Study on the Effect of A/B Site Co-Doping on the Oxygen Evolution Reaction Performance of Strontium Cobaltite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Material Synthesis
2.3. Material Characterization
2.4. Electrochemical Measurement
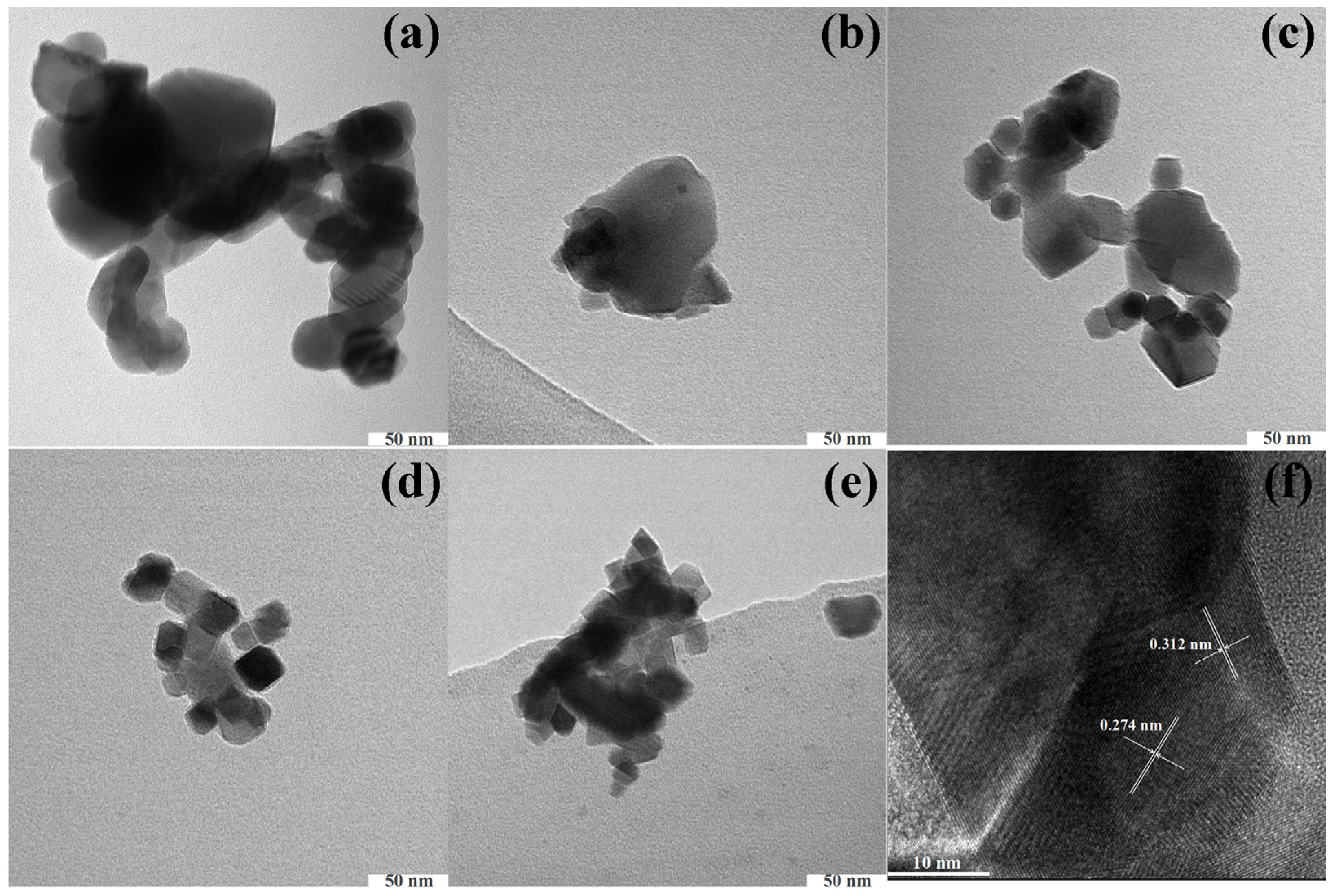
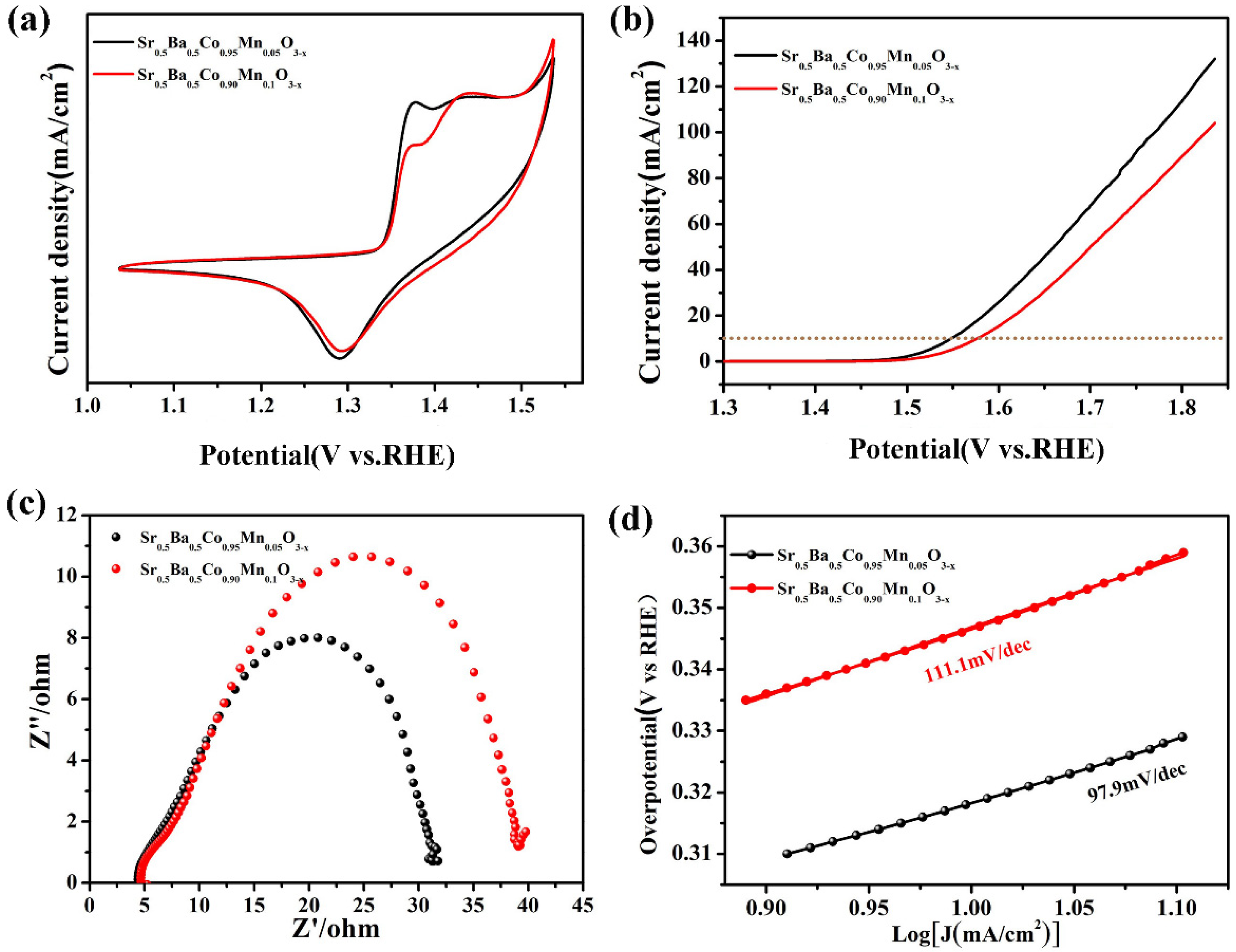
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Bockris, J. Hydrogen no longer a high cost solution to global warming: New ideas. Int. J. Hydrogen Energy 2008, 33, 2129–2131. [Google Scholar] [CrossRef]
- Li, M.; Zhuang, L.; Wang, Z.; ur Rehman, A.; Liu, L.X.; Zhu, Z. Strontium-doped lanthanum iron nickelate oxide as highly efficient electrocatalysts for oxygen evolution reaction. J. Colloid Interface Sci. 2019, 553, 813–819. [Google Scholar] [CrossRef]
- Xia, B.; Wang, T.; Ran, J.; Jiang, S.; Gao, X.; Gao, D. Optimized conductivity and spin states in N-doped LaCoO3 for oxygen electrocatalysis. ACS Appl. Mater. Interfaces 2021, 13, 2447–2454. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Dyakonov, V.; Olthof, S.; Ünlü, F.; Lê, K.M.T.; Mathur, S.; Karabanov, A.D.; Lupascu, D.C.; Herz, L.M.; Hinderhofer, A.; et al. Roadmap on organic–inorganic hybrid perovskite semiconductors and devices. APL Mater. 2021, 9, 109202. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, Y.; Yin, Y.; Tahini, H.A.; Guan, D.; Dong, F.; Lu, Q.; Smith, S.C.; Zhang, X.; Wang, H.; et al. Super-exchange interaction induced overall optimization in ferromagnetic perovskite oxides enables ultrafast water oxidation. Small 2019, 15, 1903120. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Xi, S.; Ren, X.; Zhou, Y.; Zhang, G.; Yang, H.; Du, Y.; Xu, Z.J. Tailoring the Co 3d-O 2p covalency in LaCoO3 by Fe substitution to promote oxygen evolution reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Liu, J.; Jia, E.; Wang, L.; Stoerzinger, K.A.; Zhou, H.; Tang, C.S.; Yin, X.; He, X.; Bousquet, E.; Bowden, M.E.; et al. Tuning the electronic structure of LaNiO3 through alloying with strontium to enhance oxygen evolution activity. Adv. Sci. 2019, 6, 1901073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Zhong, H.; Li, J.; Zhang, X.; Shi, J.; Cai, W.; Qu, K.; Zhu, C.; Yang, Z.; Beckman, S.P.; et al. Engineering highly active oxygen sites in perovskite oxides for stable and efficient oxygen evolution. Appl. Catal. B Environ. 2019, 256, 117817. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Díaz-Morales, O.A.; Kolb, M.J.; Koper, M.T. Why is bulk thermochemistry a good descriptor for the electrocatalytic activity of transition metal oxides. ACS Catal. 2015, 5, 869–873. [Google Scholar] [CrossRef]
- Da, Y.; Zeng, L.; Wang, C.; Gong, C.; Cui, L. A simple approach to tailor OER activity of SrxCo0.8Fe0.2O3 perovskite catalysts. Electrochim. Acta 2019, 300, 85–92. [Google Scholar] [CrossRef]
- Jeen, H.; Bi, Z.; Choi, W.S.; Chisholm, M.F.; Bridges, C.A.; Paranthaman, M.P.; Lee, H.N. Orienting oxygen vacancies for fast catalytic reaction. Adv. Mater. 2013, 25, 6459–6463. [Google Scholar] [CrossRef] [Green Version]
- Aguadero, A.; Pérez, C.D.; Alonso, J.A.; Skinner, S.J.; Kilner, J. A new family of Mo-doped SrCoO3-δ perovskites for application in reversible solid state electrochemical cells. Chem. Mater. 2016, 24, 2655–2663. [Google Scholar] [CrossRef]
- Mitra, C.; Meyer, T.; Lee, H.N.; Reboredo, F.A. Oxygen diffusion pathways in brownmillerite SrCoO2.5: Influence of structure and chemical potential. J. Chem. Phys. 2014, 141, 084710. [Google Scholar] [CrossRef] [Green Version]
- Cascos, V.; Martínez, C.R.; Alonso, J.A. New Nb-doped SrCo1−xNbxO3-δ perovskites performing ascathodes in solid-oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 14349–14354. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, Z.; Ran, R.; Jin, W.; Xu, N. A novel efficient oxide electrode for electrocatalytic oxygenreduction at 400–600 °C. Chem. Commun. 2008, 44, 5791. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.G.; Zhou, W.; Jiang, S.; Zou, J.; Shao, Z. An A-site-deficient perovskite offers high activity and stability for low-temperature solid-oxide fuel cells. ChemSusChem 2013, 6, 2249–2254. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Zhou, W.; Zhu, Y.; Chen, Y.; Shao, Z. Co-doping strategy for developing perovskite oxides as highly efficient electrocatalysts for oxygen evolution reaction. Adv. Sci. 2016, 3, 1500187. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Wei, H.; Liu, Y.; Li, L.; Yang, X.; Zhang, X.; Liu, H.; Lu, Z. Sr, Fe co-doped perovskite oxides with high performance for oxygen evolution reaction. Front. Chem. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhu, Y.; Hu, Z.; Yin, Y.; Lin, H.; Chen, C.; Zhang, X.; Shao, Z. Boosting the oxygen evolution catalytic performance of perovskites via optimizing calcination temperature. J. Mater. Chem. A 2020, 8, 6480–6486. [Google Scholar] [CrossRef]
- Shaojie, F.; Jianxin, Y. TPD/TPR investigation of the stabilization of Fe doping for SrCoO3-δ. J. Anhui Inst. Archit. Ind. 2006, 14, 45–48. [Google Scholar]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeis, J. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-doped perovskite oxide as highly efficient water oxidation electrocatalyst in alkaline solution. Adv. Funct. Mater. 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Jarvis, K.A.; Therese, S.; Ferreira, P.J.; Manthiram, A. Spinel-type lithium cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen reduction reactions. Nat. Commun. 2014, 5, 3949. [Google Scholar] [CrossRef] [Green Version]
- Xiaoguang, D.; Chao, S.; Jie, M.; Zhong, Y.; Shao, Z.; Wang, S.; Sun, H. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals. Appl. Catal. B Environ. 2018, 220, 626–634. [Google Scholar]
- Peña, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.; Myers, D. Activity–stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Jeong, M.G.; Oh, I.H.; Nam, K.W.; Jung, H.G. Role of strontium as doping agent in LaMn0.5Ni0.5O3 for oxygen electro-catalysis. J. Ind. Eng. Chem. 2020, 85, 94–101. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, M.; Liang, F.; Smith, S.C.; Zhu, Z. High activity and durability of novel perovskite electrocatalysts for water oxidation. Mater. Horiz. 2015, 2, 495–501. [Google Scholar] [CrossRef]
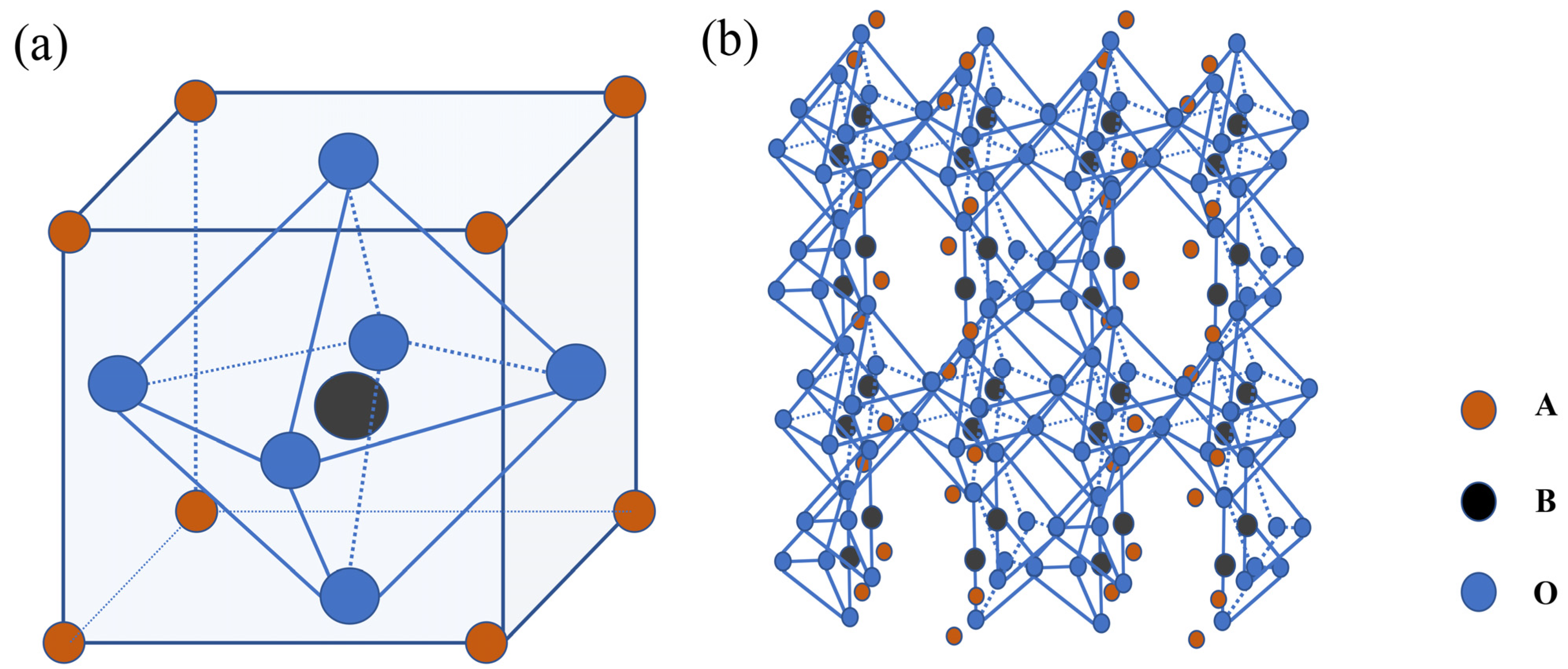
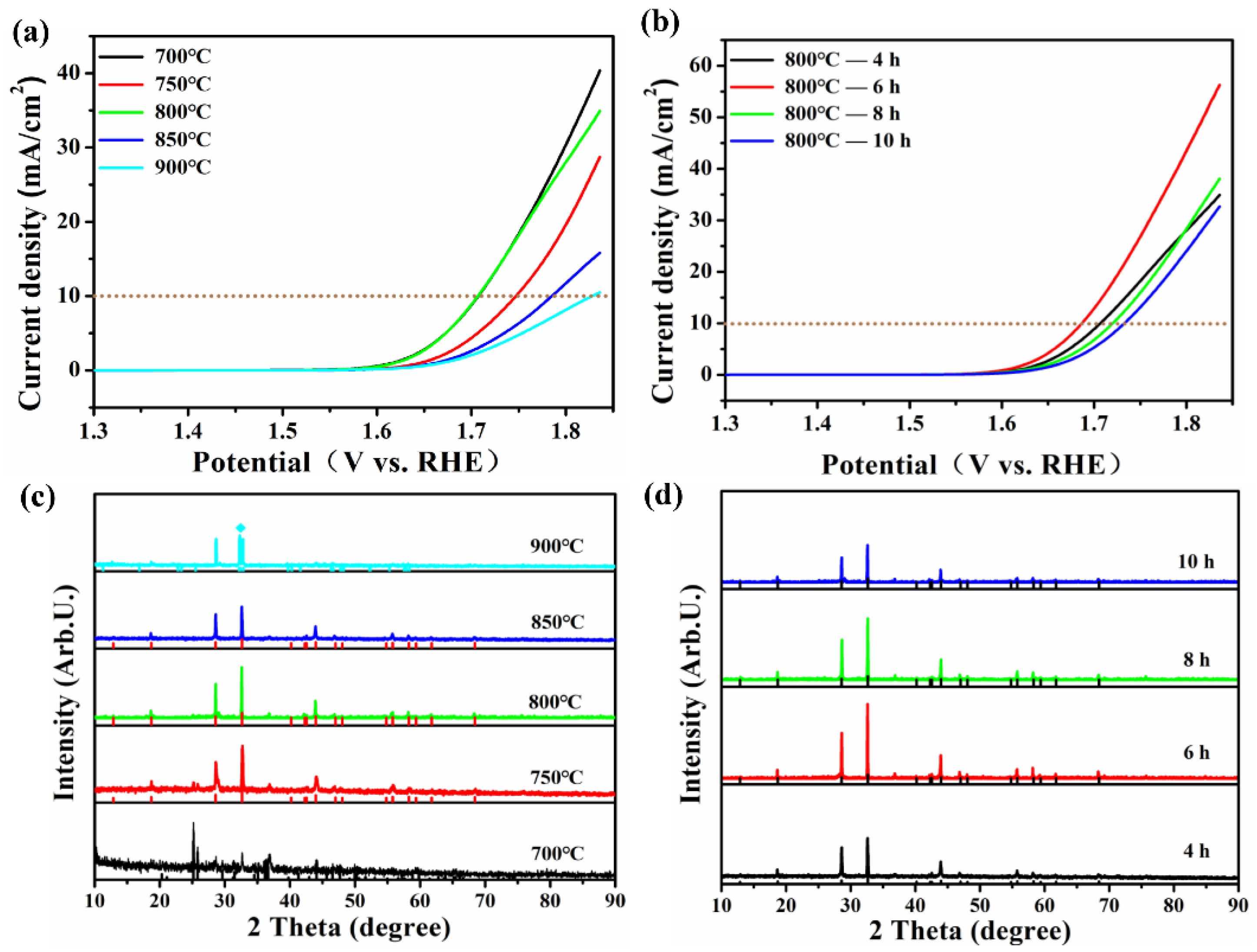

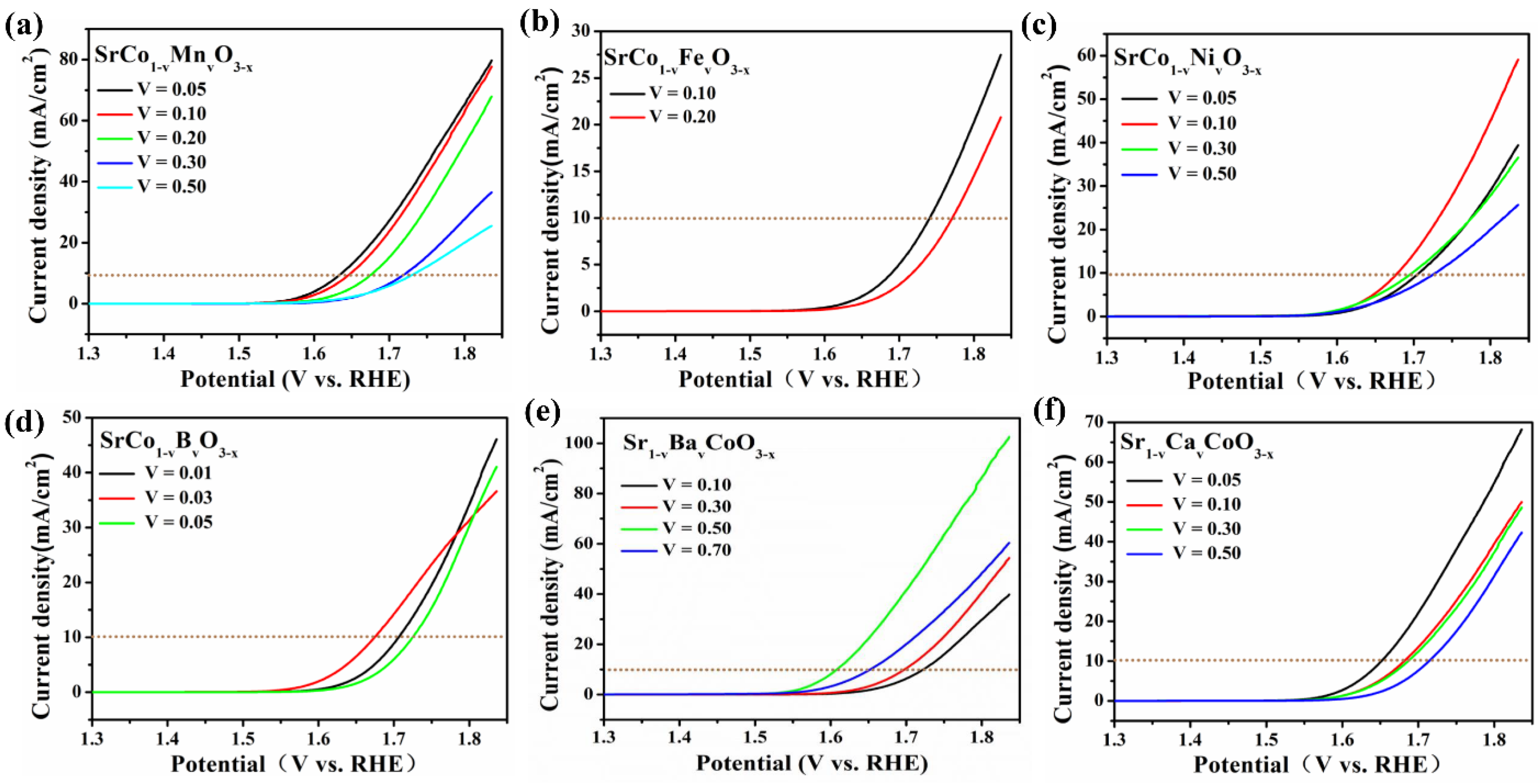
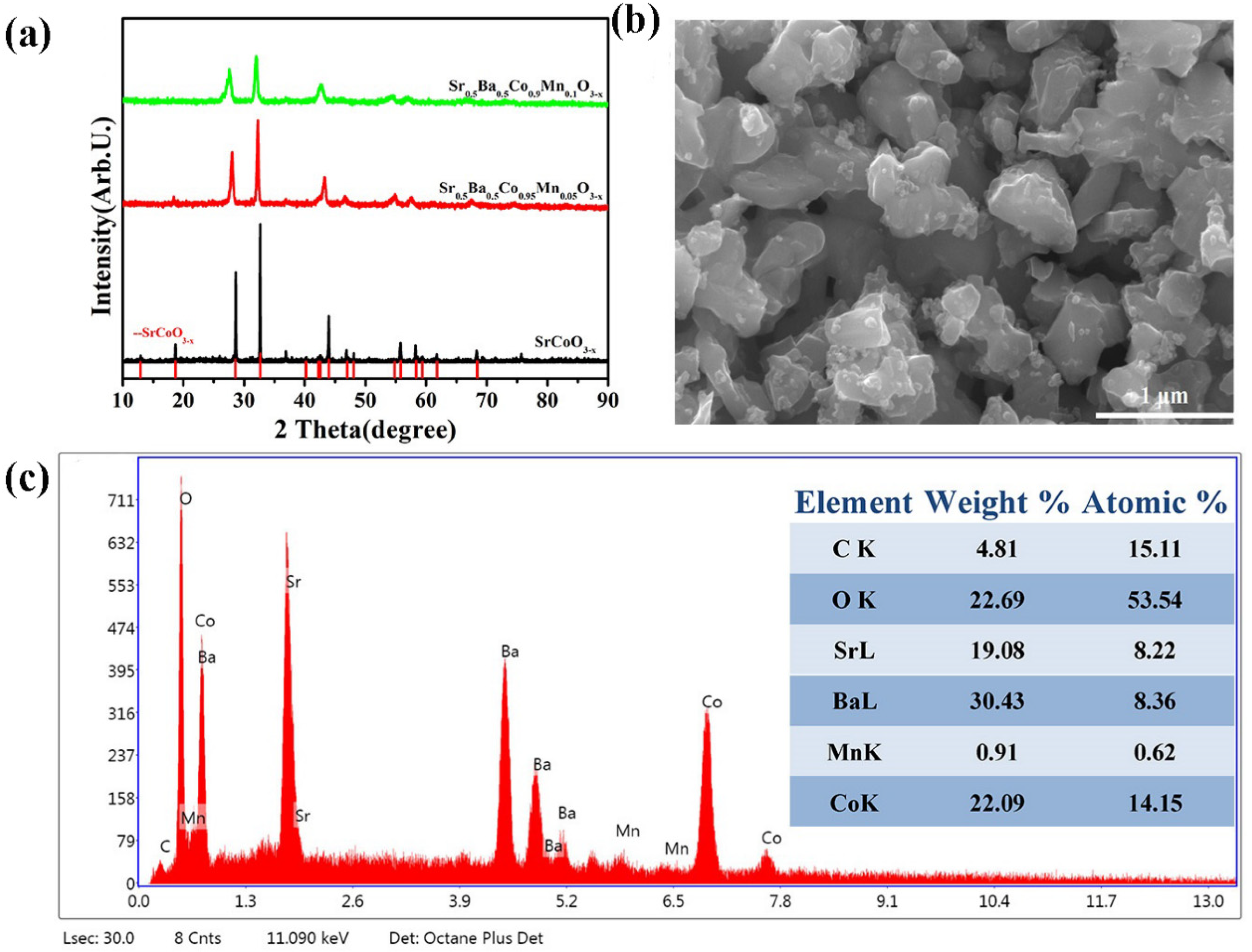


| Catalyst | Co2+% | Co3+% | Co3+/Co2+ | OL% | Mn3+ | Mn4+ | Mn3+/Mn4+ |
|---|---|---|---|---|---|---|---|
| SrCoO3−x | 44 | 56 | 1.27 | 14 | —— | —— | —— |
| SrCo0.95Mn0.05O3−x | 42 | 58 | 1.38 | 21 | 54 | 46 | 1.17 |
| Sr0.5Ba0.5CoO3−x | 22 | 78 | 3.54 | 27 | —— | —— | —— |
| Sr0.5Ba0.5Co0.95Mn0.05O3−x | 26 | 74 | 2.84 | 32 | 58 | 42 | 1.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Mu, X.; Ren, Y.; Guo, J.; Wei, H.; Liu, H.; Lu, Z. Study on the Effect of A/B Site Co-Doping on the Oxygen Evolution Reaction Performance of Strontium Cobaltite. Metals 2022, 12, 991. https://doi.org/10.3390/met12060991
Song S, Mu X, Ren Y, Guo J, Wei H, Liu H, Lu Z. Study on the Effect of A/B Site Co-Doping on the Oxygen Evolution Reaction Performance of Strontium Cobaltite. Metals. 2022; 12(6):991. https://doi.org/10.3390/met12060991
Chicago/Turabian StyleSong, Shihao, Xiaoming Mu, Yanwei Ren, Jia Guo, Haifei Wei, Heyan Liu, and Zunming Lu. 2022. "Study on the Effect of A/B Site Co-Doping on the Oxygen Evolution Reaction Performance of Strontium Cobaltite" Metals 12, no. 6: 991. https://doi.org/10.3390/met12060991
APA StyleSong, S., Mu, X., Ren, Y., Guo, J., Wei, H., Liu, H., & Lu, Z. (2022). Study on the Effect of A/B Site Co-Doping on the Oxygen Evolution Reaction Performance of Strontium Cobaltite. Metals, 12(6), 991. https://doi.org/10.3390/met12060991





