Magnetic-Field-Induced Strain Enhances Electrocatalysis of FeCo Alloys on Anode Catalysts for Water Splitting
Abstract
:1. Introduction
2. Experimental Process
2.1. Materials Preparations
2.2. Material Characterizations
2.3. Electrochemical Measurements
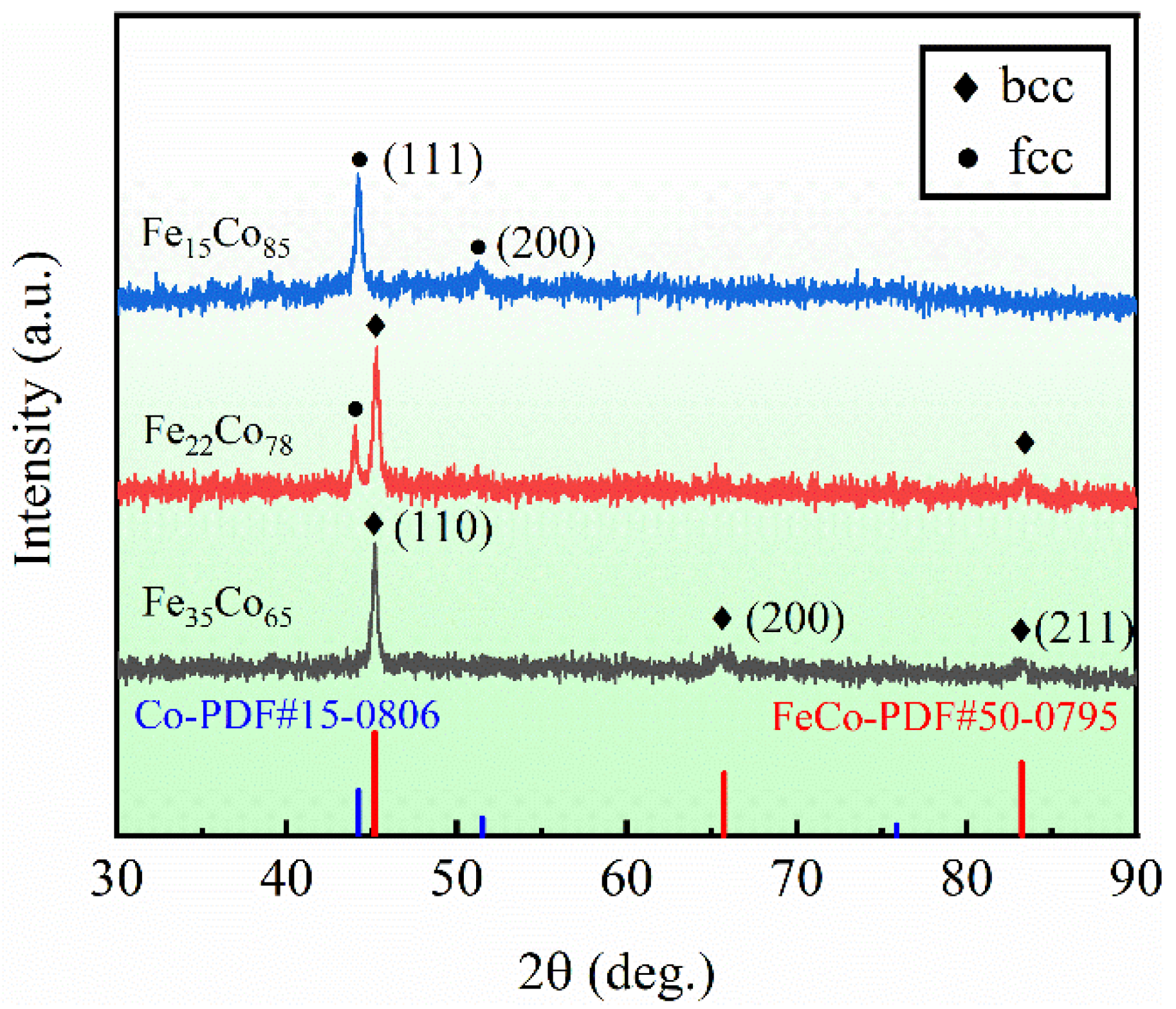
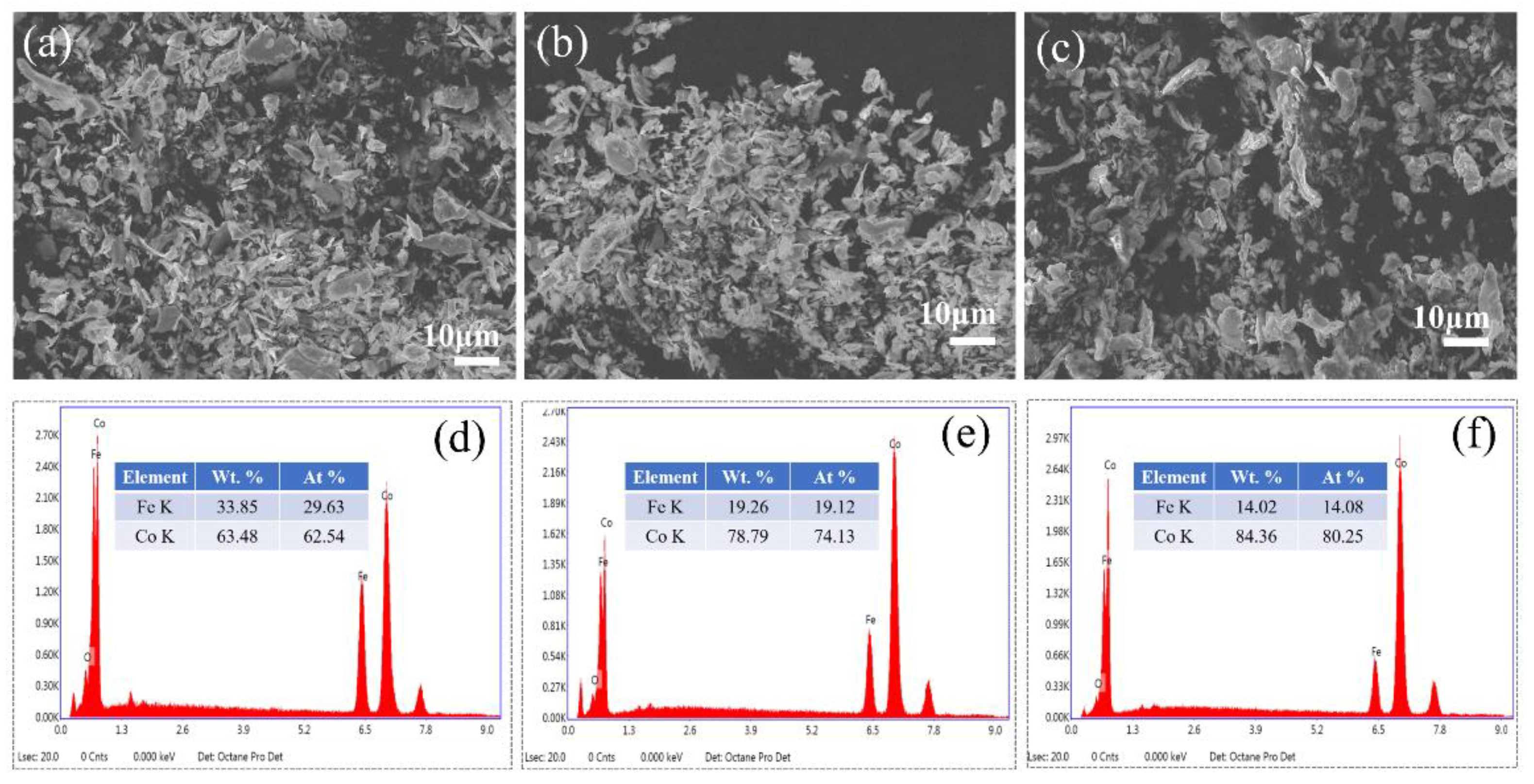
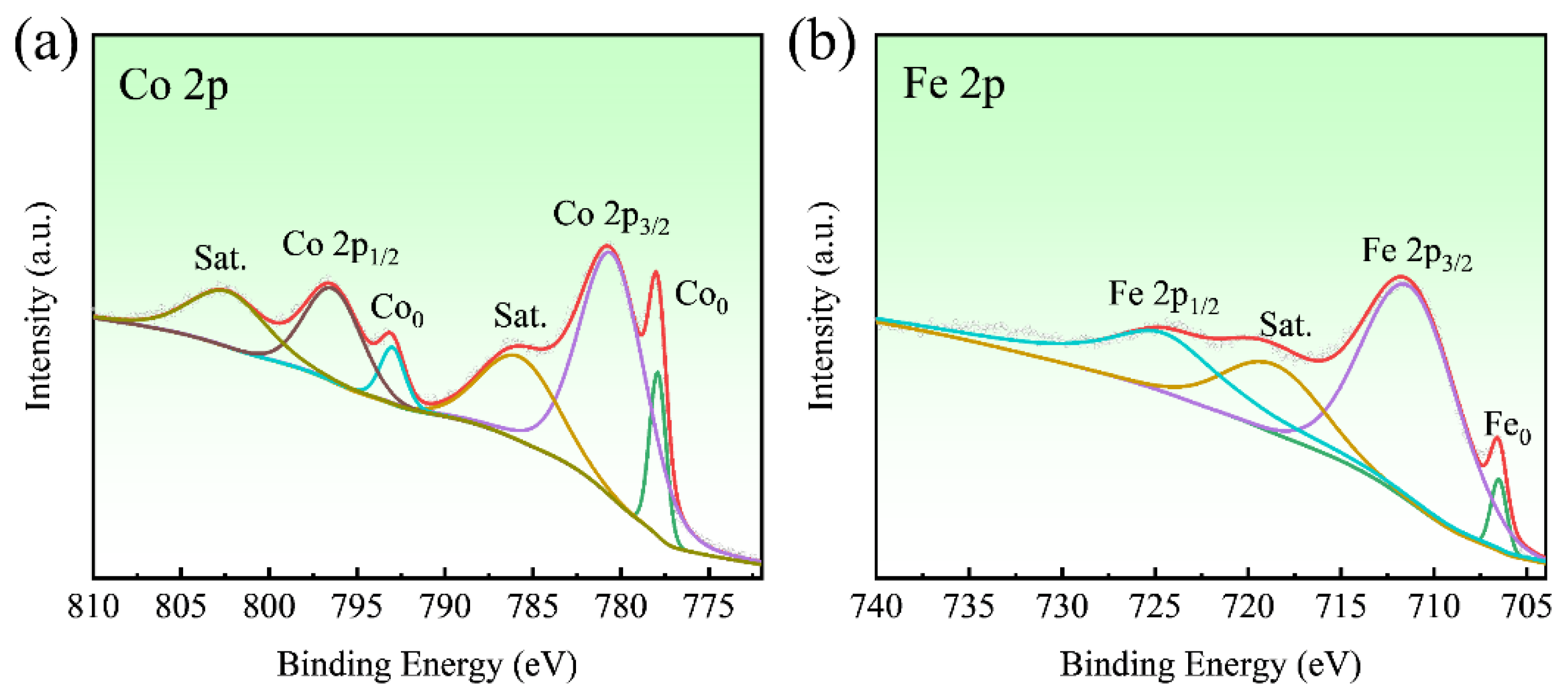
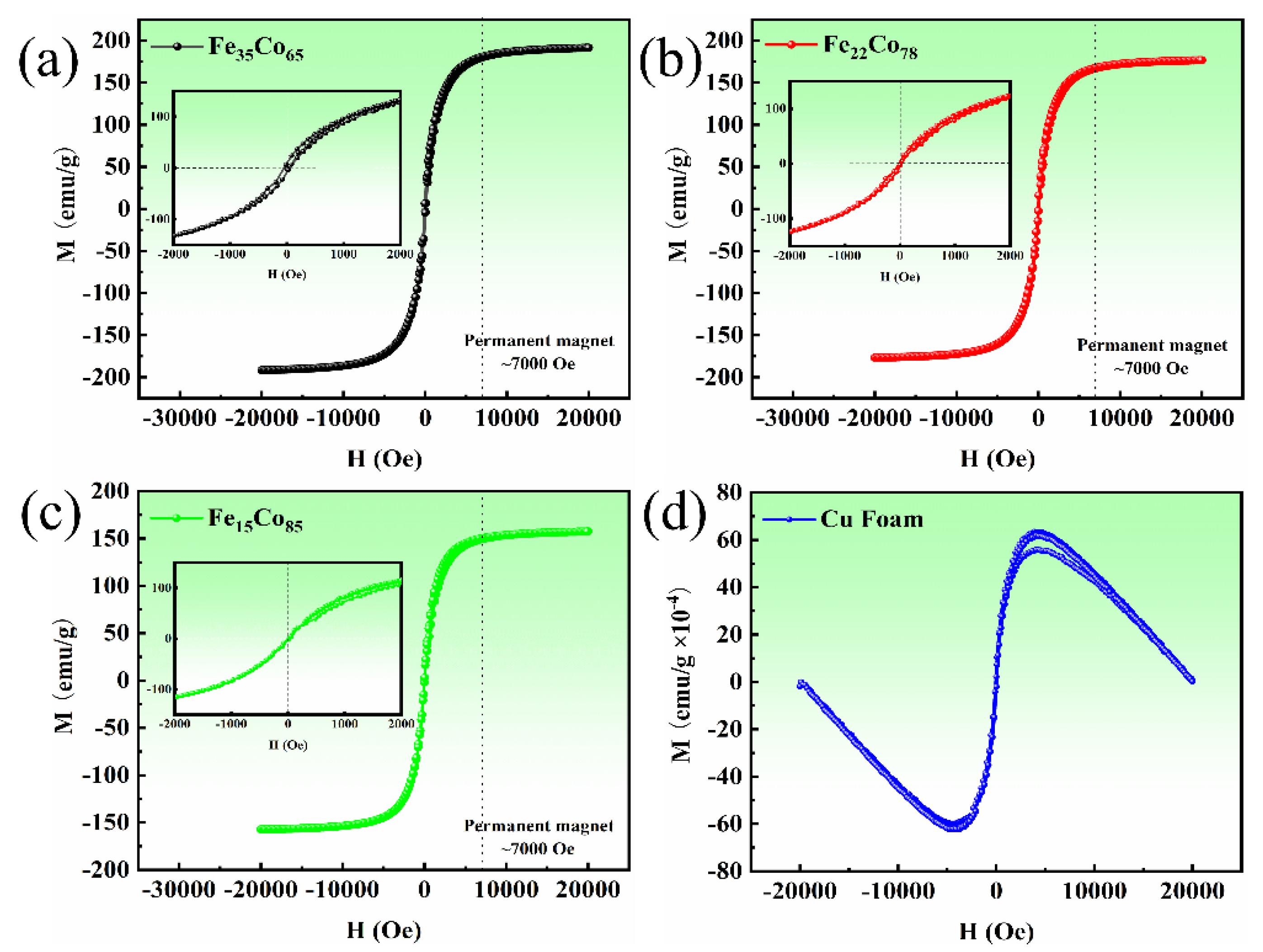
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, R.; Chen, H.; Wang, H.; Wei, P.; Huang, H.; Sun, X. Recent advances in the development of water oxidation electrocatalysts at mild pH. Small 2019, 15, 1805103. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Zhu, C.; Du, D.; Lin, Y. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 2019, 48, 3181–3192. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.; Wang, E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt–iron (oxy) hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Lyu, F.; Wang, Q.; Choi, S.M.; Yin, Y. Noble-metal-free electrocatalysts for oxygen evolution. Small 2019, 15, 1804201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, X.; Dastafkan, K.; Zhao, C. Design of multi-metallic-based electrocatalysts for enhanced water oxidation. ChemPhysChem 2019, 20, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, Y.; Chen, H.M.; Hu, Z.; Hung, S.F.; Ma, N.; Shao, Z. An amorphous nickel–iron-based electrocatalyst with unusual local structures for ultrafast oxygen evolution reaction. Adv. Mater. 2019, 31, 1900883. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, G.; Yao, C.; Chen, H.; Hu, J.; Zhu, Y.; Liang, W. Porous amorphous FeCo alloys as pre-catalysts for promoting the oxygen evolution reaction. J. Alloys Compd. 2020, 828, 154465. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.H.; Wang, X.Y.; Zhang, J.; Han, B.H. N-doped graphitic carbon shell-encapsulated FeCo alloy derived from metal–polyphenol network and melamine sponge for oxygen reduction, oxygen evolution, and hydrogen evolution reactions in alkaline media. J. Colloid Interface Sci. 2021, 581, 362–373. [Google Scholar] [CrossRef]
- Mattioli, G.; Giannozzi, P.; Amore Bonapasta, A.; Guidoni, L. Reaction pathways for oxygen evolution promoted by cobalt catalyst. J. Am. Chem. Soc. 2013, 135, 15353–15363. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, Z.; Guo, Z. Water electrolysis enhanced by super gravity field for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 3198–3205. [Google Scholar] [CrossRef]
- Liu, G.; Li, P.; Zhao, G.; Wang, X.; Kong, J.; Liu, H.; Ye, J. Promoting active species generation by plasmon-induced hot-electron excitation for efficient electrocatalytic oxygen evolution. J. Am. Chem. Soc. 2016, 138, 9128–9136. [Google Scholar] [CrossRef]
- Su, R.; Hsain, H.A.; Wu, M.; Zhang, D.; Hu, X.; Wang, Z.; Pennycook, S.J. Nano-Ferroelectric for High Efficiency Overall Water Splitting under Ultrasonic Vibration. Angew. Chem. Int. Ed. 2019, 58, 15076–15081. [Google Scholar] [CrossRef]
- Wang, J.; Yan, M.; Zhao, K.; Liao, X.; Wang, P.; Pan, X.; Mai, L. Field effect enhanced hydrogen evolution reaction of MoS2 nanosheets. Adv. Mater. 2017, 29, 1604464. [Google Scholar] [CrossRef]
- Garcés-Pineda, F.A.; Blasco-Ahicart, M.; Nieto-Castro, D.; López, N.; Galán-Mascarós, J.R. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy 2019, 4, 519–525. [Google Scholar] [CrossRef]
- Niether, C.; Faure, S.; Bordet, A.; Deseure, J.; Chatenet, M.; Carrey, J.; Rouet, A. Improved water electrolysis using magnetic heating of FeC–Ni core–shell nanoparticles. Nat. Energy 2018, 3, 476–483. [Google Scholar] [CrossRef]
- Gatard, V.; Deseure, J.; Chatenet, M. Use of magnetic fields in electrochemistry: A selected review. Curr. Opin. Electrochem. 2020, 23, 96–105. [Google Scholar] [CrossRef]
- Elias, L.; Hegde, A.C. Effect of magnetic field on HER of water electrolysis on Ni–W alloy. Electrocatalysis 2017, 8, 375–382. [Google Scholar] [CrossRef]
- Westsson, E.; Picken, S.; Koper, G. The Effect of Magnetic Field on Catalytic Properties in Core-Shell Type Particles. Front. Chem. 2020, 8, 163. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Peng, J.; Zhang, W.; Peng, K. Magnetic field enhancing electrocatalysis of Co3O4/NF for oxygen evolution reaction. J. Power Sources 2019, 433, 226704. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, T.; Liu, Y.; Zhang, W.; Yin, Z.; Ji, Z.; Wei, J. Magnetic Field Enhanced 4-electron Pathway of the Well-aligned Co3O4/ECNFs in the Oxygen Reduction Reaction. ChemSusChem 2018, 11, 580–588. [Google Scholar] [CrossRef]
- Hunter, D.; Osborn, W.; Wang, K.; Wang, K.; Kazantseva, N.; Hattrick-Simpers, J.; Suchoski, R.; Takeuchi, I. Giant magnetostriction in annealed Co1−xFex thin-films. Nat. Commun. 2011, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Wang, H.; Zhang, T.; He, Y.; Coey, J.M.D.; Jiang, C. Tailoring the heterogeneous magnetostriction in Fe-Co alloys. J. Alloys Compd. 2017, 699, 200–209. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, S. Strain engineering of metal-based nanomaterials for energy electrocatalysis. Chem. Soc. Rev. 2019, 48, 3265–3278. [Google Scholar] [CrossRef]
- Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Kodama, D.; Shinoda, K.; Sato, K.; Konno, Y.; Joseyphus, R.J.; Motomiya, K.; Jeyadevan, B. Chemical synthesis of sub-micrometer-to nanometer-sized magnetic FeCo dice. Adv. Mater. 2006, 18, 3154–3159. [Google Scholar] [CrossRef]
- Szumiata, T.; Gzik-Szumiata, M.; Brzózka, K.; Górka, B.; Gawroński, M.; Finkel, A.C.; Morley, N.A. Interface influence on the properties of Co90Fe10 films on soft magnetic underlayers–Magnetostrictive and Mössbauer spectrometry studies. J. Magn. Magn. Mater. 2016, 401, 943–948. [Google Scholar] [CrossRef]
- Brzózka, K.; Szumiata, T.; Olekšáková, D.; Kollár, P. Structural and Magnetic Properties of Mechanically Alloyed Fe50Co50 Systems. Acta Phys. Pol. A. 2020, 137, 726–729. [Google Scholar] [CrossRef]
- Wu, L.Z.; Ding, J.; Jiang, H.B.; Chen, L.F.; Ong, C.K. Particle size influence to the microwave properties of iron based magnetic particulate composites. J. Magn. Magn. Mater. 2005, 285, 233–239. [Google Scholar] [CrossRef]
- Song, J.; Lv, X.; Jiao, Y.; Wang, P.; Xu, M.; Li, T.; Zhang, Z. Catalyst nanoarchitecturing via functionally implanted cobalt nanoparticles in nitrogen doped carbon host for aprotic lithium-oxygen batteries. J. Power Sources 2018, 394, 122–130. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Kashyap, V.; Bhange, S.N.; Baek, J.B.; Kurungot, S. Nanoporous graphene enriched with Fe/Co-N active sites as a promising oxygen reduction electrocatalyst for anion exchange membrane fuel cells. Adv. Funct. Mater. 2016, 26, 2150–2162. [Google Scholar] [CrossRef]
- Xiao, X.; Li, X.; Yu, G.; Wang, J.; Yan, G.; Wang, Z.; Guo, H. FeCox alloy nanoparticles encapsulated in three-dimensionally N-doped porous carbon/multiwalled carbon nanotubes composites as bifunctional electrocatalyst for zinc-air battery. J. Power Sources 2019, 438, 227019. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, L.; Liu, H.; Chen, T.; Yin, S.; Liu, M. Effects of magnetic field on water electrolysis using foam electrodes. Int. J. Hydrog. Energy 2019, 44, 1352–1358. [Google Scholar] [CrossRef]
- Iida, T.; Matsushima, H.; Fukunaka, Y. Water electrolysis under a magnetic field. J. Electrochem. Soc. 2007, 154, E112. [Google Scholar] [CrossRef]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting carbon nitride and titanium carbide nanosheets for high-performance oxygen evolution. Angew. Chem. 2016, 128, 1150–1154. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Puente Santiago, A.R.; Hong, Y.; Zhang, N.; Cano, M.; Rodriguez-Castellon, E.; Noveron, J.C. Tuning of trifunctional NiCu bimetallic nanoparticles confined in a porous carbon network with surface composition and local structural distortions for the electrocatalytic oxygen reduction, oxygen and hydrogen evolution reactions. J. Am. Chem. Soc. 2020, 142, 14688–14701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, M.; Guo, M.; Hong, A.; Yu, T.; Luo, X.; Wang, S. Magnetic enhancement for hydrogen evolution reaction on ferromagnetic MoS2 catalyst. Nano Lett. 2020, 20, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Rajamathi, C.R.; Kumar, N.; Li, G.; Sun, Y.; Shekhar, C.; Rao, C.N.R. Effect of magnetic field on the hydrogen evolution activity using non-magnetic Weyl semimetal catalysts. Dalton Trans. 2020, 49, 3398–3402. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wu, T.; Sun, Y.; Li, Y.; Xian, G.; Liu, X.; Xu, Z.J. Spin-polarized oxygen evolution reaction under magnetic field. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.; Zhang, Y.; Xia, S.; Yu, J.; Ding, B. Direct magnetic reinforcement of electrocatalytic ORR/OER with electromagnetic induction of magnetic catalysts. Adv. Mater. 2021, 33, 2007525. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Yang, J.; Yang, Y.; Wang, R.; Li, S.; Ji, S. The effect of the internal magnetism of ferromagnetic catalysts on their catalytic activity toward oxygen reduction reaction under an external magnetic field. Ionics 2016, 22, 2195–2202. [Google Scholar] [CrossRef] [Green Version]
- Monzon, L.M.A.; Rode, K.; Venkatesan, M.; Coey, J.M.D. Electrosynthesis of iron, cobalt, and zinc microcrystals and magnetic enhancement of the oxygen reduction reaction. Chem. Mater. 2012, 24, 3878–3885. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, S.; Jia, Y.; Xiong, X.; Yang, H.; Liu, S.; Liu, B. NiFe hydroxide lattice tensile strain: Enhancement of adsorption of oxygenated intermediates for efficient water oxidation catalysis. Angew. Chem. Int. Ed. 2019, 58, 736–740. [Google Scholar] [CrossRef]
- Han, M.; Li, S.; Li, C.; Wu, J.; Han, J.; Wang, N.; Liang, H. Strain-modulated Ni3Al alloy promotes oxygen evolution reaction. J. Alloys Compd. 2020, 844, 156094. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ren, Y.; Wang, K.; Mu, X.; Song, S.; Guo, J.; Yang, X.; Lu, Z. Magnetic-Field-Induced Strain Enhances Electrocatalysis of FeCo Alloys on Anode Catalysts for Water Splitting. Metals 2022, 12, 800. https://doi.org/10.3390/met12050800
Liu H, Ren Y, Wang K, Mu X, Song S, Guo J, Yang X, Lu Z. Magnetic-Field-Induced Strain Enhances Electrocatalysis of FeCo Alloys on Anode Catalysts for Water Splitting. Metals. 2022; 12(5):800. https://doi.org/10.3390/met12050800
Chicago/Turabian StyleLiu, Heyan, Yanwei Ren, Kai Wang, Xiaoming Mu, Shihao Song, Jia Guo, Xiaojing Yang, and Zunming Lu. 2022. "Magnetic-Field-Induced Strain Enhances Electrocatalysis of FeCo Alloys on Anode Catalysts for Water Splitting" Metals 12, no. 5: 800. https://doi.org/10.3390/met12050800
APA StyleLiu, H., Ren, Y., Wang, K., Mu, X., Song, S., Guo, J., Yang, X., & Lu, Z. (2022). Magnetic-Field-Induced Strain Enhances Electrocatalysis of FeCo Alloys on Anode Catalysts for Water Splitting. Metals, 12(5), 800. https://doi.org/10.3390/met12050800




