Oxidation Characteristics of Nickel-Based Superalloy Powders Exposed at Ambient Condition
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Analysis of Adsorbed Gas on the Initial Powder Surface
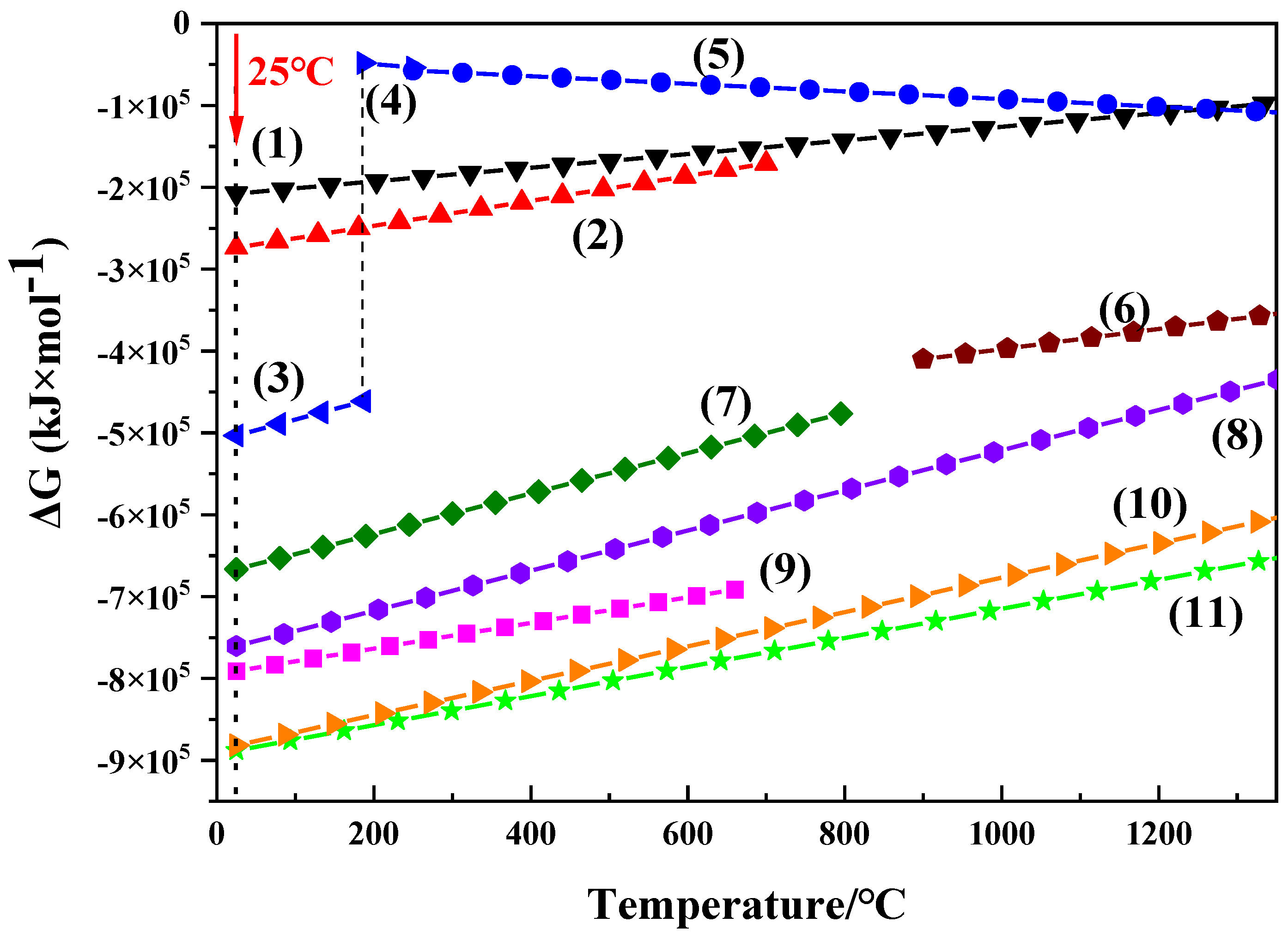
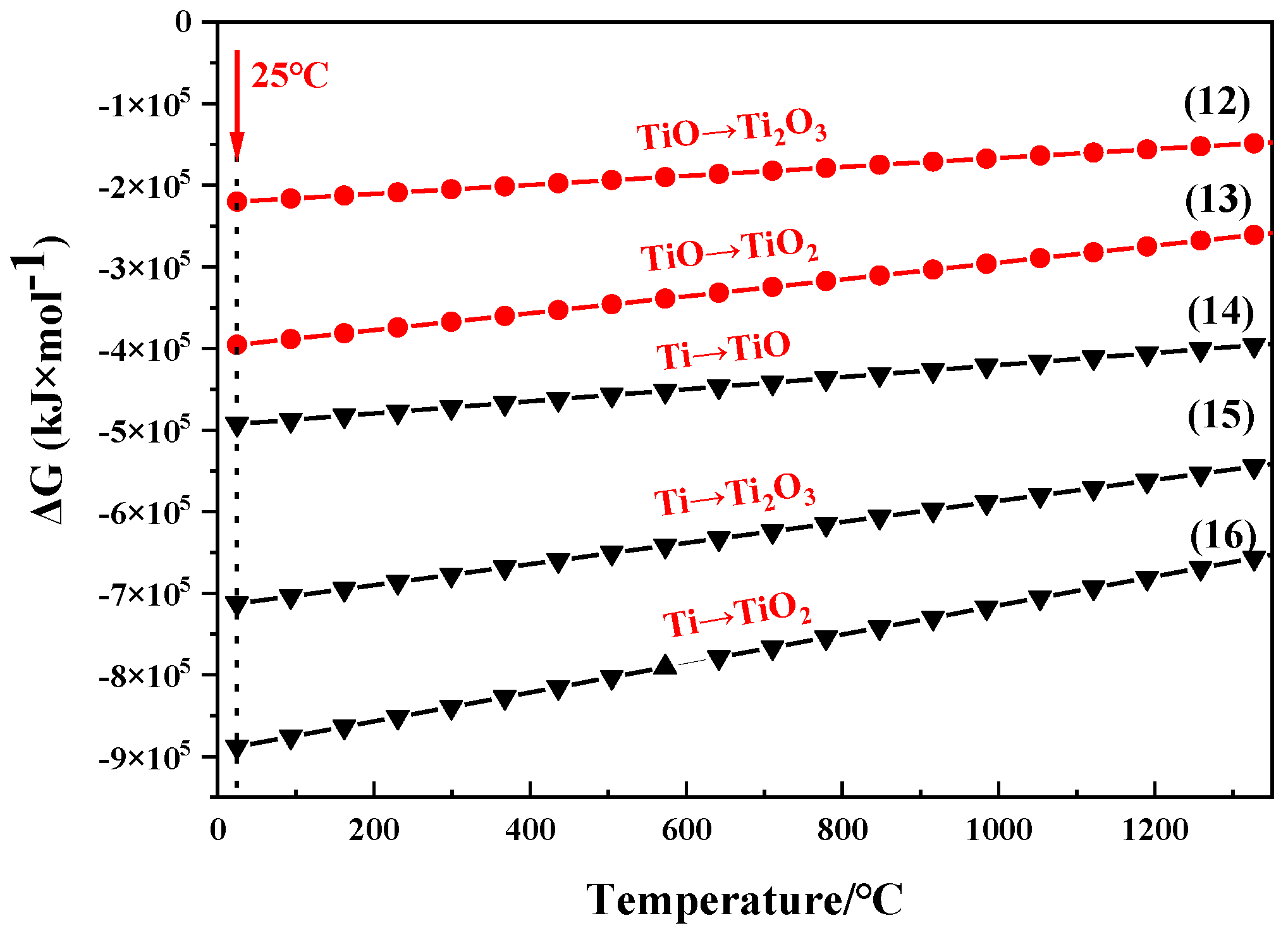
3.2. Thermodynamics Calculation of Oxidation Processes
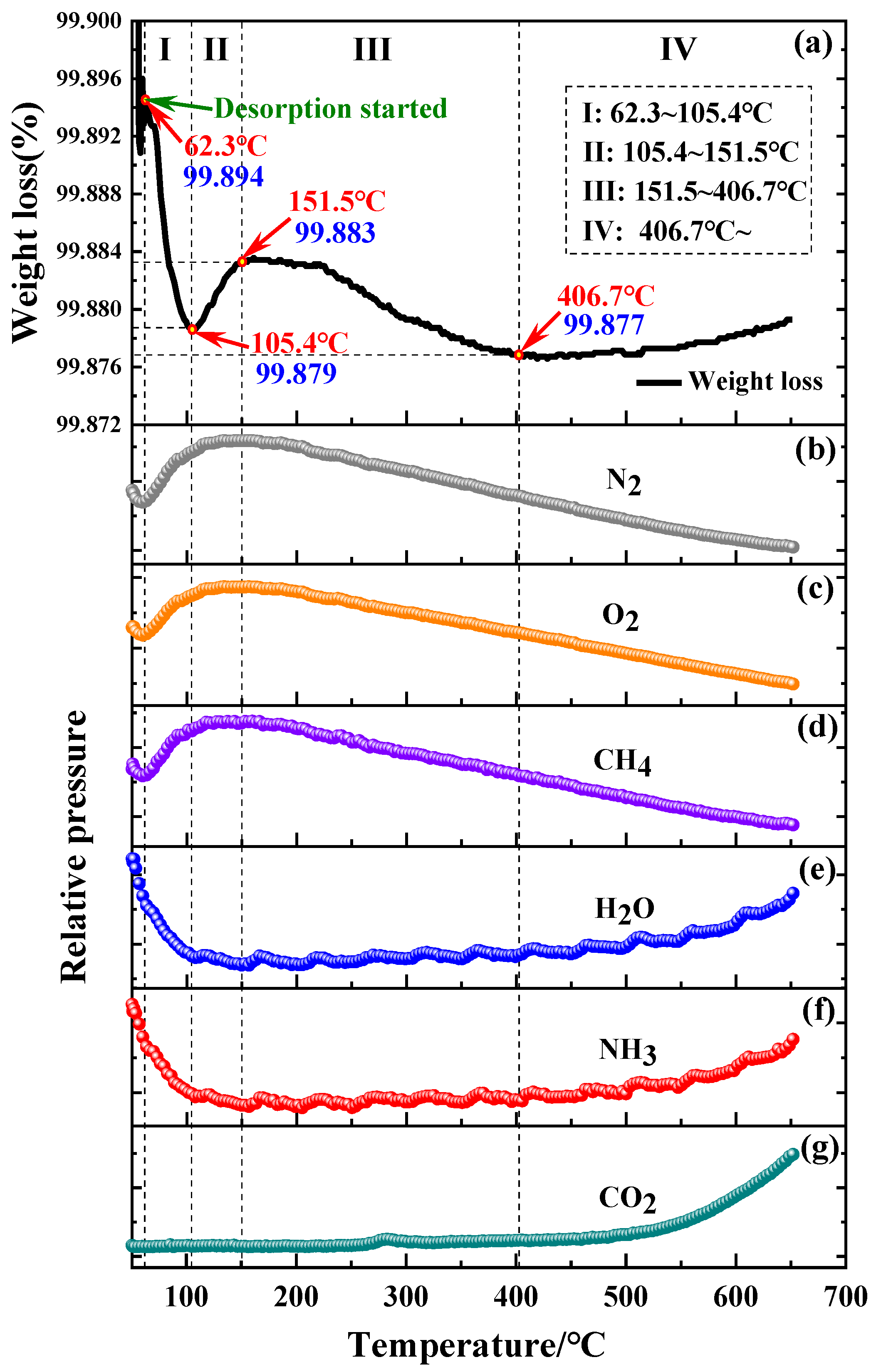
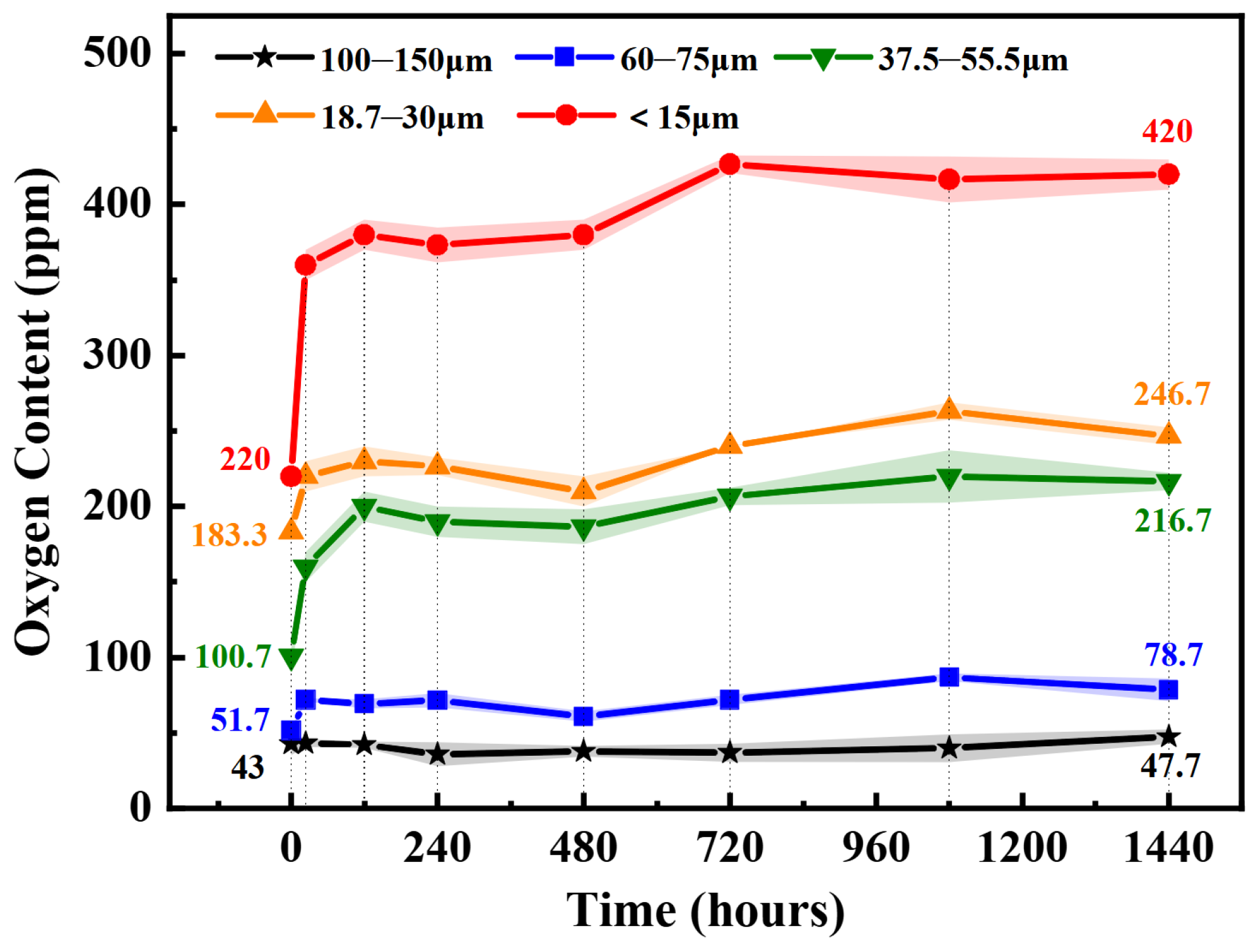
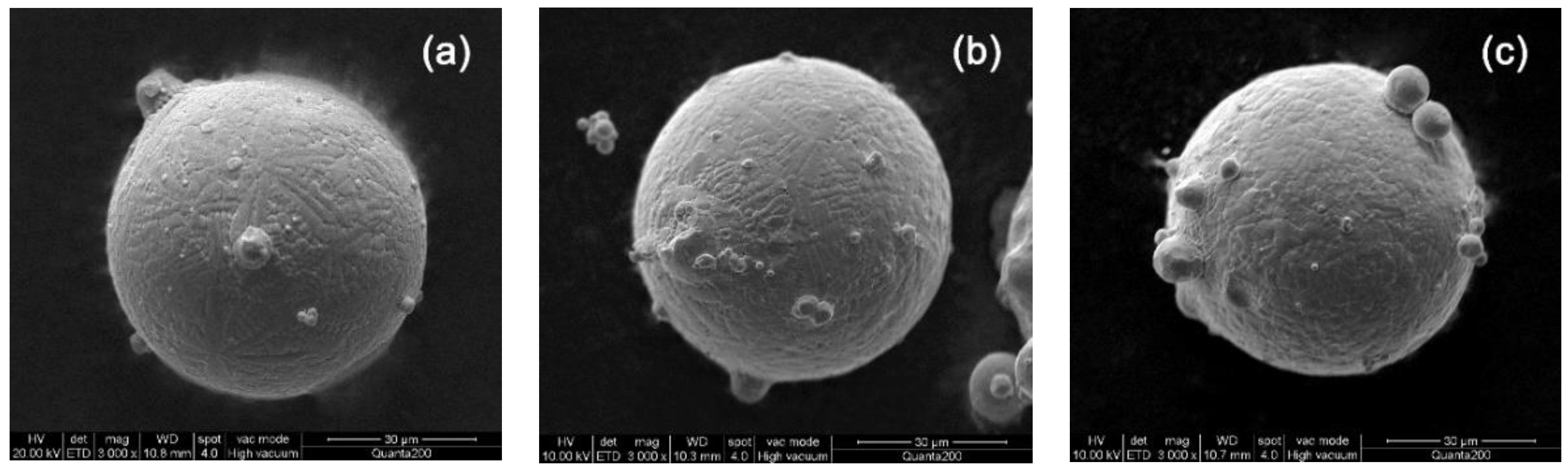
3.3. Oxidation Kinetics of Powder at Ambient Temperature
3.4. Characterization of the State of Oxygen
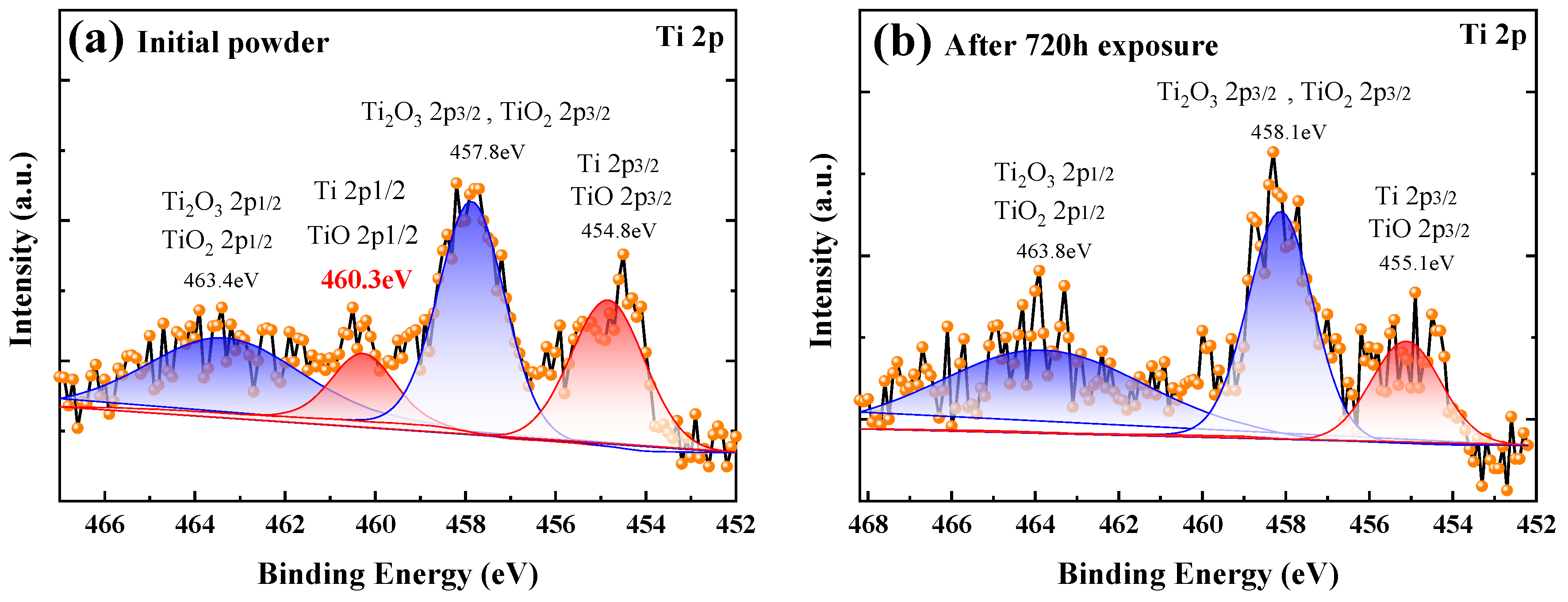
3.5. Characterization of the Oxidation state of Titanium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.R.; Wang, X.C.; Zhong, B.; Wei, D.S.; Jiang, X.H. Estimation of fatigue parameters in total strain life equation for powder metallurgy superalloy FGH96 and other metallic materials. Int. J. Fatigue. 2019, 122, 116–124. [Google Scholar] [CrossRef]
- Umeda, T.; Okane, T.; Kurz, W. Phase selection during solidification of peritectic alloys. Acta Mater. 1996, 44, 4209–4216. [Google Scholar] [CrossRef]
- Yao, C.G.; Meng, S.; Li, X.L.; Yi, D.Q.; Lü, H.J.; Wang, B. Effects of powder oxygen content on mechanical properties and microstructure of FGH4169 alloy. Mater. Sci. Eng. Powder Metall. 2017, 22, 33–40. [Google Scholar]
- Warren, R.; Ingesten, N.G.; Winberg, L.; Ronnhult, T. Particle surfaces and prior particle boundaries in Hf modified PM astroloy. Powder Metall. 1984, 27, 141–146. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Y.W.; Chi, Y.; Liu, J.T.; Jia, J.; Han, S.B. Effect of content of Hf and Zr on equilibrium phase and PPB in FGH96 P/M superalloy. Trans. Mater. Heat Treat. 2013, 34, 60–67. [Google Scholar]
- Zhang, Y.W.; Liu, J.T. Development in powder metallurgy superalloy. Mater. China 2013, 32, 1–11. [Google Scholar]
- Prakash, T.L.; Chari, Y.N.; Bhagiradha Rao, E.S.; Thamburaj, R. Microstructures and mechanical properties of hot isostatically pressed powder metallurgy alloy APK-1. Metall. Trans. A 1983, 14, 733–742. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Liu, C.K.; Zhao, W.X.; Zheng, Z.; Zhong, Y. Prior particle boundary of PM FGH96 superalloy and its in-situ high-cycle fatigue at elevated temperature. J. Aeronaut. Mater. 2017, 37, 83–89. [Google Scholar]
- Qin, S.Y.; Yan, L.G.; Zhang, X.F. Removing prior particle boundaries in a powder superalloy based on the interaction between pulsed electric current and chain-like structure. J. Mater. Sci. Technol. 2021, 87, 95–100. [Google Scholar] [CrossRef]
- Qiu, C.L.; Attallah, M.M.; Wu, X.H.; Andrews, P. Influence of hot isostatic pressing temperature on microstructure and tensile properties of a nickel-based superalloy powder. Mater. Sci. Eng. A 2013, 564, 176–185. [Google Scholar] [CrossRef]
- Appa Rao, G.A.; Srinvas, M.; Sarma, D.S. Effect of oxygen content of powder on microstructure and mechanical properties of hot isostatically pressed superalloy Inconel 718. Mater. Sci. Eng. A 2006, 435, 84–99. [Google Scholar]
- Zeoli, N.; Gu, S. Computational validation of an isentropic plug nozzle design for gas atomisation. Comput. Mater. Sci. 2008, 42, 245–258. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Z.; Xu, W.Y.; Zhang, G.Q. The study of argon atomized superalloy powders. Powder Metall. Ind. 2010, 20, 1–5. [Google Scholar]
- Tan, L.M.; Li, Y.P.; Liu, C.Z.; Yang, C.; Ding, H.H.; Huang, L.; Liu, F.; Qin, Z.J.; Jiang, L. The evolution history of superalloy powders during hot consolidation and plastic deformation. Mater. Charact. 2018, 140, 30–38. [Google Scholar] [CrossRef]
- Liu, N.; Li, Z.; Zhang, G.Q.; Yuan, H.; Xu, W.Y.; Zhang, Y. Oxidation characteristics of nickel-based superalloy powders prepared by argon gas atomization. Chin. J. Rare Met. 2011, 32, 481–485. [Google Scholar]
- Gao, Z.J.; Zhang, G.Q.; Li, Z.; Yuan, H.; Xu, W.Y.; Liu, N. Effect of size distribution and oxygen content of powder on microstructure of HIPed superalloy FGH96. Chin. J. Rare Met. 2012, 36, 665–670. [Google Scholar]
- Xu, W.Y.; Li, Z.; Liu, Y.F.; Zhang, L.; Zhang, G.Q. Influence of temperature on the oxidation behaviors of the nickel-based superalloy powders. Powder Metall. Technol. 2020, 38, 192–196. [Google Scholar]
- Shimizu, K.; Kobayashi, K.; Thompson, G.E.; Wood, G.C. The apparent induction period for γ-Al2O3 development in thermal oxide films on aluminium. Oxid. Met. 1991, 36, 1–13. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J.; Dietrich, J.R.; Senkov, O.N. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, X.Q.; Li, Q.M.; Gao, X.; Liu, W.L. Application of themogravimetric mass spectrometry system with vacuum replacement technology in the thermal decomposition of sodium azide. Anal. Instrum. 2019, 1, 32–35. [Google Scholar]
- Guo, W.M.; Wu, J.T.; Chen, G.S.; Zhou, B.; Zhao, M.H. The influence of vacuum degasing pretreatment on microstructure and properties of superalloy FGH95. J. Aeronaut. Mater. 2003, 23, 21–24. [Google Scholar]
- Kong, L.R.; Zhang, S.Y. Theoretical explanation of the special temperature dependence of rate constant for oxidation of carbon. Univ. Chem. 2016, 31, 84–88. [Google Scholar] [CrossRef]
- Hu, B.F.; Li, H.Y. Microstructure of argon-atomized FGH95 and imported René95 superalloy powders after heat treatment. Chin. J. Eng. 1987, 9, 12–18. [Google Scholar]
- Ma, W.B.; Liu, G.Q.; Hu, B.F.; Zhang, Y.W.; Liu, J.T. Prior particle boundary and its effect on tensile properties of PM FGH96 superalloy. Mater. Sci. Eng. Powder Metall. 2013, 18, 1–7. [Google Scholar]
- Liang, Y.J.; Che, M.C. Handbook of Thermodynamics of Inorganic Materials; Northeast University Press: Liaoning, China, 1993; pp. 449–479. ISBN 7-81006-532-7. [Google Scholar]
- Shen, Y.L.; Guo, M.L.; Xia, X.H.; Shao, G.S. Role of materials chemistry on the electrical/electronic properties of CuO thin films. Acta Mater. 2015, 85, 122–131. [Google Scholar] [CrossRef]
- Fang, P.J.; Xu, Y.; Li, X.G.; Chen, Y. Influence of atomizing gas and cooling rate on solidification characterization of nickel-based superalloy powders. Rare Met. Mater. Eng. 2018, 47, 423–430. [Google Scholar]
- Wang, P.W.; Woo, J.; Avila, M.; Garicia, J.; Bronson, A.; Varma, S.K. In situ surface oxidation of Ti44Al11Nb alloy at room temperature. J. Mater. Sci. 2003, 38, 489–497. [Google Scholar] [CrossRef]
- Merritt, R.R.; Hyde, B.G.; Bursil, L.A.; Philp, D.K. The thermodynamics of titanium+oxygen system: An isothermal gravimetric study of the composition range Ti3O5 to TiO2 at 1304K. Philos. Trans. R. Soc. A 1973, 274, 627–661. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Jiao, D.; Qiu, W.; Liu, Z.; Xu, W.; Li, Z.; Zhang, G. Oxidation Characteristics of Nickel-Based Superalloy Powders Exposed at Ambient Condition. Metals 2022, 12, 972. https://doi.org/10.3390/met12060972
Zhong W, Jiao D, Qiu W, Liu Z, Xu W, Li Z, Zhang G. Oxidation Characteristics of Nickel-Based Superalloy Powders Exposed at Ambient Condition. Metals. 2022; 12(6):972. https://doi.org/10.3390/met12060972
Chicago/Turabian StyleZhong, Weijie, Dongling Jiao, Wanqi Qiu, Zhongwu Liu, Wenyong Xu, Zhou Li, and Guoqing Zhang. 2022. "Oxidation Characteristics of Nickel-Based Superalloy Powders Exposed at Ambient Condition" Metals 12, no. 6: 972. https://doi.org/10.3390/met12060972
APA StyleZhong, W., Jiao, D., Qiu, W., Liu, Z., Xu, W., Li, Z., & Zhang, G. (2022). Oxidation Characteristics of Nickel-Based Superalloy Powders Exposed at Ambient Condition. Metals, 12(6), 972. https://doi.org/10.3390/met12060972






