Round-Robin Measurement of Surface Tension for Liquid Titanium by Electromagnetic Levitation (EML) and Electrostatic Levitation (ESL)
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Electromagnetic Levitation (EML)
2.2. Electrostatic Levitation (ESL)
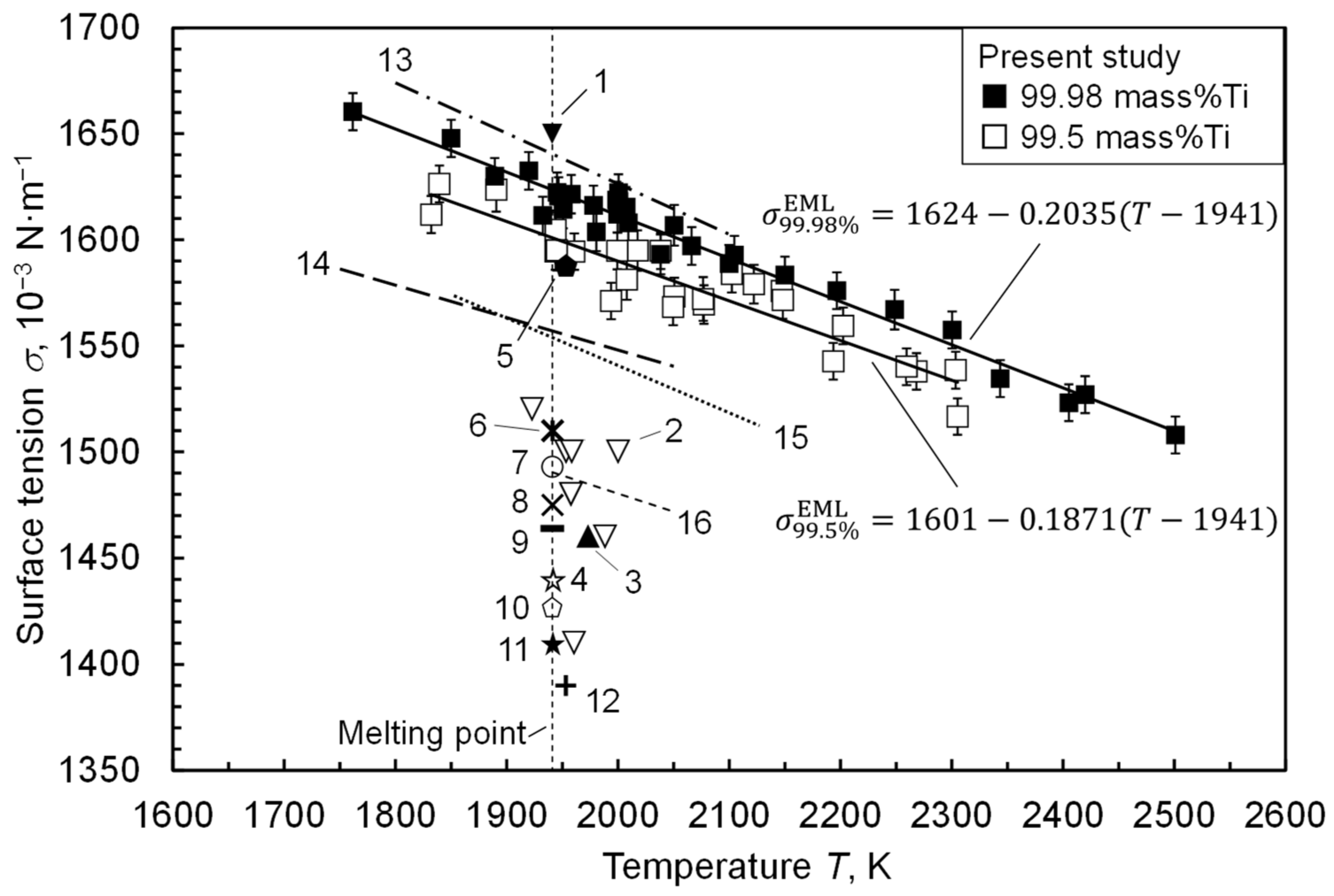
3. Results
4. Discussion
4.1. Influence of Atmospheric Gas Species on the Surface Tension of Liquid Titanium
4.2. Round-Robin Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, B.C. The Surface Tension of Liquid Transition Metals at Their Melting Points. Trans. Metall. Soc. AIME 1963, 227, 1175–1183. [Google Scholar]
- Brillo, J.; Wessing, J.; Kobatake, H.; Fukuyama, H. Surface tension of liquid Ti with adsorbed oxygen and its prediction. J. Mol. Liq. 2019, 290, 111226. [Google Scholar] [CrossRef]
- Zhu, J.; Kamiya, A.; Yamada, T.; Shi, W.; Naganuma, K.; Mukai, K. Surface tension, wettability and reactivity of molten titanium in Ti/yttria-stabilized zirconia system. Mater. Sci. Eng. A 2002, 327, 117–127. [Google Scholar] [CrossRef]
- Flint, O. Surface tension by pendant drop technique. J. Nucl. Mater. 1965, 16, 260–270. [Google Scholar] [CrossRef]
- Tille, J.; Kelly, J.C. The surface tension of liquid titanium. Br. J. Appl. Phys. 1963, 14, 717–719. [Google Scholar] [CrossRef]
- Elyutin, V.P.; Maurah, M. Density and Surface Tension of Liquid Titanium. Izvest. Akad. Nauk SSSR Otdel. Tekh. Nauk 1955, 4, 129–131. [Google Scholar]
- Long, J.; Wang, Y.; Zeng, Y.; Xiong, X.; Li, X.; Lun, H.; Ye, Z.; Hu, J.; Gao, S.; Chen, S.; et al. Surface Tension Measurements of Liquid Metals by the Quasi-Containerless Pendant Drop Method. Vacuum 2022, 200, 111045. [Google Scholar] [CrossRef]
- Man, K.F. Surface Tension Measurements of Liquid Metals by the Quasi-Containerless Pendant Drop Method. Int. J. Thermophys. 2000, 21, 793–804. [Google Scholar] [CrossRef]
- Namba, S.; Isobe, T. Surface Tension Measurements of Liquid Metals by the Quasi-Containerless Pendant Drop Method. Sci. Pap. IPCR 1963, 57, 51–54. [Google Scholar]
- Maurakh, M.A. Surface Tension of Titanium, Zirconium & Vanadium. Trans. Indian Inst. Met. 1964, 14, 209–225. [Google Scholar]
- Kostikov, V.I.; Grigoriev, G.A.; Arkhipkin, V.I.; Agaev, A.D. Surface Tension Measurements of High-Temperature Group IV metals. Izv. Vyss. Chem. Met. 1972, 3, 25–27. [Google Scholar]
- Peterson, A.W.; Kedesdy, H.; Keck, P.H.; Schwarz, E. Surface Tension of Titanium, Zirconium, and Hafnium. J. Appl. Phys. 1958, 29, 213–216. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.P.; Chang, J.; Wei, B. Experimental Study of Surface Tension, Specific Heat and Thermal Diffusivity of Liquid and Solid Titanium. Chem. Phys. Lett. 2015, 639, 105–108. [Google Scholar] [CrossRef]
- Paradis, P.-F.; Ishikawa, T.; Yoda, S. Non-Contact Measurements of Surface Tension and Viscosity of Niobium, Zirconium, and Titanium Using an Electrostatic Levitation Furnace. Int. J. Thermophys. 2002, 23, 825–842. [Google Scholar] [CrossRef]
- Wessing, J.J.; Brillo, J. Density, Molar Volume, and Surface Tension of Liquid Al-Ti. Metall. Mater. Trans. A 2017, 48, 868–882. [Google Scholar] [CrossRef] [Green Version]
- Amore, S.; Brillo, J.; Egry, I.; Novakovic, R. Surface Tension of Liquid Cu-Ti Binary Alloys Measured by Electromagnetic Levitation and Thermodynamic Modelling. Appl. Surf. Sci. 2011, 257, 7739–7745. [Google Scholar] [CrossRef]
- Ozawa, S.; Takahashi, S.; Suzuki, S.; Sugawara, H.; Fukuyama, H. Influence of oxygen partial pressure on surface tension and its temperature coefficient of molten iron. J. Appl. Phys. 2011, 109, 014902. [Google Scholar] [CrossRef]
- Ozawa, S.; Morohoshi, K.; Hibiya, T.; Fukuyama, H. Influence of oxygen partial pressure on surface tension of molten silver. J. Appl. Phys. 2010, 107, 014910. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, S.; Nishimura, M.; Kuribayashi, K. Surface Tension of Molten Silver in Consideration of Oxygen Adsorption Measured by Electromagnetic Levitation. J. Microgravity Sci. Appl. 2016, 33, 330310. [Google Scholar]
- Ozawa, S.; Takahashi, S.; Watanabe, N.; Fukuyama, H. Influence of Oxygen Adsorption on Surface Tension of Molten Nickel Measured under Reducing Gas Atmosphere. Int. J. Thermophys. 2014, 35, 1705–1711. [Google Scholar] [CrossRef]
- Ozawa, S.; Nagasaka, Y.; Itakura, M.; Sugisawa, K.; Seimiya, Y. Influence of oxygen adsorption from atmosphere on surface tension of liquid silicon. J. Appl. Phys. 2021, 130, 135101. [Google Scholar] [CrossRef]
- Morohoshi, K.; Ozawa, S.; Tagawa, T.; Hibiya, T. Measurement of Surface Tension for Molten Silver by Oscillating Droplet Method Using Electromagnetic Levitation Furnace. J. Jpn. Inst. Met. 2008, 72, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Egry, I.; Ricci, E.; Novakovic, R.; Ozawa, S. Surface tension of liquid metals and alloys—Recent developments. Adv. Colloid Interface Sci. 2010, 159, 198–212. [Google Scholar] [CrossRef]
- Ozawa, S.; Suzuki, S.; Iwasaka, Y.; Sugawara, K.; Morohoshi, K.; Fukuyama, H.; Hibiya, T. Surface tension measurement of high temperature melt by oscillating droplet method in consideration of droplet rotation. In Proceedings of the Current Advances in Materials and Processes: Report of the ISIJ Meeting (CAMP-ISIJ), Kyoto, Japan, 30 March 2009; Volume 22, p. 22901. [Google Scholar]
- Takenaga, N.; Ozawa, S.; Hibiya, T.; Kobatake, H.; Fukuyama, H.; Awaji, S.; Watanabe, M. Measurement of Surface Tension for Molten Silicon by Electromagnetic Levitation Combined with Static Magnetic Field. In Proceedings of the 57th International Astronautical Congress, Valencia, Spain, 2–10 October 2006. [Google Scholar]
- Ozawa, S.; Koda, T.; Adachi, M.; Morohoshi, K.; Watanabe, M.; Hibiya, T. The influence of temporal phase difference of m = ±2 oscillations on surface frequency analysis for levitated droplets. J. Appl. Phys. 2009, 106, 034907. [Google Scholar] [CrossRef]
- Rhim, W.-K.; Chung, S.K.; Barber, D.; Man, K.F.; Gutt, G.; Rulison, A.; Spjut, R.E. An electrostatic levitator for high-temperature containerless materials processing in 1-g. Rev. Sci. Instrum. 1993, 64, 2961–2970. [Google Scholar] [CrossRef] [Green Version]
- Rhim, W.-K.; Paradis, P.-F. Laser-induced rotation of a levitated sample in vacuum. Rev. Sci. Instrum. 1999, 70, 4652–4655. [Google Scholar] [CrossRef] [Green Version]
- Paradis, P.-F.; Ishikawa, T.; Yoda, S. Position stability analysis of electrostatically levitated samples for thermophysical and structural properties measurements of materials. Space Technol. 2002, 22, 81–92. [Google Scholar]
- Ozawa, S.; Kawanobe, Y.; Kuribayashi, K.; Nagasawa, T. Influence of Trace Impurities on Oxygen Activity for High Purity Nitrogen Gas Processed by Zirconia Oxygen Pump. Int. J. Microgravity Sci. Appl. 2016, 33, 330124. [Google Scholar]
- Rayleigh, L. On the Capillary Phenomena of Jets. Proc. R. Soc. Lond. 1979, 29, 71–97. [Google Scholar]
- Cummings, D.L.; Blackburn, D.A. Oscillations of magnetically levitated aspherical droplets. J. Fluid Mech. 1991, 224, 395–416. [Google Scholar] [CrossRef]
- Ozawa, S.; Kudo, Y.; Kuribayashi, K.; Watanabe, Y.; Ishikawa, T. Precise Density Measurement of Liquid Titanium by Electrostatic Levitator. Mater. Trans. 2017, 50, 1664–1669. [Google Scholar] [CrossRef] [Green Version]
- ISO. Guide to the Expression of Uncertainty in Measurement; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Rhim, W.-K.; Ohsaka, K.; Paradis, P.-F.; Spjut, R.E. Noncontact technique for measuring surface tension and viscosity of molten materials using high temperature electrostatic levitation. Rev. Sci. Instrum. 1999, 70, 2796–2801. [Google Scholar] [CrossRef] [Green Version]
- Lord Rayleigh, F.R.S. On the Equilibrium of Liquid Conducting Masses charged with Electricity. Philos. Mag. 1882, 14, 184–186. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.Q.; Beard, K.V. Small-Amplitude Oscillations of Electrostatically Levitated Drops. Proc. R. Soc. Lond. A 1990, 430, 133–150. [Google Scholar]
- Belyanchikov, L.N. Thermodynamics of Titanium-Based Melts: II. Oxygen in Liquid Titanium. Russ. Metall. 2010, 2010, 1156–1163. [Google Scholar] [CrossRef]



| Al | C | Ca | Cr | Cu | Fe | K | Mg | Mn |
|---|---|---|---|---|---|---|---|---|
| 0.69 | <10 | <1 | 0.39 | 0.4 | 0.35 | <0.01 | <0.01 | 0.1 |
| N | Ni | O | S | Si | Th | U | V | Zn |
| <10 | 0.54 | 110 | <10 | 1.4 | <0.0001 | <0.0001 | 0.03 | <0.05 |
| C | Fe | H | N | O |
|---|---|---|---|---|
| 800 | 2000 | 150 | 300 | 1800 |
| Source of Uncertainty | Value ± | Divisor | Standard Uncertainty, u(i) | Sensitivity Coefficient, c(i) | Uncertainty Contribution, us(i) |
|---|---|---|---|---|---|
| Density, ρ [33] | 62.36 kg·m−3 | 2 | 31.18 kg·m−3 | −6.423 × 10−6 m3·s−2 | −2.003 × 10−4 N·m−1 |
| Sample mass, M | 7.277 × 10−7 kg | 1 | 7.277 × 10−7 Kg | 3976 s−2 | 2.893 × 10−3 N·m−1 |
| Translation frequency of m = −1 for the l = 1 mode, f1,−1 | 6.104 × 10−2 s−1 | 3.524 × 10−2 s−1 | 1.618 × 10−3 kg·s−1 | 5.702 × 10−5 N·m−1 | |
| Translation frequency of m = 0 for the l = 1 mode, f1,0 | 6.104 × 10−2 s−1 | 3.524 × 10−2 s−1 | 2.325 × 10−3 kg·s−1 | 8.193 × 10−5 N·m−1 | |
| Translation frequency of m = +1 for the l = 1 mode, f1,+1 | 6.104 × 10−2 s−1 | 3.524 × 10−2 s−1 | 1.823 × 10−3 kg·s−1 | 6.425 × 10−5 N·m−1 | |
| Surface oscillation frequency of m = 0 for the l = 2 mode, f2,0 | 6.104 × 10−2 s−1 | 3.524 × 10−2 s−1 | 9.349 × 10−3 kg·s−1 | 3.294 × 10−4 N·m−1 | |
| Surface oscillation frequency of m = ±1 for the l = 2 mode, f2,±1 | 6.104 × 10−2 s−1 | 3.524 × 10−2 s−1 | 2.228 × 10−2 kg·s−1 | 7.852 × 10−4 N·m−1 | |
| Surface oscillation frequency of m = ±2 for the l = 2 mode, f2,±2 | 6.104 × 10−2 s−1 | 3.524 × 10−2 s−1 | 2.531 × 10−3 kg·s−1 | 8.918 × 10−4 N·m−1 | |
| Repeatability of surface tension measurement, σrep | 3.618 × 10−3 N·m−1 | 1 | 3.618 × 10−3 N·m−1 | 1 | 3.618 × 10−3 N·m−1 |
| Combined uncertainty, uc(σ): 4.800 × 10−3 N·m−1 | |||||
| Expanded uncertainty, U: 9.600 × 10−3 N·m−1 (coverage factor kp = 2 is selected) | |||||
| Source of Uncertainty | Value ± | Divisor | Standard Uncertainty, u(i) | Sensitivity Coefficient, c(i) | Uncertainty Contribution, us(i) |
|---|---|---|---|---|---|
| Numerical fitting of droplet contour, f | 0.6131 px | 1 | 0.6131 px | −5.717 × 10−5 kg·s−2 px−1 | −3.538 × 10−5 N·m−1 |
| Diameter of droplet image, rimg | 0.5 px | 0.2887 px | −5.717 × 10−5 kg·s−2 px−1 | −1.666 × 10−5 N·m−1 | |
| Diameter of reference sphere rref | 5.0 × 10−7 m | 2.887 × 10−7 m | −8.388 kg·m−1 s−2 | −2.422 × 10−6 N·m−1 | |
| Density, ρ [33] | 65.46 kg·m−3 | 2 | 29.33 kg·m−3 | −3.876 × 10−4 m3·s−2 | −1.269 × 10−2 N·m−1 |
| Surface oscillation frequency f2c | 0.5 s−1 | 0.2887 s−1 | 1.818×10−2 kg·s−1 | 5.249 × 10−3 N·m−1 | |
| Sample charge, Q | 1.359 × 10−11 c | 1 | 1.359 × 10−11 c | −3.346 × 107 kg·s−2·c−1 | −4.547 × 10−4 N·m−1 |
| Correction factor, F | 3.098 × 10−10 | 1 | 3.098 × 10−10 | 1.581 N·m−1 | 4.898 × 10−10 N·m−1 |
| Repeatability of surface tension measurement, σrep | 2.781 × 10−3 N·m−1 | 1 | 2.781 × 10−3 N·m−1 | 1 | 2.781 × 10−3 N·m−1 |
| Combined uncertainty, uc(σ): 14.02 × 10−3 N·m−1 | |||||
| Expanded uncertainty, U: 28.03 × 10−3 N·m−1 (coverage factor kp = 2 is selected) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seimiya, Y.; Kudo, Y.; Shinazawa, R.; Watanabe, Y.; Ishikawa, T.; Ozawa, S. Round-Robin Measurement of Surface Tension for Liquid Titanium by Electromagnetic Levitation (EML) and Electrostatic Levitation (ESL). Metals 2022, 12, 1129. https://doi.org/10.3390/met12071129
Seimiya Y, Kudo Y, Shinazawa R, Watanabe Y, Ishikawa T, Ozawa S. Round-Robin Measurement of Surface Tension for Liquid Titanium by Electromagnetic Levitation (EML) and Electrostatic Levitation (ESL). Metals. 2022; 12(7):1129. https://doi.org/10.3390/met12071129
Chicago/Turabian StyleSeimiya, Yusaku, Yu Kudo, Ryo Shinazawa, Yuki Watanabe, Takehiko Ishikawa, and Shumpei Ozawa. 2022. "Round-Robin Measurement of Surface Tension for Liquid Titanium by Electromagnetic Levitation (EML) and Electrostatic Levitation (ESL)" Metals 12, no. 7: 1129. https://doi.org/10.3390/met12071129
APA StyleSeimiya, Y., Kudo, Y., Shinazawa, R., Watanabe, Y., Ishikawa, T., & Ozawa, S. (2022). Round-Robin Measurement of Surface Tension for Liquid Titanium by Electromagnetic Levitation (EML) and Electrostatic Levitation (ESL). Metals, 12(7), 1129. https://doi.org/10.3390/met12071129






