Optimizing Metallographic Etchants for Ancient Gold and Silver Materials
Abstract
:1. Introduction
- (a)
- Gold-aqua regia: The problem of poorly defined metallographic images and blurred grain boundaries often occurs (Figure S1a). Some researchers have suggested that this phenomenon is caused by over-etching or impurities on the surface of the sample and have responded by properly polishing the sample after etching. However, this treatment may result in new secondary scratches (Figure S1b) and relies on the researchers’ personal experience of the experimental procedure, which is not suitable for application;
- (b)
- Gold/silver-ammonium persulfate + potassium cyanide: Although this etchant is suitable for precious metal alloys such as platinum, gold and silver [20], KCN is a highly toxic chemical and may pose a potential health risk to the researcher;
- (c)
- Silver-ammonia + hydrogen peroxide: The oxidation–reduction reaction between two components of this etchant forms N2, which leads to failure (in fact, the etchant is usually only effective for approximately 10 min) and can cause considerable inconvenience when many samples must be etched (Figure S1c). Additionally, when using this etchant many bubbles are formed, creating “hollow drums” on the surface of the sample and preventing the etchant from continuously reacting with the metal, which results in unsatisfactory etching results;
- (d)
- Silver-acidified potassium dichromate: The formulation of this etchant is complex and must be diluted in a certain proportion when used. In addition, although the sample is washed after etching, some reagent remains on the surface and creates a slow, continuous dissolution, which can require repolishing the sample after a period of time (this problem also occurs with other etchants).
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Metallographic Observation
2.3. SEM–EDS Analysis
3. Results and Discussion
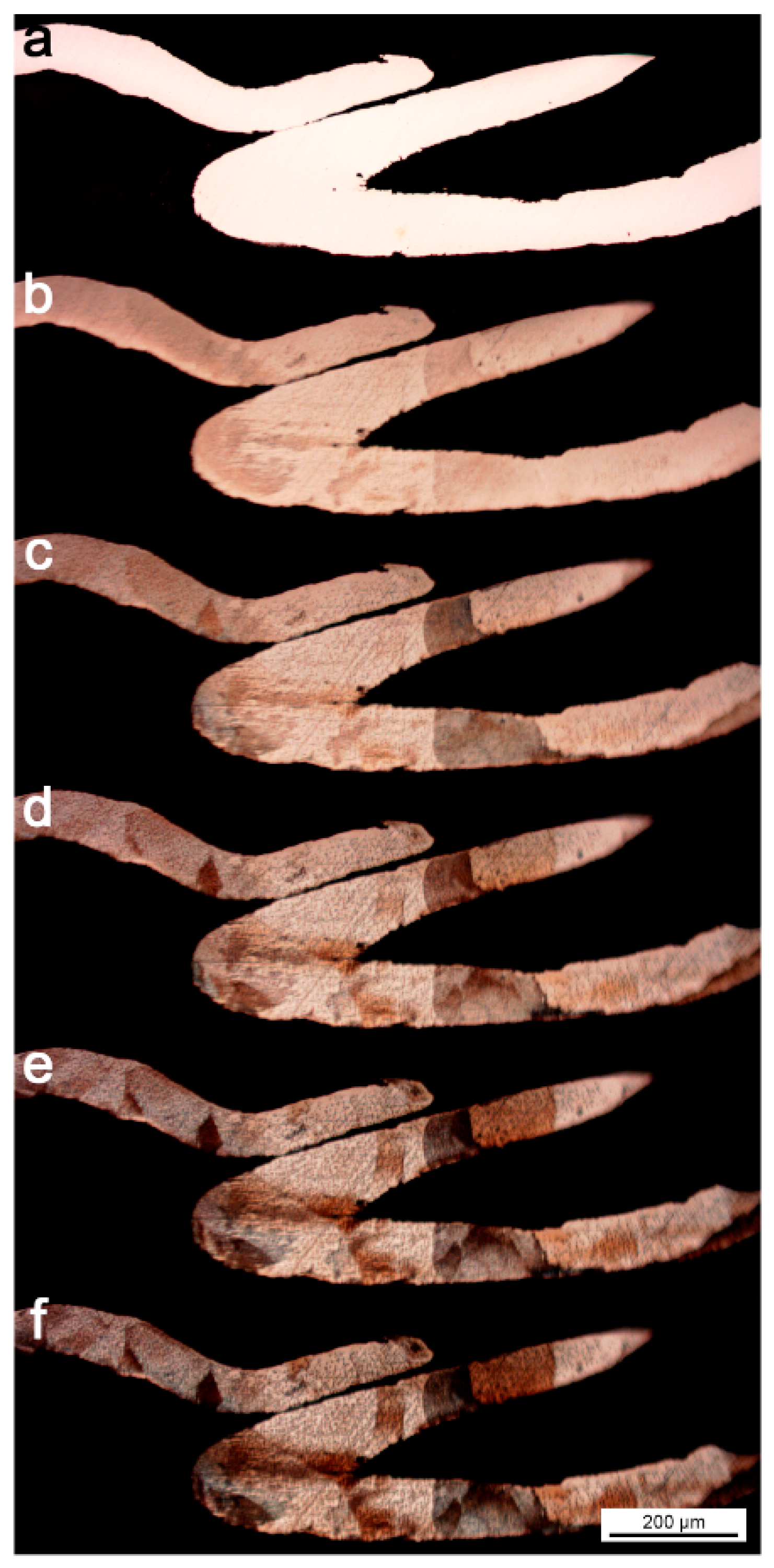
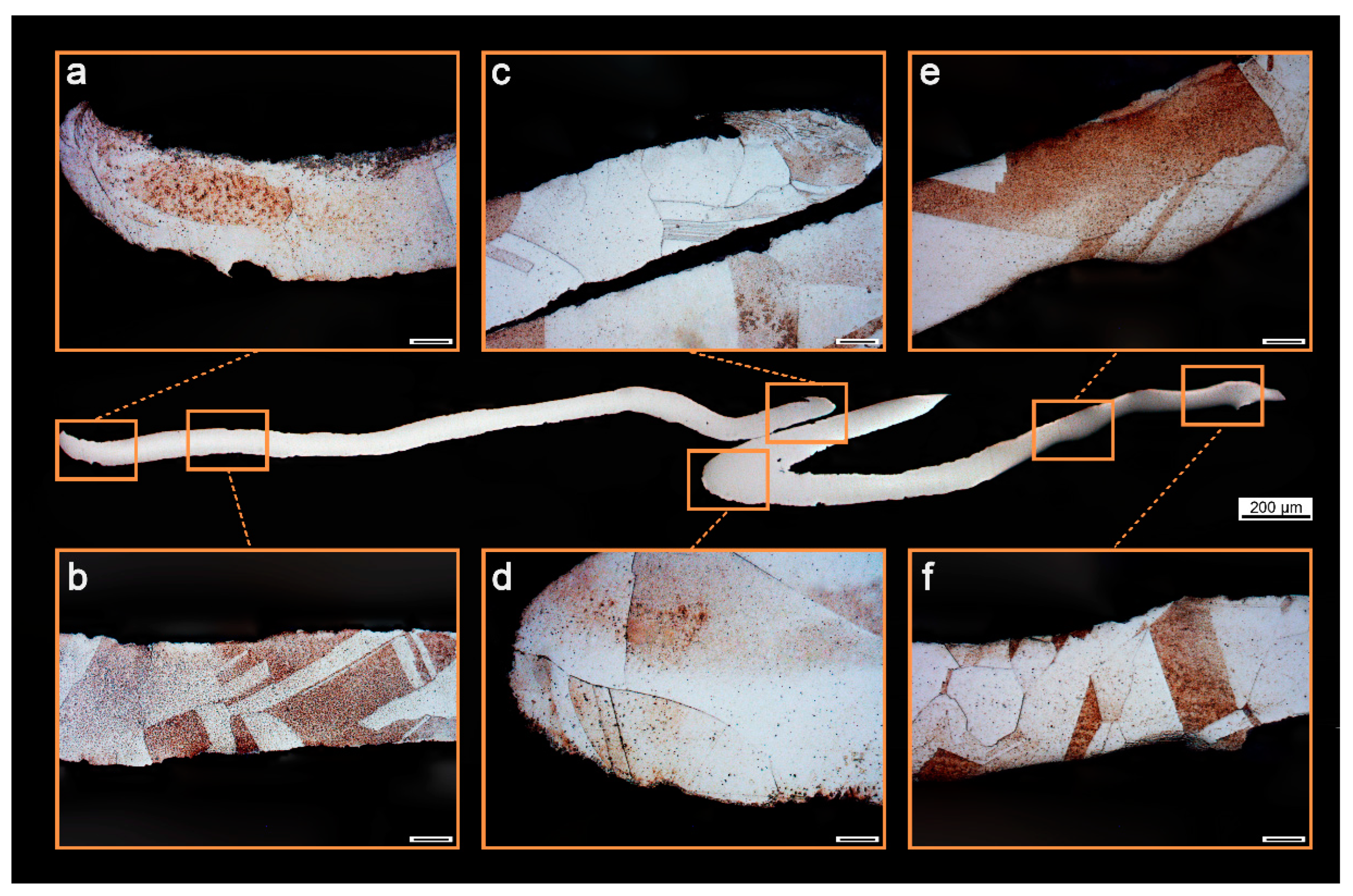
3.1. Kinetic Control Study of Etching Ancient Gold Materials with Aqua Regia
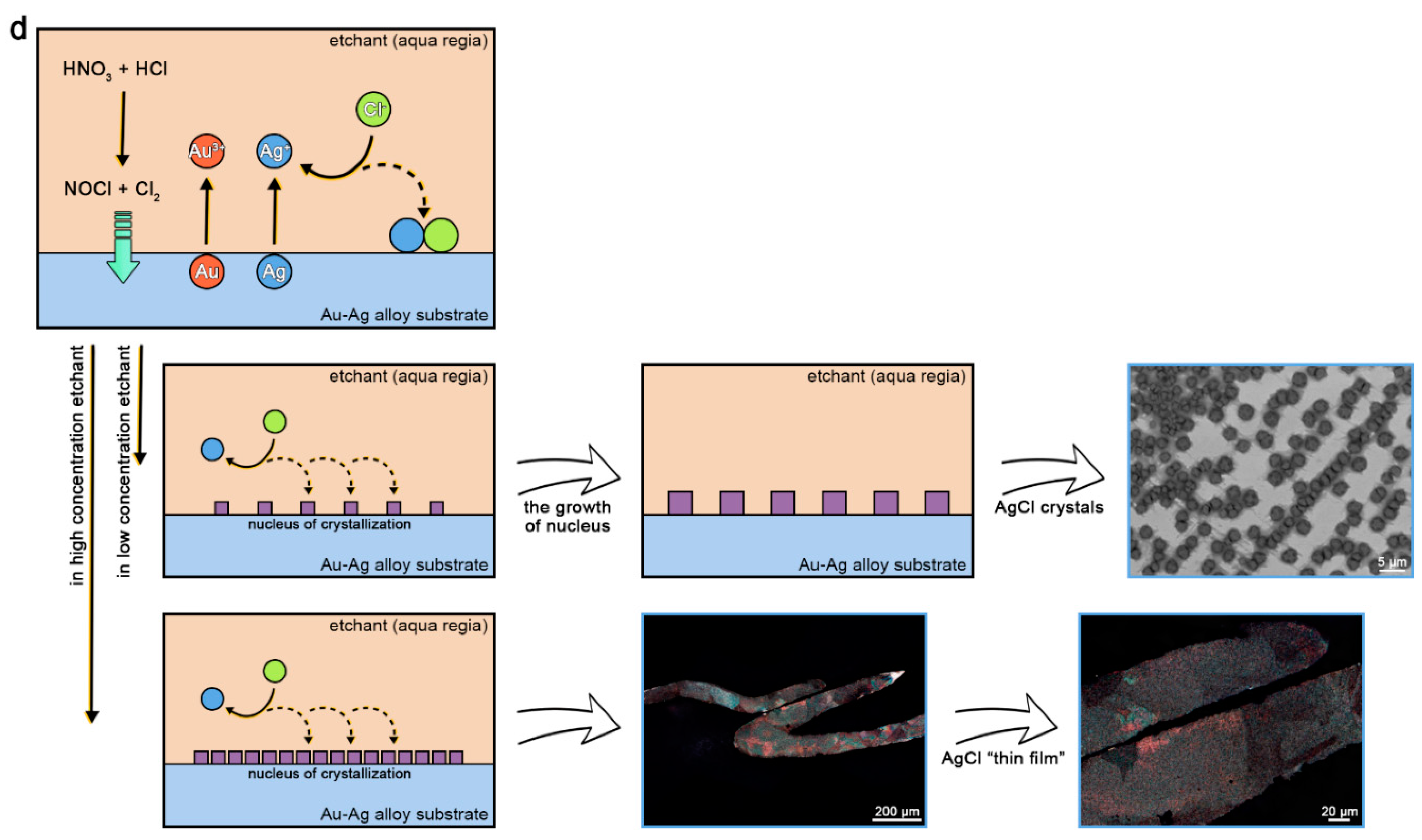
3.2. Removal of Silver Chloride Impurities
3.3. Optimizing the Etching Methods for Ancient Au–Ag Alloys
- (a)
- Rapid etching: The etchant is pure aqua regia, and the etching time is approximately 10 s. The morphology of the AgCl impurities is a “thin-film” pattern. Both concentrated ammonia and 1 mol/L Na2S2O3 solution can be used to efficiently remove AgCl impurities;
- (b)
- Local etching: The etchant is diluted aqua regia, the dilution ratio can be adjusted according to the composition of the Au–Ag alloy samples (1:1.5–1:2.5 ranging), and the length of etching is 30–100 s according to the dilution ratio. The advantage of this method is that the etching process of the region of interest can be observed under the metallographic microscope, and the end point of the etching can be flexibly controlled. The morphology of the AgCl impurities is a “particulate crystal” pattern, which requires a long immersion time with concentrated ammonia or 1 mol/L Na2S2O3 solution; thus, ammonia may face the problem of volatilization, and it is better to use Na2S2O3 solution.
3.4. Optimizing the Etchant Formulations for Ancient Silver Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chikashige, M. A Study of ancient Eastern bronze mirrors. Hist. Rev. 1918, 3, 178–203. [Google Scholar]
- Shigeru, K.; Yamauchi, S. Chemical studies of ancient mirrors. J. Orient. Stud. 1937, 8, 11–31. [Google Scholar]
- Yetts, W.P. Problems of Chinese bronzes. J. R. Cent. Asian Soc. 1931, 18, 399–402. [Google Scholar] [CrossRef]
- Scott, D.A. Metallography and Microstructure of Ancient and Historic Metals (Translated by Tian, X.; Ma, Q.); Chinese Translation Science Press: Beijing, China, 2012; pp. 69–78. [Google Scholar]
- Sun, S.; Han, R.; Li, X. Microstructure Atlas of Ancient Chinese Metallic Materials—Non-Ferrous Metals Volume; Science Press: Beijing, China, 2011; pp. 25–36. [Google Scholar]
- Yu, Y. Principles of Metallurgy (Upper), 3rd ed.; Metallurgical Industry Press: Beijing, China, 2020; pp. 521–533. [Google Scholar]
- Yuan, Z.; Dai, Q. Metallic Materials, 3rd ed.; Chemical Industry Press: Beijing, China, 2019; pp. 3–17. [Google Scholar]
- Cho, N.; Lee, H.; Lee, J. Microstructure and heat treatment of Early Iron Age cast iron axes excavated from the Sinpung site, Wanju, Jeonbuk, in the Korean Peninsula. Archaeol. Anthropol. Sci. 2019, 11, 2611–2621. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Golan, O.; Tal, O. An archaeometallurgical study of 13th-century arrowheads and bolts from the Crusader Castle of Arsuf/Arsur. Archaeometry 2013, 55, 235–257. [Google Scholar] [CrossRef]
- Haubner, R.; Strobl, S.; Zbiral, J.; Gusenbauer, C.; Pintz, U. Metallurgical characterization of a coated Roman iron coin by analytical investigations. Archaeometry 2016, 58, 441–452. [Google Scholar] [CrossRef]
- Park, J.-S.; Rehren, T. Large-scale 2nd to 3rd century AD bloomery iron smelting in Korea. J. Archaeol. Sci. 2011, 38, 1180–1190. [Google Scholar] [CrossRef]
- Park, J.-S.; Honeychurch, W.; Chunag, A. Iron technology and medieval nomadic communities of East Mongolia. Archaeol. Anthropol. Sci. 2019, 11, 555–565. [Google Scholar] [CrossRef]
- Gelegdorj, E.; Chunag, A.; Gordon, R.B.; Park, J.-S. Transitions in cast iron technology of the nomads in Mongolia. J. Archaeol. Sci. 2007, 34, 1187–1196. [Google Scholar] [CrossRef]
- Vander Voort, G.F. Metallography: Principles and Practice; ASM International: Novelty, OH, USA, 2004; p. 642. [Google Scholar]
- Mödlinger, M.; Trebsche, P. Work on the cutting edge: Metallographic investigation of Late Bronze Age tools in southeastern Lower Austria. Archaeol. Anthropol. Sci. 2021, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, P.; Vernet, J.; Voland, G.; Ghiara, G. Metallographic investigation of Early Bronze Age armbands from Western Switzerland (ca. 2200-1500 BC): New highlights about early manufacturing processes. Archaeol. Anthropol. Sci. 2020, 12, 215. [Google Scholar] [CrossRef]
- Sáenz-Samper, J.; Martinón-Torres, M. Depletion gilding, innovation and life-histories: The changing colours of Nahuange metalwork. Antiquity 2017, 91, 1253–1267. [Google Scholar] [CrossRef]
- Oudbashi, O.; Hasanpour, A.; Jahanpoor, A.; Rahjoo, Z. Microscopic and microanalytical study on Sasanian metal objects from Western Iran: A case study. Sci. Technol. Archaeol. Res. 2017, 3, 194–205. [Google Scholar] [CrossRef] [Green Version]
- Oudbashi, O.; Hessari, M. Iron Age tin bronze metallurgy at Marlik, Northern Iran: An analytical investigation. Archaeol. Anthropol. Sci. 2017, 9, 233–249. [Google Scholar] [CrossRef]
- Scott, D.A. The La Tolita-Tumaco Culture: Master Metalsmiths in Gold and Platinum. Lat. Am. Antiq. 2011, 22, 65–95. [Google Scholar] [CrossRef]
- Monge Soares, A.M.; Valério, P.; Silva, R.J.C.; Cerqueira Alves, L.; De Fátima Araújo, M. Early Iron Age gold buttons from South-Western Iberian Peninsula. Identification of a gold metallurgical workshop. Trab. Prehist. 2010, 67, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, L.; Zhou, X.; Li, P.; Xiao, L. Protection and restoration of gold and silver inlaid belt hook unearthed from Feihu village, Pujiang County, Chengdu. Sci. Conserv. Archaeol. 2021, 33, 75–82. [Google Scholar]
- Shao, A.; Mei, J.; Chen, K.; Zhou, G.; Wang, H. Preliminary analysis of the metal ornaments unearthed from the Majiayuan Graveyard of the warring states in Zhangjiachuan. Cult. Relics 2010, 10, 88–96. [Google Scholar]
- Wu, H. Analysis on Production Process of Gold Mask Unearthed from Poma Graveyard in Zhaosu County. Master’s Thesis, Northwest University, Shaanxi, China, December 2019. [Google Scholar]
- Meng, X.; Mei, J.; Dong, Y.; Feng, S.; Han, C. The preliminary analysis of metal tools excavated from tomb M10 from Xiangyang Chenpo burial site in Hubei. Jianghan Archaeol. 2009, 4, 106–113. [Google Scholar]
- Xie, X.; Li, Y.; Jing, Y.; Wang, X. A preliminary study on some metal objects unearthed from Yihe-Nur M1, Zhengxiangbai Banner, Inner Mongolia. Steppe Cult. Relics 2020, 2, 110–117. [Google Scholar]
- Xiang, X.; Bai, X. Grain refinement of 18-Carat Au-Ag-Cu-Zn alloy containing cerium. Int. J. Met. 2015, 10, 100–105. [Google Scholar]
- Merkel, S.W. Evidence for the widespread use of dry silver ore in the Early Islamic period and its implications for the history of silver metallurgy. J. Archaeol. Sci. 2021, 135, 105478. [Google Scholar] [CrossRef]
- Northover, P.; Northover, S.; Wilson, A. Microstructures of ancient and historic silver. In Proceedings of the International Council of Museums ICOM-CC, Edinburgh, UK, 16–20 September 2013. [Google Scholar]
- Wang, Y. Scientific Study on Decorative Sheetmetal Artifacts Unearthed in the Region of the Chu Culture of the Eastern Zhou Period. Ph.D. Thesis, University of Science and Technology, Beijing, China, December 2019. [Google Scholar]
- Mortazavi, M.; Naghavi, S.; Khanjari, R.; Agha-Aligol, D. Metallurgical study on some Sasanian silver coins in Sistan Museum. Archaeol. Anthropol. Sci. 2018, 10, 1831–1840. [Google Scholar] [CrossRef]
- Shalev, S.; Shechtman, D.; Shilstein, S.S. A study of the composition and microstructure of silver hoards from Tel Beth-Shean, Tel Dor, and Tel Miqne, Israel. Archaeol. Anthropol. Sci. 2014, 6, 221–225. [Google Scholar] [CrossRef]
- Török, b.; Benke, M.; Mertinger, V.; Barkóczy, P.; Kovács, A.; Hoppál, K.; Kovács, P. Complex metallographic study on Gepid bronze and silver buckles from the Great Hungarian Plain (5–6th cent.). STAR: Sci. Technol. Archaeol. Res. 2017, 3, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Faieta, R.; Guida, G.; Vidale, M. A preliminary note on the metallography and chemical analysis of silver samples from the Mahboubian Collection, London. Iran 2018, 56, 144–147. [Google Scholar] [CrossRef]
- Lei, Y.; Li, H.; Yu, M. Gold mask excavated from No.5 pit of the sacrificial area at the Sanxingdui site. Sichuan Cult. Relics 2022, 2, 107–118. [Google Scholar]
- Zhou, B.; Peng, W. Tusi cemetery of the Yang Family at Xinpu Town, Zunyi City, Guizhou Province. Archaeology 2015, 7, 87–100. [Google Scholar]
- Chen, G.; Sha, C.; Liu, B.; Zhang, W.; Wang, S. A brief report on the excavation of the Murong Zhi tomb of the Tuyu Hun royal Family during the early Tang Dynasty in Gansu Province. Archaeol. Cult. Relics 2021, 2, 15–38. [Google Scholar]
- Massucci, M.; Clegg, S.L.; Brimblecombe, P. Equilibrium partial pressures, thermodynamic properties of aqueous and solid phases, and Cl2 production from aqueous HCl and HNO3 and their mixtures. J. Phys. Chem. A 1999, 103, 4209–4226. [Google Scholar] [CrossRef]
- Ye, T. Principles and Applications of Chemical Crystallization Processes, 3rd ed.; Beijing University of Technology Press: Beijing, China, 2020; pp. 23–48. [Google Scholar]
- Luo, Q. Coordination Chemistry; Science Press: Beijing, China, 2012; pp. 135–139. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods—Fundamentals and Applications, 2nd ed.; Chemical Industry Press: Beijing, China, 2005; pp. 32–38. [Google Scholar]





| Ag+ Ligand (X) | Coordination Number (n) | Accumulative Stability Constant (lgβn) |
|---|---|---|
| NH3 | 1, 2 | 3.24, 7.05 |
| S2O32− | 1, 2 | 8.82, 13.46 |
| CN− | 2, 3, 4 | 21.1, 21.7, 20.6 |
| Br− | 1, 2, 3, 4 | 4.38, 7.33, 8.00, 8.73 |
| SCN− | 1, 2, 3, 4 | 4.6, 7.57, 9.08, 10.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Gong, Z.; Lu, H.; Zhang, W.; Ma, Y.; Yang, X.; Xie, Z.; Hu, G.; Hu, D. Optimizing Metallographic Etchants for Ancient Gold and Silver Materials. Metals 2022, 12, 1229. https://doi.org/10.3390/met12071229
Liu S, Gong Z, Lu H, Zhang W, Ma Y, Yang X, Xie Z, Hu G, Hu D. Optimizing Metallographic Etchants for Ancient Gold and Silver Materials. Metals. 2022; 12(7):1229. https://doi.org/10.3390/met12071229
Chicago/Turabian StyleLiu, Shengyu, Zisang Gong, Haizi Lu, Wei Zhang, Yanru Ma, Xiaolin Yang, Zhenda Xie, Gang Hu, and Dongbo Hu. 2022. "Optimizing Metallographic Etchants for Ancient Gold and Silver Materials" Metals 12, no. 7: 1229. https://doi.org/10.3390/met12071229






