Effect of Grain Orientation on Hydrogen Embrittlement Behavior of Interstitial-Free Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogen Diffusion Coefficient
2.2. Slow Strain Rate Tensile Test
2.3. Hydrogen Microprint Technique Method Test
2.4. Microstructural Characterization
3. Results
3.1. Microstructure of IF Steel
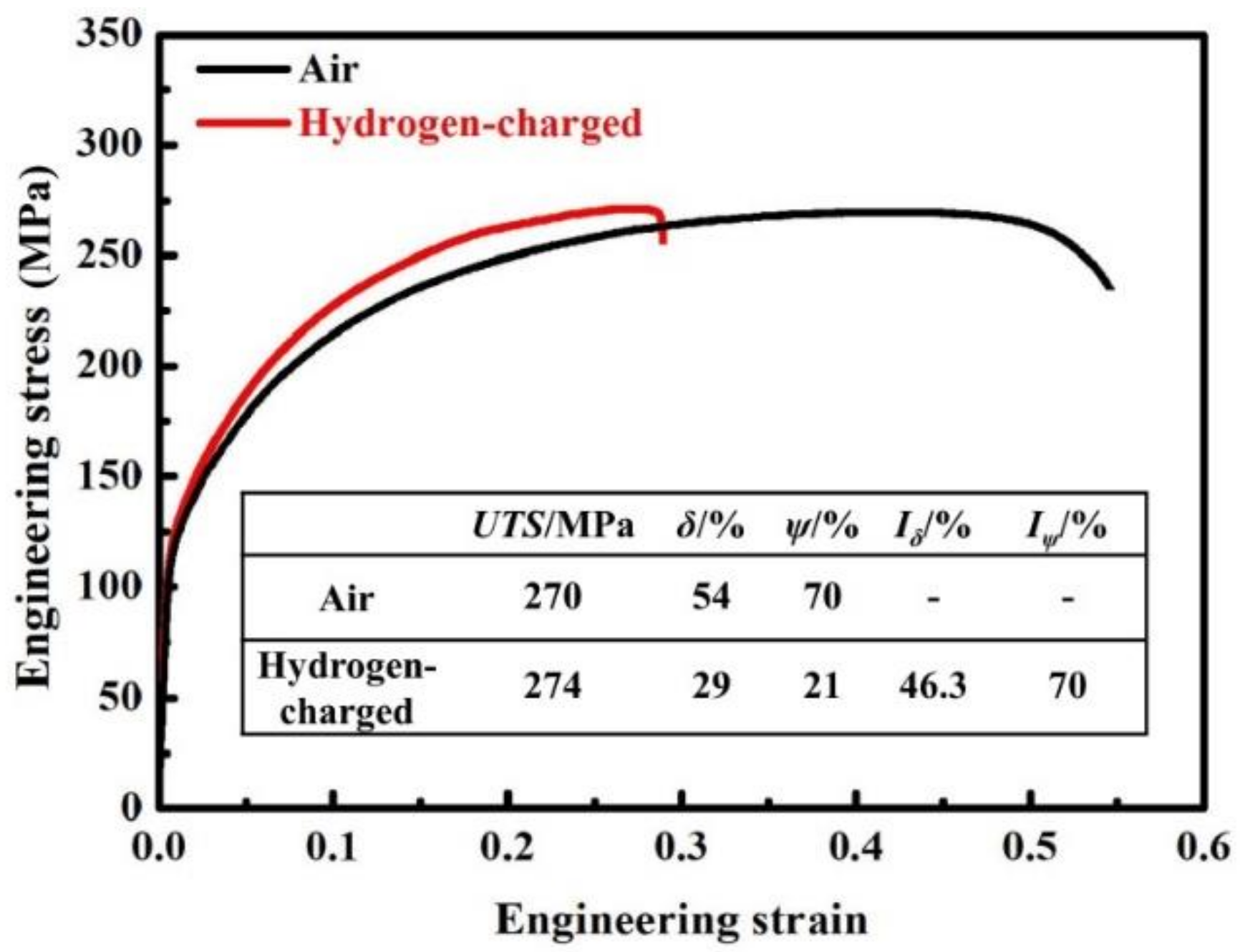
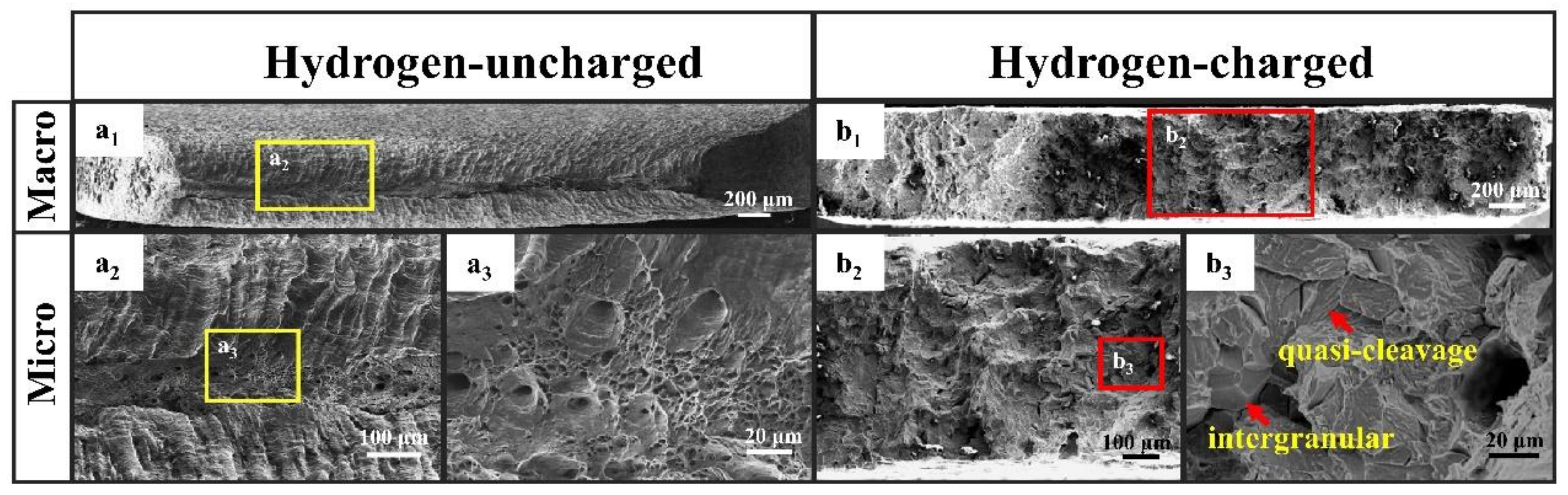
3.2. Mechanical Properties and HE Susceptibility
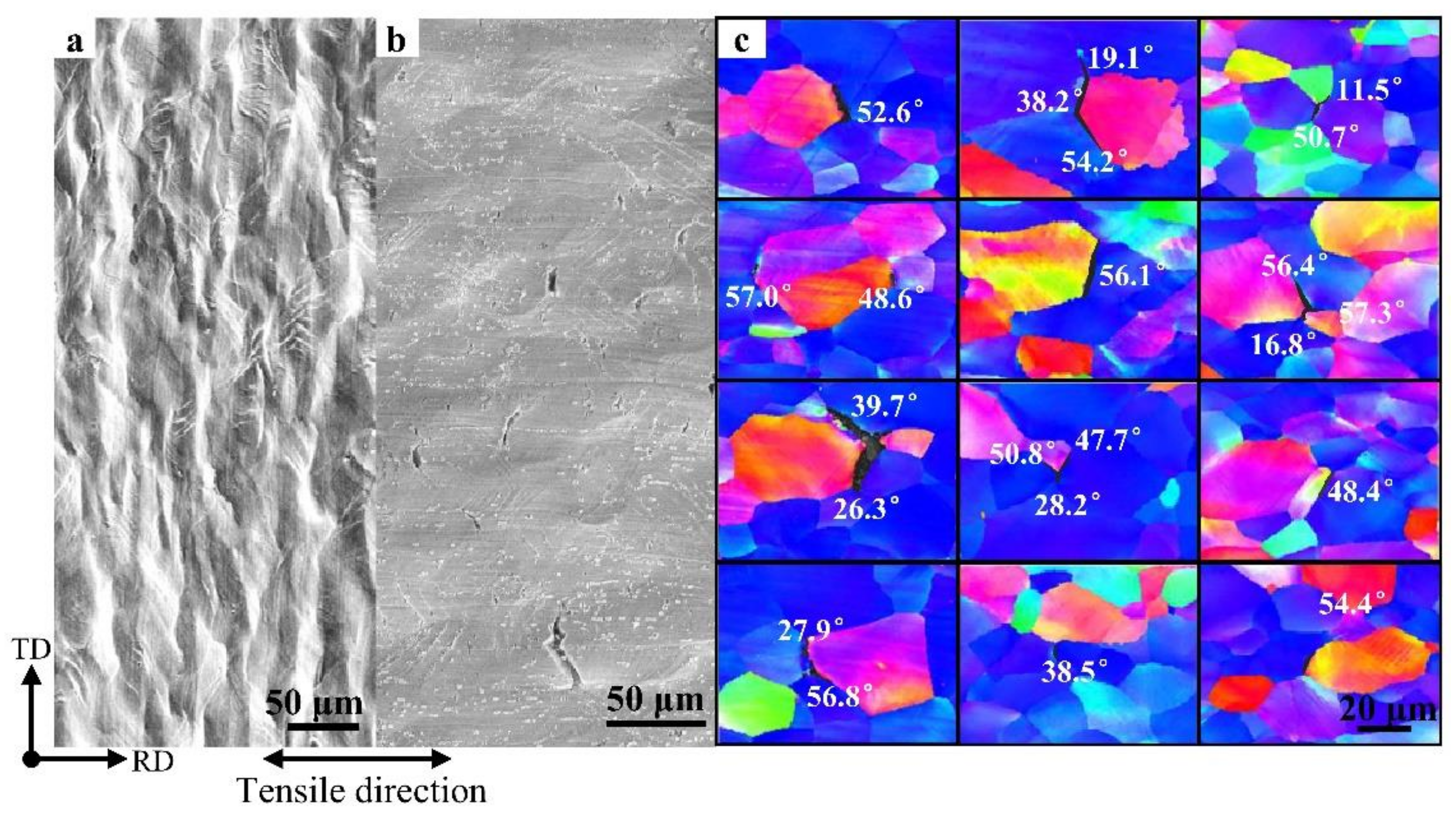
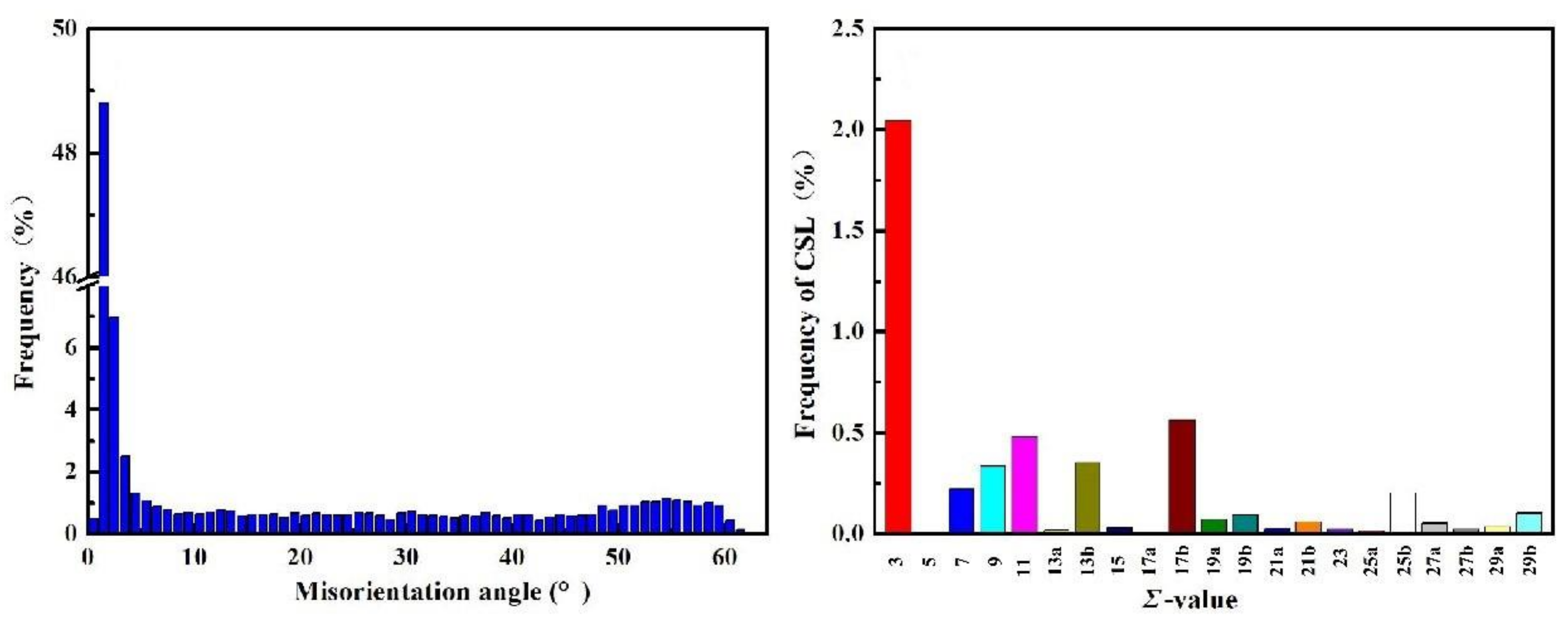
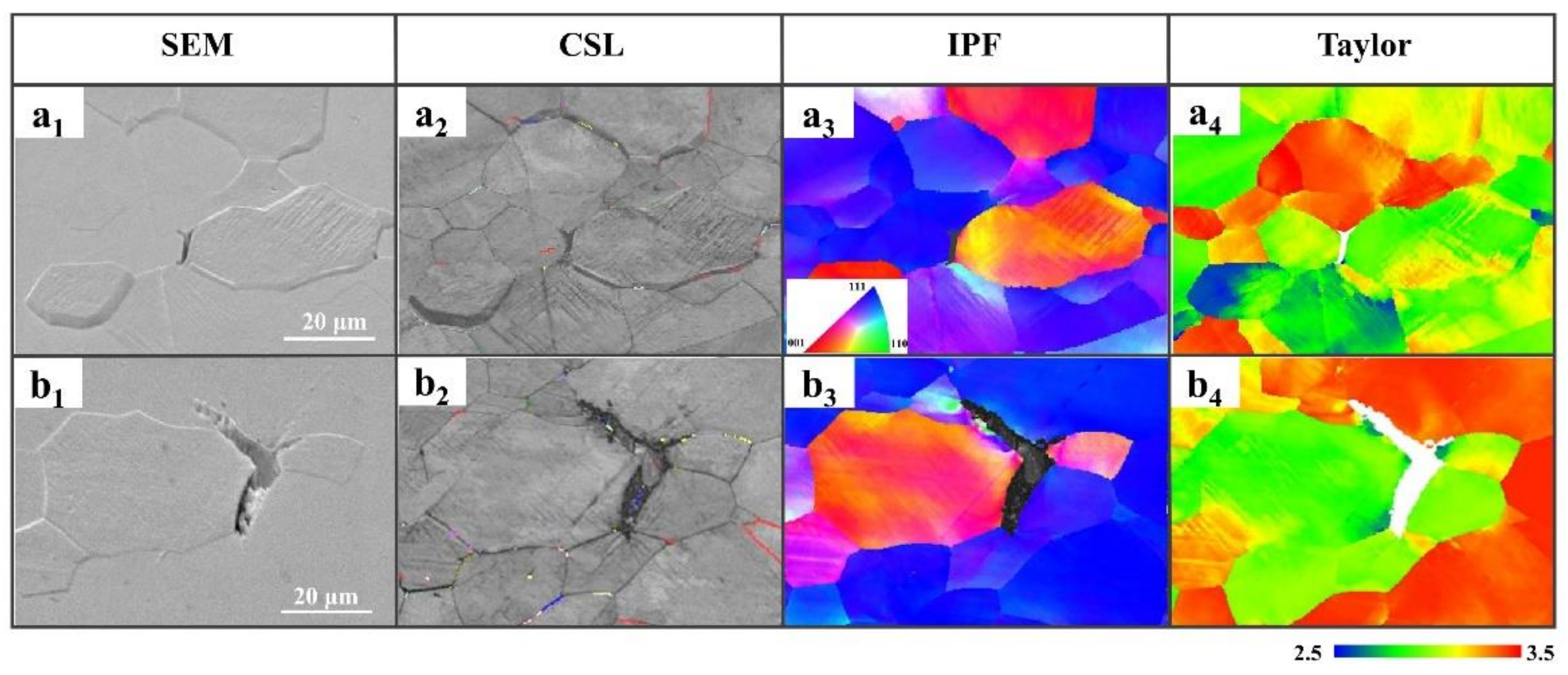
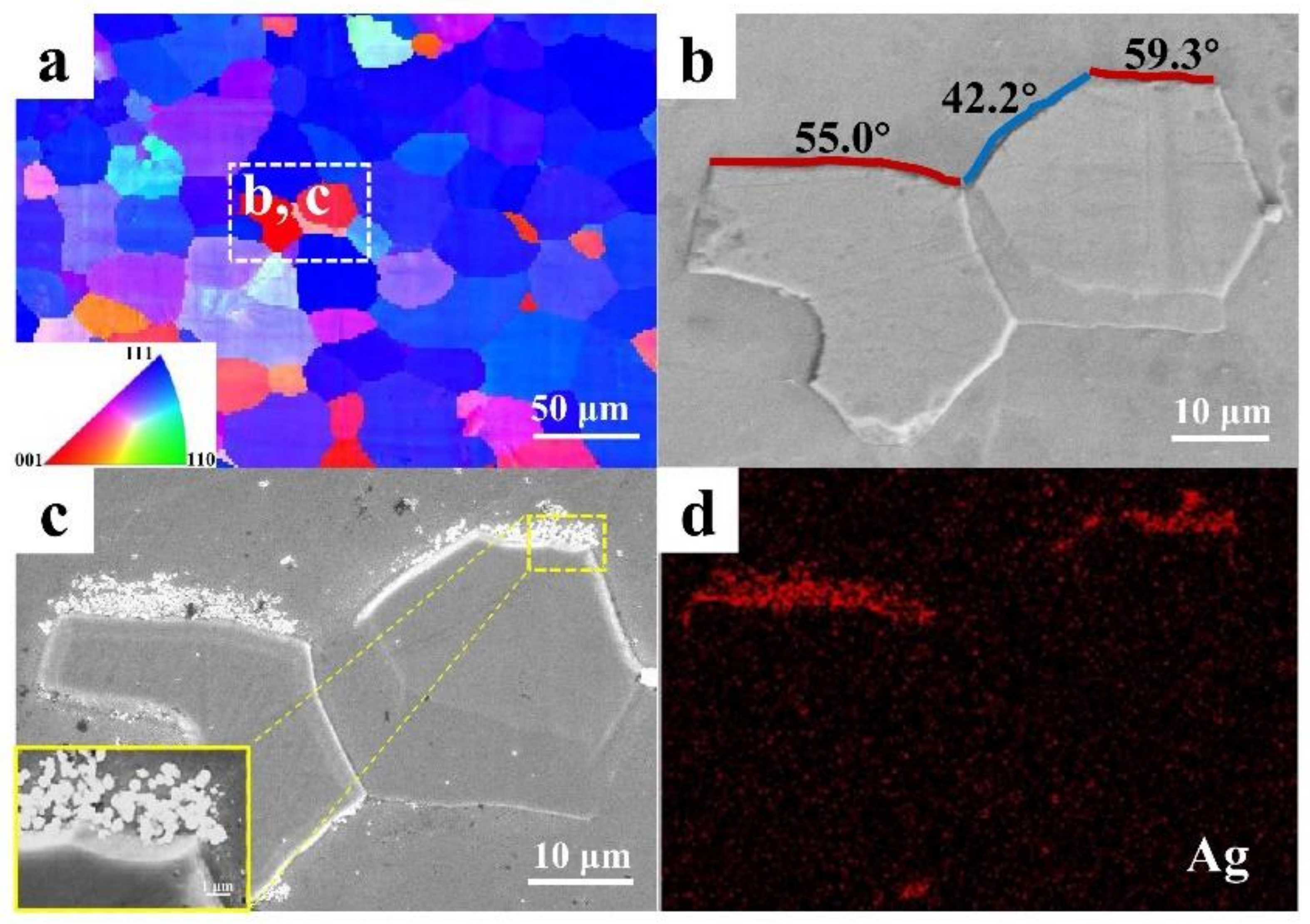
3.3. Orientation Statistics of Hydrogen-Induced Microcracks
3.4. The HMT Results of Hydrogen-Induced Microcracks
4. Discussion
4.1. The Orientation Dependence of HE Susceptibility
4.2. The Effect of Taylor Factor on Crack Propagation
5. Conclusions
- (1)
- IF steels showed obvious HE susceptibility after the SSRT test with hydrogen charging, and their decrease in the reduction in area reached 70% (Iψ), which may severely damage the materials’ deep drawing performance and uniform deformation capacity in applications.
- (2)
- Hydrogen diffusion and segregation in IF steel were dependent on the crystal orientation. Hydrogen preferentially agglomerated around {100} || ND-oriented grains in ferrite grains under the condition of a high concentration of diffusible hydrogen, which makes the {100} || ND brittle. Grains with a {100} || ND orientation were susceptible to hydrogen and easily cracked along the GBs, while grains with a {111} || ND orientation were insusceptible to hydrogen and resistant to cracking. The Σ3 GBs hindered crack propagation. {111} || ND-oriented grains with a high Taylor factor (>3) are difficult to deform and crack.
- (3)
- The misorientation of GBs at 50–60° angles in IF steels was prone to cracking in a hydrogen environment. This study confirms that HICs usually propagate along GBs with a large misorientation.
- (4)
- For the purpose of textural design, the best resistance against crack propagation results in minimizing the {100} plane || ND and increasing the desirable {111} || ND fiber components that are close to the compact planes. The formation of strong covalent bonds between two lattices linked together by a close-packed plane leads to obstacles to crack propagation and increases HIC resistance. Although there are differences between the laboratory (rapid evaluation) and engineering applications (much longer service time), we still believe that it may be one of the effective methods to improve the HE resistance of IF steel by appropriate rolling and heat treatment processes to optimize the microstructure (microtexture and GB characteristics), such as reducing the {100} || ND micro-oriented texture.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Kan, B.; Xu, J.; Li, J. The effect of second tempering on hydrogen embrittlement of ultra-high-strength steel. Metall. Mater. Trans. A 2020, 51, 2811–2821. [Google Scholar] [CrossRef]
- Nagao, A.; Smith, C.D.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. Interpretation of hydrogen-induced fracture surface morphologies for lath martensitic steel. Procedia Mater. Sci. 2014, 3, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Nagao, A.; Martin, M.L.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The effect of nanosized (Ti,Mo)C precipitates on hydrogen embrittlement of tempered lath martensitic steel. Acta Mater. 2014, 74, 244–254. [Google Scholar] [CrossRef]
- Shi, R.; Chen, L.; Wang, Z.; Yang, X.S.; Qiao, L.; Pang, X. Quantitative investigation on deep hydrogen trapping in tempered martensitic steel. J. Alloys Compd. 2021, 854, 157218. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D. Mechanisms of hydrogen trapping in austenitic, duplex, and super martensitic stainless steels. J. Alloys Compd. 2017, 720, 451–459. [Google Scholar] [CrossRef]
- Nagao, A.; Smith, C.D.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The role of hydrogen in hydrogen embrittlement fracture of lath martensitic steel. Acta Mater. 2012, 60, 5182–5189. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Hydrogen embrittlement behavior at different strain rates in low-carbon martensitic steel. Mater. Today Proc. 2015, 2, S735–S738. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of strain rate on hydrogen embrittlement in low-carbon martensitic steel. Int. J. Hydrog. Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Zhao, H.; Wang, L.; Jin, X. Hydrogen trapping sites and hydrogen-induced cracking in high strength quenching & partitioning (Q&P) treated steel. Int. J. Hydrog. Energy 2014, 39, 13031–13040. [Google Scholar]
- Liu, P.W.; Wu, J.K. Hydrogen susceptibility of an interstitial free steel. Mater. Lett. 2003, 57, 1224–1228. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. Hydrogen embrittlement of low carbon structural steel at macro-, micro-and nano-levels. Int. J. Hydrog. Energy 2020, 45, 2145–2156. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of low carbon structural steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Fu, H.; Wang, W.; Chen, X.; Pia, G.; Li, J. Fractal and multifractal analysis of fracture surfaces caused by hydrogen embrittlement in high-Mn twinning/transformation-induced plasticity steels. Appl. Surf. Sci. 2019, 470, 870–881. [Google Scholar] [CrossRef]
- Łabanowski, J.; Świerczyńska, A.; Topolska, S. Effect of microstructure on mechanical properties and corrosion resistance of 2205 duplex stainless steel. Pol. Marit. Res. 2014, 4, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, M.; Silva, C.C.; de Abreu, H.F.G. Effect of crystallographic orientations on the hydrogen-induced cracking resistance improvement of API 5L X70 pipeline steel under various thermomechanical processing. Corros. Sci. 2016, 111, 121–131. [Google Scholar] [CrossRef]
- Masoumi, M.; Tavares, S.S.M.; Pardal, J.M.; Martins, T.R.B.; da Silva, M.J.G.; de Abreu, H.F.G. The role of microstructure and grain orientations on intergranular cracking susceptibility of UNS 17400 martensitic stainless steel. Eng. Fail. Anal. 2017, 79, 198–207. [Google Scholar] [CrossRef]
- Masoumi, M.; Santos, L.P.M.; Bastos, I.N.; Tavares, S.S.; da Silva, M.J.; de Abreu, H.F.G. Texture and grain boundary study in high strength Fe–18Ni–Co steel related to hydrogen embrittlement. Mater. Des. 2016, 91, 90–97. [Google Scholar] [CrossRef]
- Masoumi, M.; Coelho, H.L.F.; Tavares, S.S.M.; Silva, C.C.; De Abreu, H.F.G. Effect of grain orientation and boundary distributions on hydrogen-induced cracking in low-carbon-content steels. Jom 2017, 69, 1368–1374. [Google Scholar] [CrossRef]
- Masoumi, M.; Silva, C.C.; Béreš, M.; Ladino, D.H.; de Abreu, H.F.G. Role of crystallographic texture on the improvement of hydrogen-induced crack resistance in API 5L X70 pipeline steel. Int. J. Hydrog. Energy 2017, 42, 1318–1326. [Google Scholar] [CrossRef]
- Masoumi, M.; Herculano, L.F.G.; de Abreu, H.F.G. Study of texture and microstructure evaluation of steel API 5L X70 under various thermomechanical cycles. Mater. Sci. Eng. A 2015, 639, 550–558. [Google Scholar] [CrossRef]
- Masoumi, M.; Ariza, E.A.; Sinatora, A.; Goldenstein, H. Role of crystallographic orientation and grain boundaries in fatigue crack propagation in used pearlitic rail steel. Mater. Sci. Eng. A 2018, 722, 147–155. [Google Scholar] [CrossRef]
- Venegas, V.; Caleyo, F.; Baudin, T.; Espina-Hernandez, J.H.; Hallen, J.M. On the role of crystallographic texture in mitigating hydrogen-induced cracking in pipeline steels. Corros. Sci. 2011, 53, 4204–4212. [Google Scholar] [CrossRef]
- Venegas, V.; Caleyo, F.; Baudin, T.; Hallen, J.M.; Penelle, R. Role of microtexture in the interaction and coalescence of hydrogen-induced cracks. Corros. Sci. 2009, 51, 1140–1145. [Google Scholar] [CrossRef]
- Ghosh, A.; Kundu, S.; Chakrabarti, D. Effect of crystallographic texture on the cleavage fracture mechanism and effective grain size of ferritic steel. Scr. Mater. 2014, 81, 8–11. [Google Scholar] [CrossRef]
- Schreiber, A.; Rosenkranz, C.; Lohrengel, M.M. Grain-dependent anodic dissolution of iron. Electrochim. Acta 2007, 52, 7738–7745. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Rahman, K.M.M.; Ouellet, R.; Szpunar, J.A. An extensive study of hydrogen-induced cracking susceptibility in an API X60 sour service pipeline steel. Int. J. Hydrog. Energy 2016, 41, 4185–4197. [Google Scholar] [CrossRef]
- Fu, H.; Wang, W.; Chen, X.; Pia, G.; Li, J. Grain boundary design based on fractal theory to improve intergranular corrosion resistance of TWIP steels. Mater. Des. 2020, 185, 108253. [Google Scholar] [CrossRef]
- Seita, M.; Hanson, J.P.; Gradečak, S.; Demkowicz, M.J. The dual role of coherent twin boundaries in hydrogen embrittlement. Nat. Commun. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Pérez, T.E.; García, J.O. Direct observation of hydrogen evolution in the electron microscope scale. Scr. Metall. 1982, 16, 161–164. [Google Scholar] [CrossRef]
- Ovejero-García, J. Hydrogen microprint technique in the study of hydrogen in steels. J. Mater. Sci. 1985, 20, 2623–2629. [Google Scholar] [CrossRef]
- Chen, S.S.; Wu, T.I.; Wu, J.K. Effects of deformation on hydrogen degradation in a duplex stainless steel. J. Mater. Sci. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Yalçì, H.K.; Edmonds, D.V. Application of the hydrogen microprint and the microautoradiography techniques to a duplex stainless steel. Mater. Charact. 1995, 34, 97–104. [Google Scholar] [CrossRef]
- Ronevich, J.A.; Speer, J.G.; Krauss, G.; Matlock, D.K. Improvement of the hydrogen microprint technique on AHSS steels. Metallogr. Microstruct. Anal. 2012, 1, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Ohmisawa, T.; Uchiyama, S.; Nagumo, M. Detection of hydrogen trap distribution in steel using a microprint technique. J. Alloys Compd. 2003, 356, 290–294. [Google Scholar] [CrossRef]
- Allen, Q.S.; Nelson, T.W. Microstructural evaluation of hydrogen embrittlement and successive recovery in advanced high strength steel. J. Mater. Processing Technol. 2019, 265, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, M.; Ueno, A.; Kishimoto, H. Analysis of hydrogen behaviour in crack growth tests of γ-TiAl by means of the hydrogen microprint technique. Intermetallics 2004, 12, 23–31. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Szpunar, J.A. Modeling of texture development during recrystallization of interstitial free steel. Acta Mater. 1997, 45, 2425–2434. [Google Scholar] [CrossRef]
- Hutchinson, W.B. Recrystallisation textures in iron resulting from nucleation at grain boundaries. Acta Metall. 1989, 37, 1047–1056. [Google Scholar] [CrossRef]
- Hutchinson, W.B. Development and control of annealing textures in low-carbon steels. Int. Met. Rev. 1984, 29, 25–42. [Google Scholar] [CrossRef]
- Ray, R.K.; Jonas, J.J.; Hook, R.E. Cold rolling and annealing textures in low carbon and extra low carbon steels. Int. Mater. Rev. 1994, 39, 129–172. [Google Scholar] [CrossRef]
- Chen, L.; Ma, Z.; Shi, R.; Su, Y.; Qiao, L.; Wang, L. Comprehensive effect of hydrostatic compressive stress in retained austenite on mechanical properties and hydrogen embrittlement of martensitic steels. Int. J. Hydrog. Energy 2020, 45, 22102–22112. [Google Scholar] [CrossRef]
- Yang, J.; Huang, F.; Guo, Z.; Rong, Y.; Chen, N. Effect of retained austenite on the hydrogen embrittlement of a medium carbon quenching and partitioning steel with refined microstructure. Mater. Sci. Eng. A 2016, 665, 76–85. [Google Scholar] [CrossRef]
- Roe, R.J. Description of crystallite orientation in polycrystalline materials. III. General solution to pole figure inversion. J. Appl. Phys. 1965, 36, 2024–2031. [Google Scholar] [CrossRef]
- De Knijf, D.; Nguyen-Minh, T.; Petrov, R.H.; Kestens, L.A.I.; Jonas, J.J. Orientation dependence of the martensite transformation in a quenched and partitioned steel subjected to uniaxial tension. J. Appl. Crystallogr. 2014, 47, 1261–1266. [Google Scholar] [CrossRef]
- Rugg, D.; Dixon, M.; Dunne, F.P.E. Effective structural unit size in titanium alloys. J. Strain Anal. Eng. Des. 2007, 42, 269–279. [Google Scholar] [CrossRef]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface energies of elemental crystals. Sci. Data 2016, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.G.; Wang, Z.J.; Sun, J.; Li, J.; Ma, E.; Shan, Z.W. In situ study of the initiation of hydrogen bubbles at the aluminium metal/oxide interface. Nat. Mater. 2015, 14, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, M.; Sinatora, A.; Goldenstein, H. Role of microstructure and crystallographic orientation in fatigue crack failure analysis of a heavy haul railway rail. Eng. Fail. Anal. 2019, 96, 320–329. [Google Scholar] [CrossRef]
- Ilyin, A.M. Some features of grain boundary segregations in sensitized austenitic stainless steel. J. Nucl. Mater. 1998, 252, 168–170. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Asundi, M.K. Precipitation hardening in 350 grade maraging steel. Metall. Trans. A 1993, 24, 2429–2442. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Atkinson, J.D.; Akid, R.; Parkins, R.N. Crack interaction, coalescence and mixed mode fracture mechanics. Fatigue Fract. Eng. Mater. Struct. 1996, 19, 427–439. [Google Scholar] [CrossRef]
- Kan, B.; Wu, W.; Yang, Z.; Li, J. Stress-induced hydrogen redistribution and corresponding fracture behavior of Q960E steel at different hydrogen content. Mater. Sci. Eng. A 2020, 775, 138963. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Szpunar, J.A. The role of grain boundary character distribution in secondary recrystallization of electrical steels. Acta Mater. 1997, 45, 1285–1295. [Google Scholar] [CrossRef]
- Dingreville, R.; Berbenni, S. On the interaction of solutes with grain boundaries. Acta Mater. 2016, 104, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Sanayei, M.; Das, S. Microstructural aspects of intergranular and transgranular crack propagation in an API X65 steel pipeline related to fatigue failure. Eng. Fail. Anal. 2018, 94, 214–225. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Bhuyan, P.; Mandal, S. Individual and synergistic influences of microstructural features on intergranular corrosion behavior in extra-low carbon type 304L austenitic stainless steel. Corros. Sci. 2018, 139, 319–332. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Seo, H.J.; Kim, J.N.; Lee, C.S. Effect of grain boundary engineering on hydrogen embrittlement in Fe-Mn-C TWIP steel at various strain rates. Corros. Sci. 2018, 142, 213–221. [Google Scholar] [CrossRef]
- Roach, M.D.; Wright, S.I.; Lemons, J.E.; Zardiackas, L.D. An EBSD based comparison of the fatigue crack initiation mechanisms of nickel and nitrogen-stabilized cold-worked austenitic stainless steels. Mater. Sci. Eng. A 2013, 586, 382–391. [Google Scholar] [CrossRef]



| Component | C | Mn | S | P | Si | N | Al | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Content wt% | 0.0067 | 0.012 | 0.0083 | 0.0086 | 0.0083 | 0.0036 | 0.020 | 0.066 | Bal. |
| Orientation | {100} | {110} | {111} | Other |
|---|---|---|---|---|
| Percentage (%) | 4.73 | 2.93 | 78.70 | 13.64 |
| Orientation | {100} | {110} | {111} | Other |
|---|---|---|---|---|
| Average no. of grains | 166 | 103 | 2762 | 479 |
| No. of intergranular cracks | 16 | 2 | 50 | 8 |
| No. of cracks per 103 grains | 96.4 | 19.4 | 18.1 | 16.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Fu, H.; Zhang, H.; Yan, Y.; Li, J. Effect of Grain Orientation on Hydrogen Embrittlement Behavior of Interstitial-Free Steel. Metals 2022, 12, 981. https://doi.org/10.3390/met12060981
Wang W, Fu H, Zhang H, Yan Y, Li J. Effect of Grain Orientation on Hydrogen Embrittlement Behavior of Interstitial-Free Steel. Metals. 2022; 12(6):981. https://doi.org/10.3390/met12060981
Chicago/Turabian StyleWang, Wei, Hao Fu, Hailong Zhang, Yu Yan, and Jinxu Li. 2022. "Effect of Grain Orientation on Hydrogen Embrittlement Behavior of Interstitial-Free Steel" Metals 12, no. 6: 981. https://doi.org/10.3390/met12060981
APA StyleWang, W., Fu, H., Zhang, H., Yan, Y., & Li, J. (2022). Effect of Grain Orientation on Hydrogen Embrittlement Behavior of Interstitial-Free Steel. Metals, 12(6), 981. https://doi.org/10.3390/met12060981







