals 2022, 12, 436. [Google Scholar] [CrossRef]
- Zakaryan, M.; Nazaretyan, K.; Aydinyan, S.; Kharatyan, S. Joint reduction of NiO/WO3 pair and NiWO4 by Mg + C combined reducer at high heating rates. Metals 2021, 11, 1351. [Google Scholar] [CrossRef]
- Nazaretyan, K.T.; Kirakosyan, H.V.; Aydinyan, S.V.; Zakaryan, M.K.; Abovyan, L.S.; Kulak, M.; Khina, B. The influence of high-energy ball milling and nanoadditives on the kinetics of heterogeneous reaction in Ni-Al system. In Materials Science and Engineering; IOP Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 1140, p. 012052. [Google Scholar] [CrossRef]
- Zakaryan, M.K.; Nazaretyan, K.T.; Aydinyan, S.V.; Kharatyan, S.L. NiO reduction by Mg + C combined reducer at high heating rates. J. Therm. Anal. Calorim. 2021, 146, 1811–1817. [Google Scholar] [CrossRef]
- Aydinyan, S.V.; Nazaretyan, K.T.; Zargaryan, A.G.; Tumanyan, M.E.; Kharatyan, S.L. Reduction mechanism of WO3 + CuO mixture by combined Mg/C reducer. J. Therm. Anal. Calorim. 2018, 133, 261–269. [Google Scholar] [CrossRef]
- Kirakosyan, H.; Nazaretyan, K.; Aydinyan, S.; Kharatyan, S. The mechanism of joint reduction of MoO3 and CuO by combined Mg/C reducer at high heating rates. J. Compos. Sci. 2021, 5, 318. [Google Scholar] [CrossRef]
- Zakaryan, M.K.; Arzumanyan, A.S.; Kharatyan, S.L. Magnesio-carbothermic reduction of Ag2WO4. DTA/TG study. Arm. Chem. J. 2020, 73, 300–310. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaryan, M.; Nazaretyan, K.; Aydinyan, S.; Kharatyan, S. Kinetic Highlights of the Reduction of Silver Tungstate by Mg + C Combined Reducer. Metals 2022, 12, 1000. https://doi.org/10.3390/met12061000
Zakaryan M, Nazaretyan K, Aydinyan S, Kharatyan S. Kinetic Highlights of the Reduction of Silver Tungstate by Mg + C Combined Reducer. Metals. 2022; 12(6):1000. https://doi.org/10.3390/met12061000
Chicago/Turabian StyleZakaryan, Marieta, Khachik Nazaretyan, Sofiya Aydinyan, and Suren Kharatyan. 2022. "Kinetic Highlights of the Reduction of Silver Tungstate by Mg + C Combined Reducer" Metals 12, no. 6: 1000. https://doi.org/10.3390/met12061000





