The Effect of Mn on the Mechanical Properties and In Vitro Behavior of Biodegradable Zn-2%Fe Alloy
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Alloy Preparation
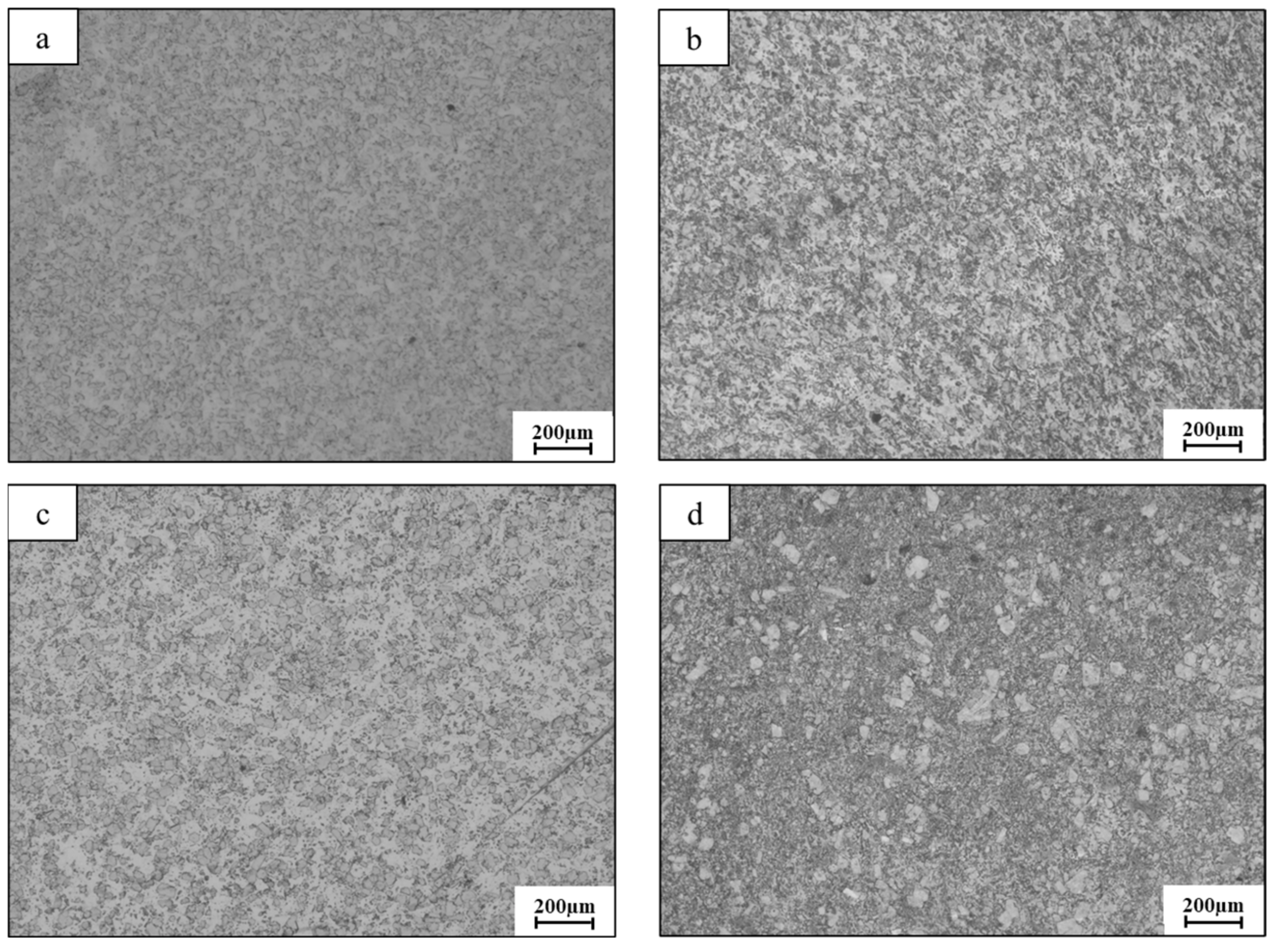
2.2. Microstructure Characterization
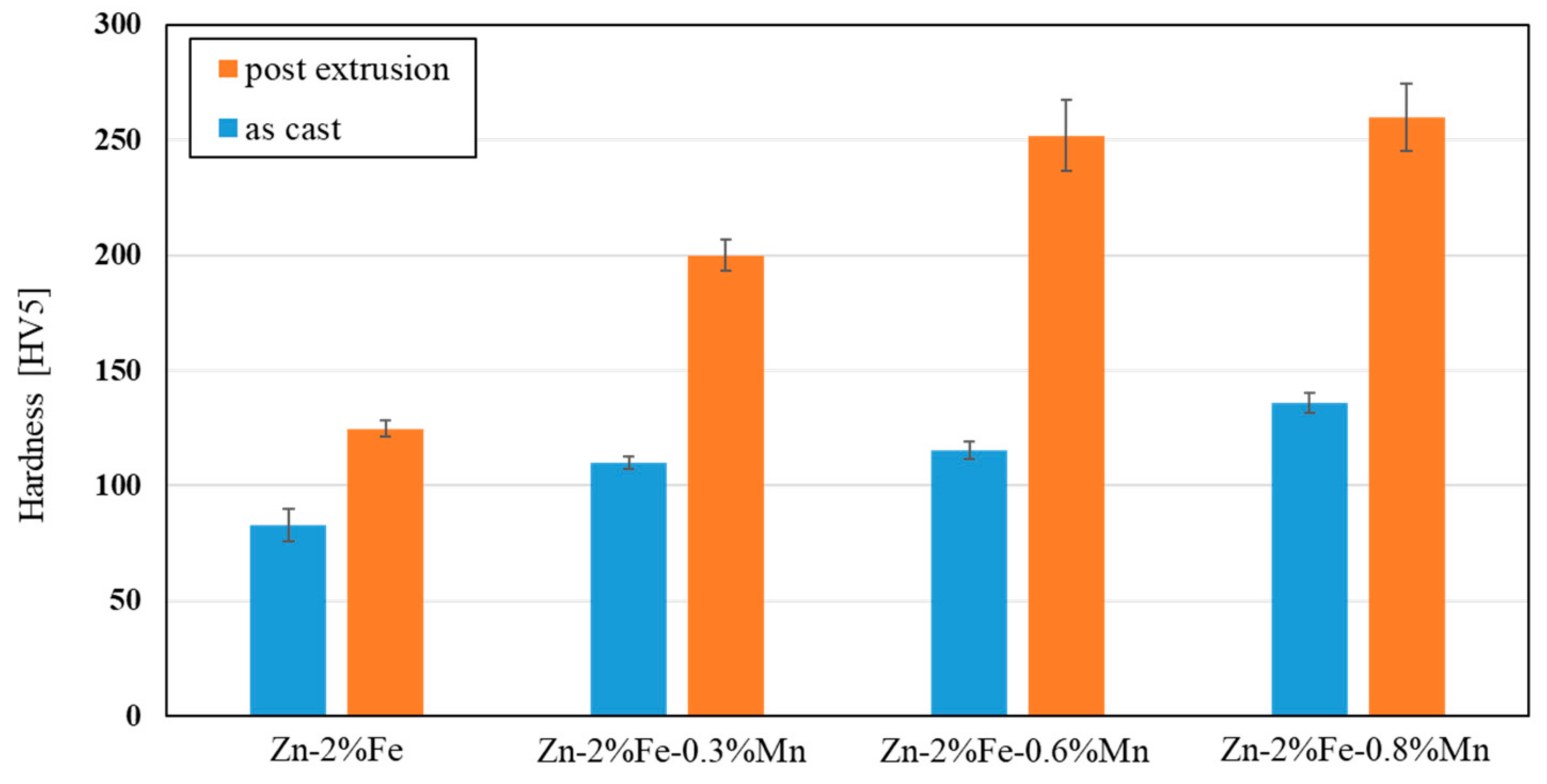
2.3. Mechanical Property Examinations
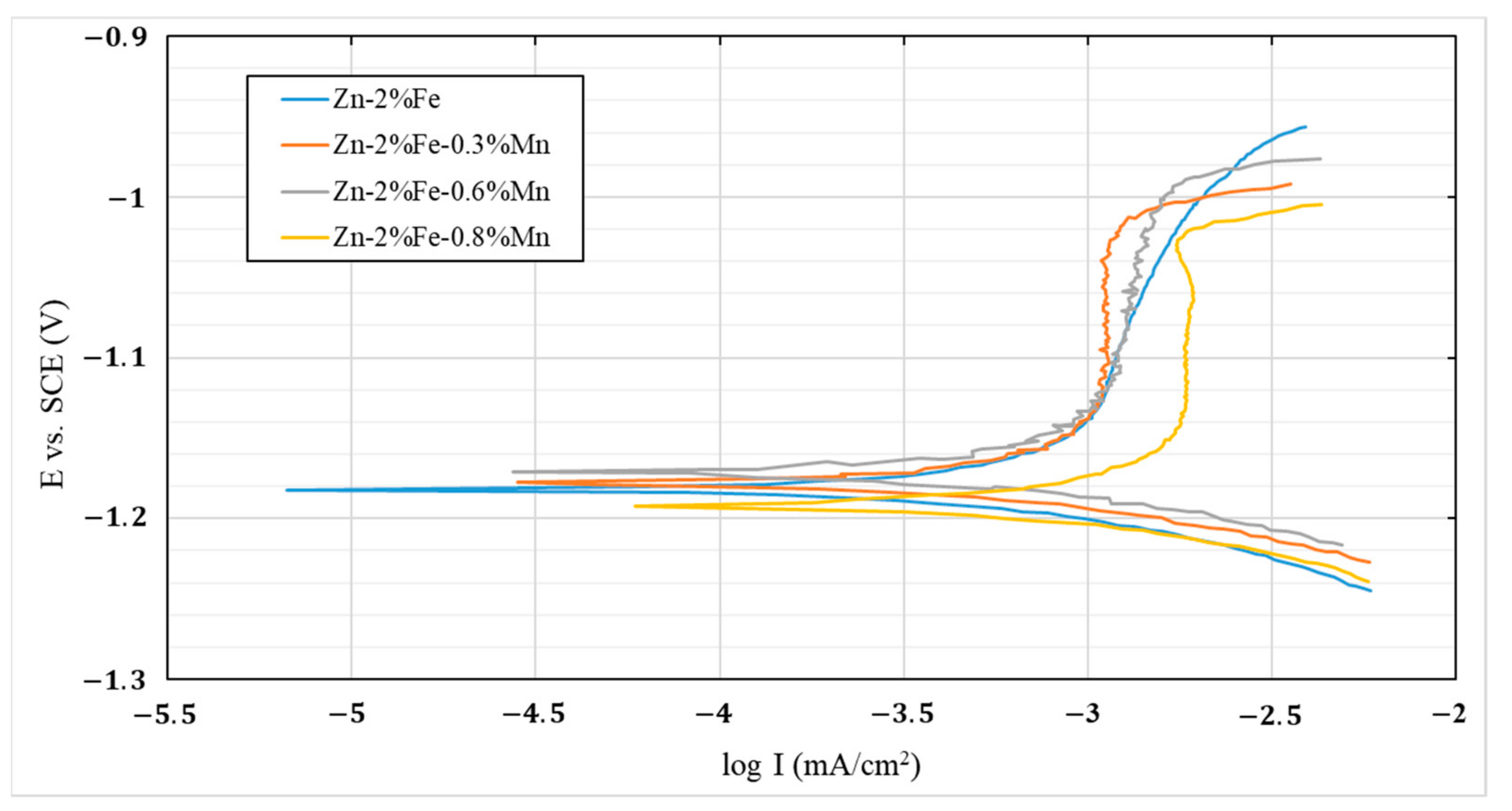
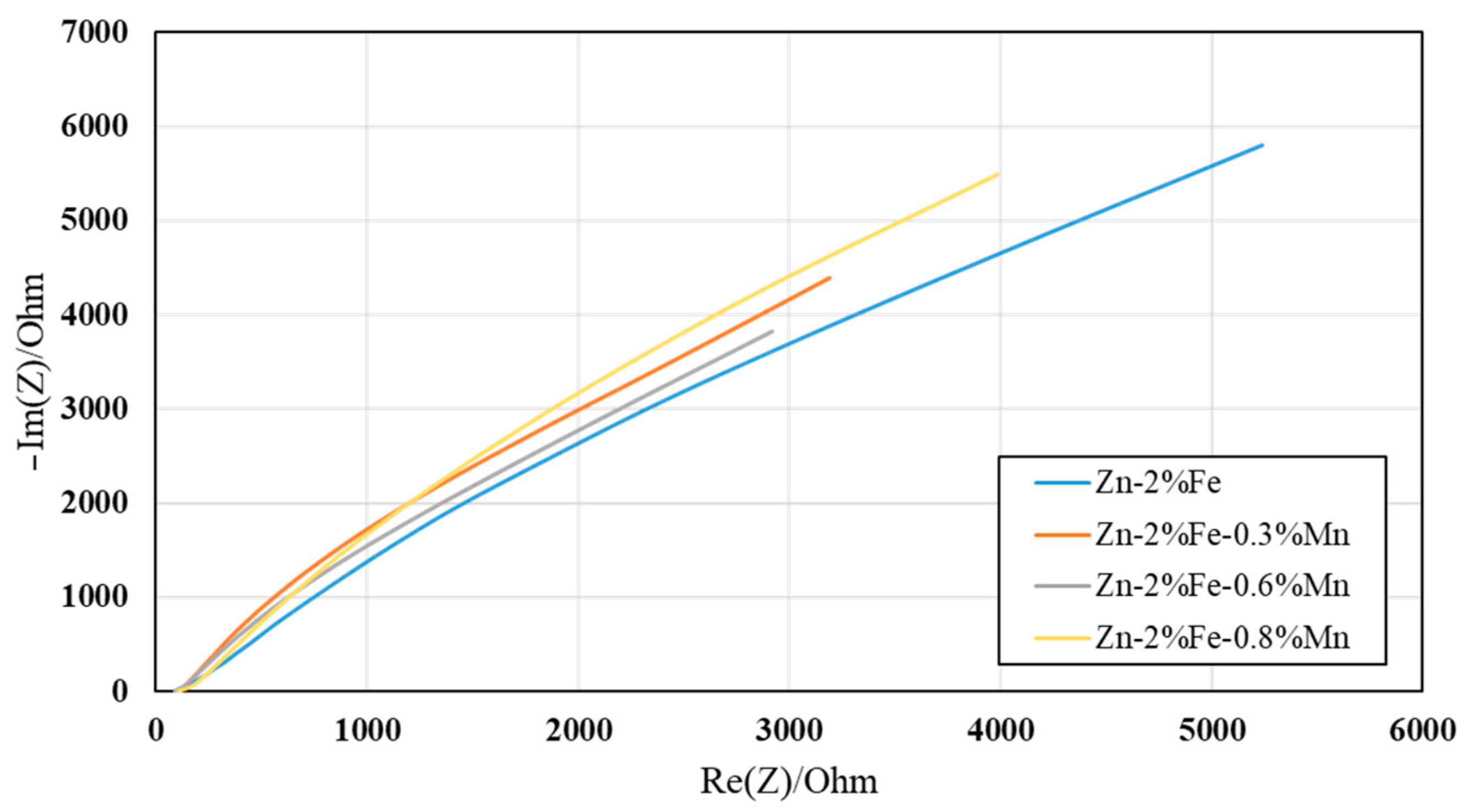
2.4. Corrosion Performance and Electrochemical Behavior
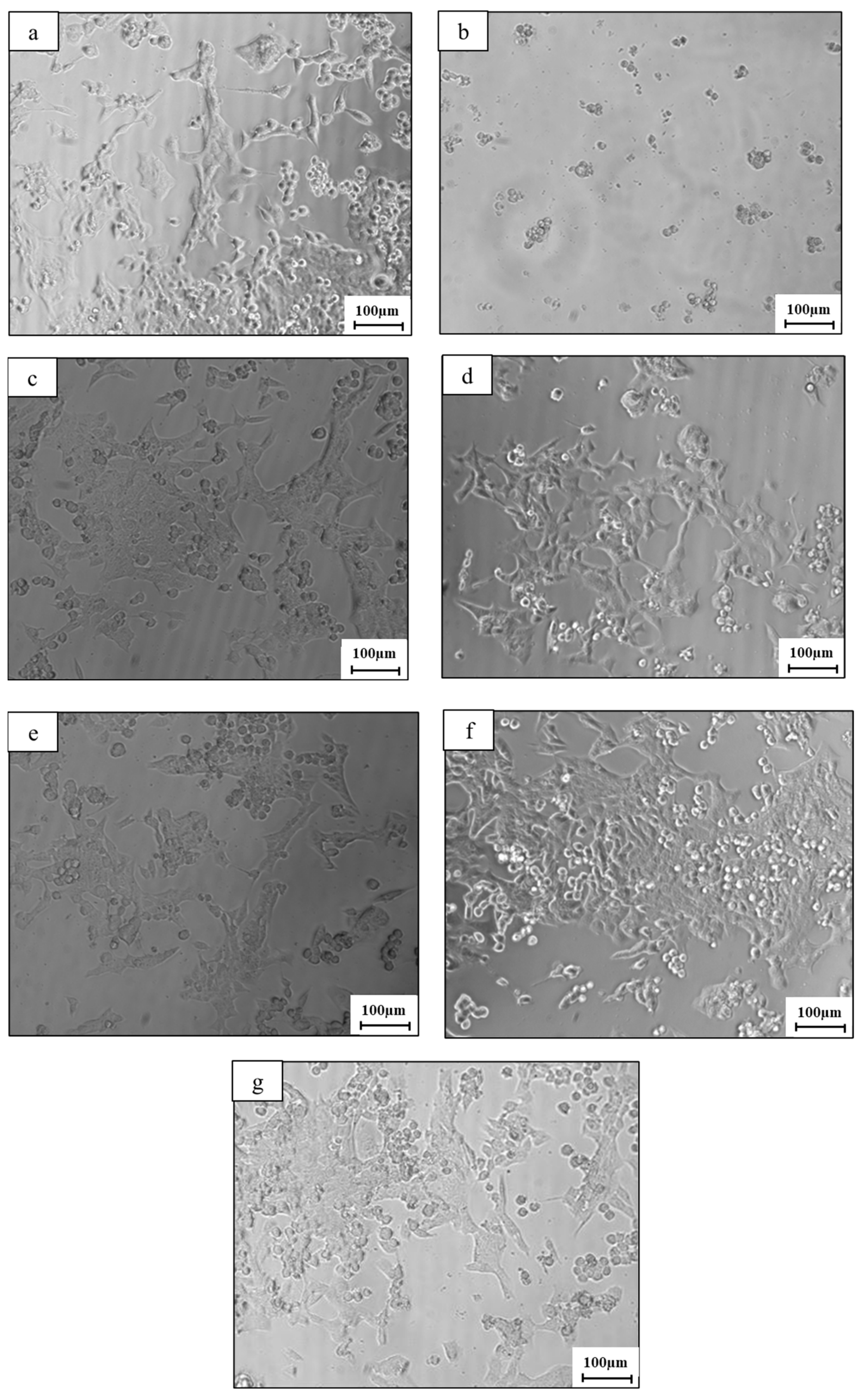
2.5. Cytotoxicity Analysis
3. Results
4. Discussion
5. Conclusions
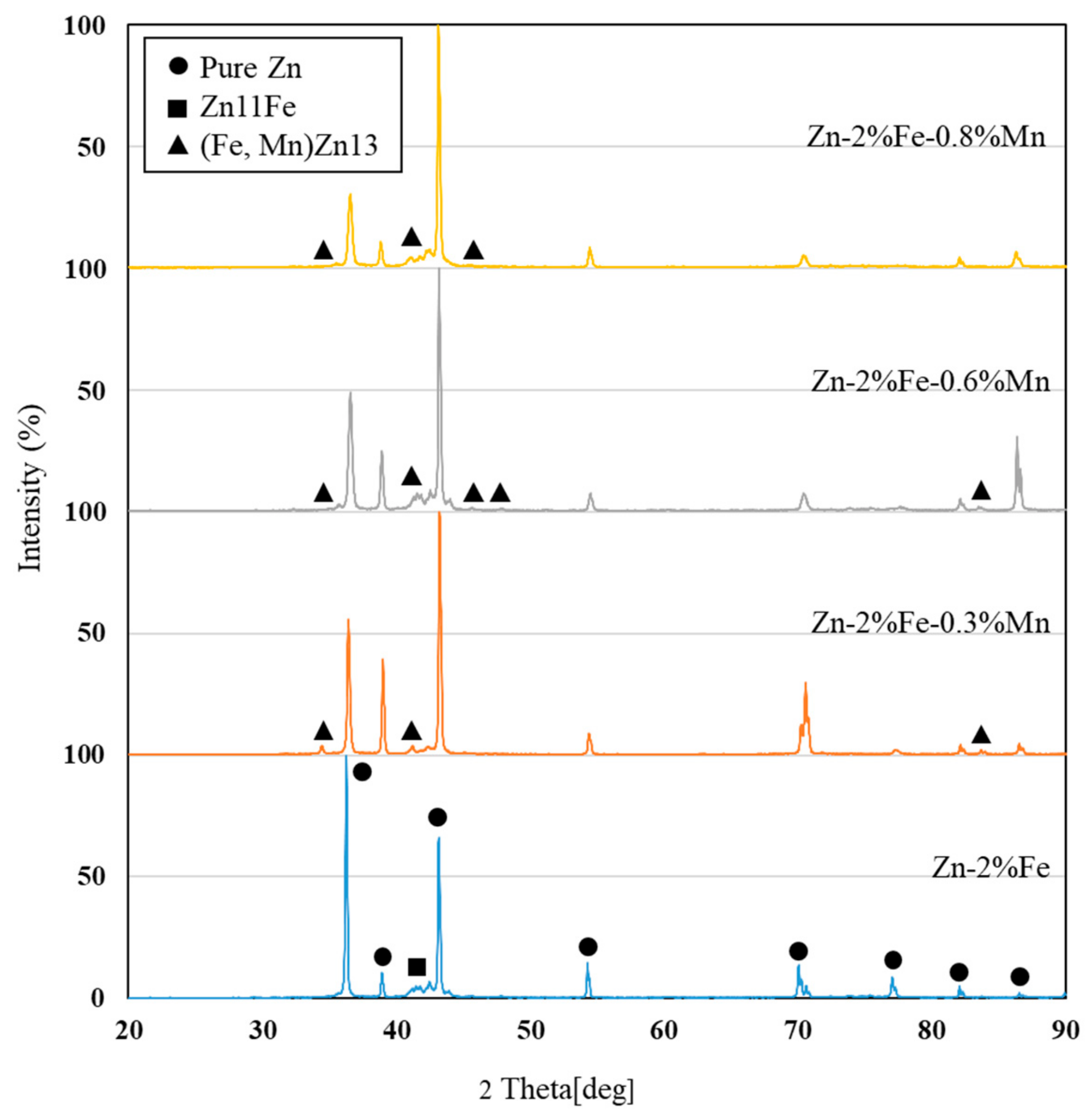
- Additions of up to 0.8% Mn to biodegradable Zn-2%Fe alloy resulted in increased hardness and tensile strength in terms of yield point and ultimate tensile strength, while slightly reducing ductility. The main mechanism of strength enhancement was solution strengthening that was amplified by the precipitation hardening of a secondary phase (Fe, Mn)Zn13.
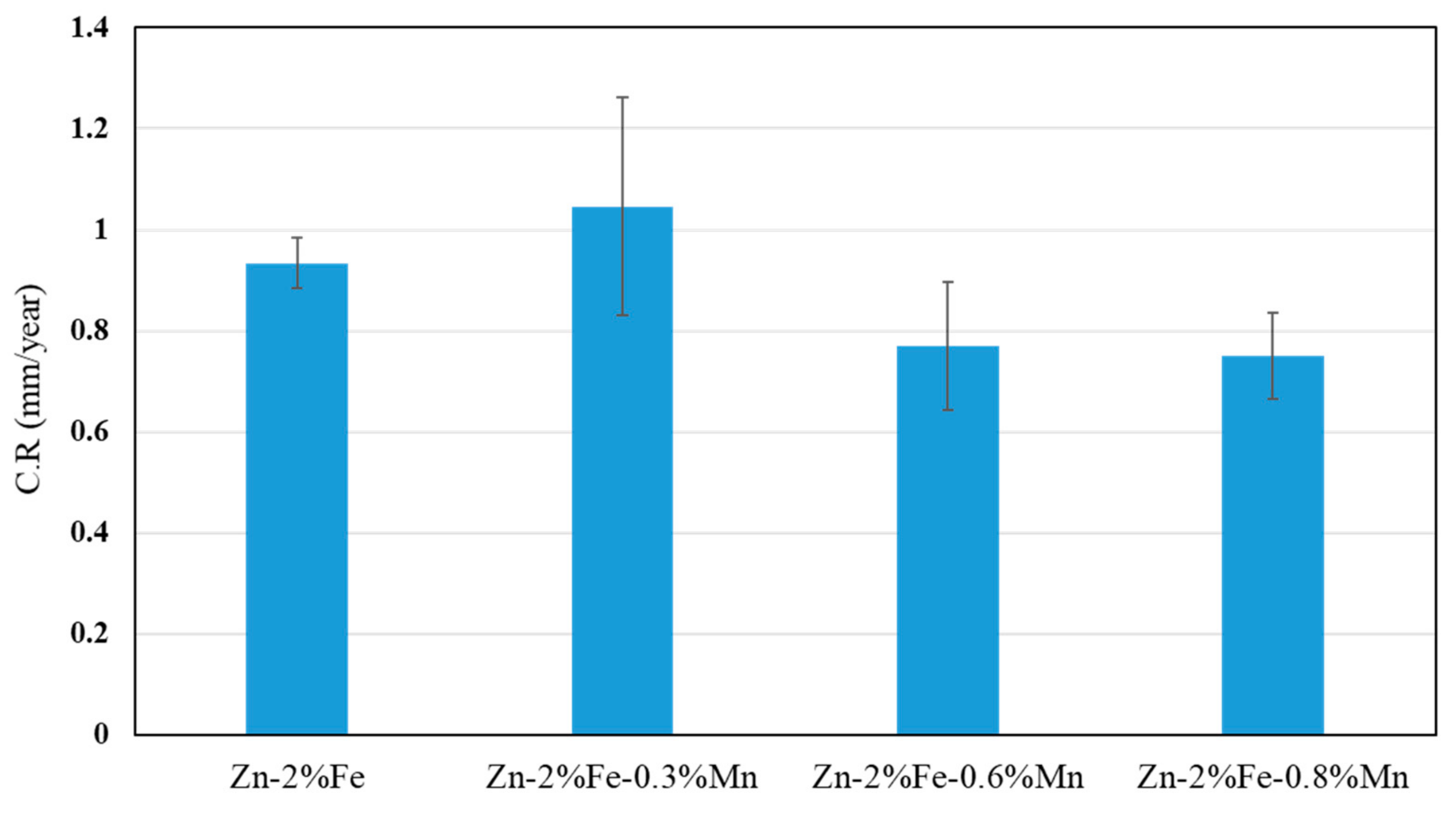
- The effect of Mn additions on the corrosion degradation of Zn-2%Fe alloy in a simulated physiological environment was insignificant. This was related to the balanced effect between reduced degradability due to Mn reaction with Fe and increased degradability attributed to the inherent microgalvanic corrosion between the Zn-based matrix and the Mn-base substance.
- Indirect cell viability assessment showed that the addition of Mn tends to increase cell viability in in vitro conditions. This may indicate that the cytotoxicity of the Zn-2%Fe-based alloy was comparatively reduced after the addition of Mn to this alloy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential Biodegradable Zn-Cu Binary Alloys Developed for Cardiovascular Implant Applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-Alloying with Mn in Zn–Mg Alloy for Future Biodegradable Metals Application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Aghion, E.; Yered, T.; Perez, Y.; Gueta, Y. The Prospects of Carrying and Releasing Drugs via Biodegradable Magnesium Foam. Adv. Eng. Mater. 2010, 12, B374–B379. [Google Scholar] [CrossRef]
- Han, C.; Fang, Q.; Shi, Y.; Tor, S.B.; Chua, C.K.; Zhou, K. Recent Advances on High-Entropy Alloys for 3D Printing. Adv. Mater. 2020, 32, e1903855. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Deng, G.; Deng, C.; Bai, Y.; Yang, Y.; Wu, W.; Chen, J.; Liu, Y.; Wang, Y.; et al. Recent progress on additive manufacturing of multi-material structures with laser powder bed fusion. Virtual Phys. Prototyp. 2022, 17, 329–365. [Google Scholar] [CrossRef]
- Shen, C.; Liu, X.; Fan, B.; Lan, P.; Zhou, F.; Li, X.; Wang, H.; Xiao, X.; Li, L.; Zhao, S.; et al. Mechanical Properties, in Vitro Degradation Behavior, Hemocompatibility and Cytotoxicity Evaluation of Zn–1.2 Mg Alloy for Biodegradable Implants. RSC Adv. 2016, 6, 86410–86419. [Google Scholar] [CrossRef]
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef]
- Aksakal, B.; Yildirim, S.; Gul, H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J. Fail. Anal. Prev. 2004, 4, 17–23. [Google Scholar] [CrossRef]
- Pospíšilová, I.; Soukupová, V.; Vojtěch, D. Influence of Calcium on the Structure and Mechanical Properties of Biodegradable Zinc Alloys. In Materials Science Forum; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2017. [Google Scholar]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design Strategy for Biodegradable Fe-Based Alloys for Medical Applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F. Effects of Alloying Elements (Mn, Co, Al, W, Sn, B, C and S) on Biodegradability and in Vitro Biocompatibility of Pure Iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef]
- Montani, M.; Demir, A.G.; Mostaed, E.; Vedani, M.; Previtali, B. Processability of pure Zn and pure Fe by SLM for biodegradable metallic implant manufacturing. Rapid Prototyp. J. 2017, 23, 514–523. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, D.; Xin, C.; Liu, X.; Lin, W.; Zhang, W.; Chen, S.; Sun, K. Characterization and in vivo evaluation of a bio-corrodible nitrided iron stent. J. Mater. Sci. Mater. Med. 2012, 24, 713–724. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-Based Alloys for Use in Osteosynthesis: Outcome of an in Vivo Study after 52 Weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Schinhammer, M.; Gerber, I.; Hänzi, A.C.; Uggowitzer, P.J. On the Cytocompatibility of Biodegradable Fe-Based Alloys. Mater. Sci. Eng. C 2013, 33, 782–789. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, E.; Yang, K. Microstructure, corrosion properties and bio-compatibility of calcium zinc phosphate coating on pure iron for biomedical application. Mater. Sci. Eng. C 2013, 34, 201–206. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. The Effects of 4%Fe on the Performance of Pure Zinc as Biodegradable Implant Material. Ann. Biomed. Eng. 2019, 47, 1400–1408. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B.; Eliezer, D.; Von Buch, F.; Schumann, S.; Friedrich, H.E. The Art of Developing New Magnesium Alloys for High Temperature Applications. In Materials Science Forum; Trans Tech Publications Ltd: Freienbach, Switzerland, 2003. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In Vitro and in Vivo Corrosion Measurements of Magnesium Alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of Biodegradation of Biocompatable Magnesium Alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In vivo performances of pure Zn and Zn–Fe alloy as biodegradable implants. J. Mater. Sci. Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Aghion, E.; Gueta, Y.; Moscovitch, N.; Bronfin, B. Effect of yttrium additions on the properties of grain-refined Mg–3%Nd alloy. J. Mater. Sci. 2008, 43, 4870–4875. [Google Scholar] [CrossRef]
- Avior, O.; Ben Ghedalia-Peled, N.; Ron, T.; Vago, R.; Aghion, E. The Effect of Ca on In Vitro Behavior of Biodegradable Zn-Fe Alloy in Simulated Physiological Environments. Metals 2020, 10, 1624. [Google Scholar] [CrossRef]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in Vitro and in Vivo Degradation Performance and Biological Response of New Biodegradable Mg–Y–Zn Alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef]
- Cha, P.R.; Han, H.S.; Yang, G.F.; Kim, Y.C.; Hong, K.H.; Lee, S.C.; Jung, J.Y.; Ahn, J.P.; Kim, Y.Y.; Cho, S.Y.; et al. Biodegradability Engineering of Biodegradable Mg Alloys: Tailoring the Electrochemical Properties and Microstructure of Constituent Phases. Sci. Rep. 2013, 3, 2367. [Google Scholar] [CrossRef] [Green Version]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Maier, P.; Hort, N. Magnesium Alloys for Biomedical Applications. Metals 2020, 10, 1328. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Han, Y.; Zhang, Z.; Qu, X.; Zhuang, Y.; Wu, Q.; Zheng, Y.; Dai, K. In Vitro and in Vivo Studies of Zn-Mn Biodegradable Metals Designed for Orthopedic Applications. Acta Biomater. 2020, 108, 358–372. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.F.; Yin, Y.X.; Shi, Z.Z.; Li, T.; Feng, X.Y.; Zhang, J.W.; Song, C.X.; Cui, X.S.; Xu, K.L.; et al. Long-Term in Vivo Study of Biodegradable Zn-Cu Stent: A 2-Year Implantation Evaluation in Porcine Coronary Artery. Acta Biomater. 2019, 97, 657–670. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Zhang, Z.; Qu, X.; Jia, X.; Wu, Q.; Han, Y.; Zheng, Y.; Dai, K. Biodegradable Zn–Sr Alloy for Bone Regeneration in Rat Femoral Condyle Defect Model: In Vitro and in Vivo Studies. Bioact. Mater. 2021, 6, 1588–1604. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The Galvanization of Biology: A Growing Appreciation for the Roles of Zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Li, Z.-L.; Bai, W.-S.; Tuoliken, A.; Yu, J.; Liu, X.-F. (Fe, Mn)Zn13 phase and its core-shell structure in novel biodegradable Zn-Mn-Fe alloys. Mater. Des. 2019, 162, 235–245. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2012, 24, 437–445. [Google Scholar] [CrossRef]
- Wei, Z.; Burwinkel, M.; Palissa, C.; Ephraim, E.; Schmidt, M.F. Antiviral Activity of Zinc Salts against Transmissible Gastroenteritis Virus in Vitro. Vet. Microbiol. 2012, 160, 468–472. [Google Scholar] [CrossRef]
- Russell, R.; Beard, J.; Cousins, R.; Dunn, J.T.; Ferland, G.; Hambidge, K.M.; Lynch, S.; Penland, J.; Ross, A.C.; Stoeker, B.J.; et al. A report of the panel. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef] [Green Version]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The Importance of Mineral Elements for Humans, Domestic Animals and Plants—A Review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Shi, Z.Z.; Yu, J.; Liu, X.F. Microalloyed Zn-Mn Alloys: From Extremely Brittle to Extraordinarily Ductile at Room Temperature. Mater. Des. 2018, 144, 343–352. [Google Scholar] [CrossRef]
- Itzhak, D.; Aghion, E. An Anodic Behaviour Study of an Analogical Sintered System of Austenitic Stainless Steel in H2SO4 Solution. Corros. Sci. 1984, 24, 145–149. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for Modifying Current Cytotoxicity Testing Standards for Biodegradable Magnesium-Based Materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical Characteristics, in Vitro Degradation, Cytotoxicity, and Antibacterial Evaluation of Zn-4.0 Ag Alloy as a Biodegradable Material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef] [Green Version]
- Bhan, S.; Lal, A.; Singh, S. The Fe-Mn-Zn system (iron-manganese-zinc). J. Phase Equilibria Diffus. 1991, 12, 667–672. [Google Scholar] [CrossRef]
- Lee, H.H.; Hiam, D. Corrosion Resistance of Galvannealed Steel. Corrosion 1989, 45, 852–856. [Google Scholar] [CrossRef]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Avedesian, M.; Baker, H. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International: Washington, DC, USA, 1999. [Google Scholar]
- Bronfin, B.; Aghion, E.; von Buch, F.; Schumann, S.; Katsir, M. Die Casting Magnesium Alloys for Elevated Temperatures Applications. In Proceedings of the Magnesium Technology 2001, New Orleans, LA, USA, 11–15 February 2001. [Google Scholar] [CrossRef]
- Vanýsek, P. Electrochemical Series. In Handbook of Chemistry and Physics, 93rd ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 5–80. [Google Scholar]
- Prasadh, S.; Gupta, M.; Wong, R. In Vitro Cytotoxicity and Osteogenic Potential of Quaternary Mg-2Zn-1Ca/X-Mn Alloys for Craniofacial Reconstruction. Sci. Rep. 2022, 12, 8259. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Li, Y.; Wei, Y.; Xi, X.; Cai, K. Microstructure, Mechanical and Bio-Corrosion Properties of Mn-Doped Mg–Zn–Ca Bulk Metallic Glass Composites. Mater. Sci. Eng. C 2013, 33, 3832–3838. [Google Scholar] [CrossRef]
- Wang, J.-L.; Wan, Y.; Ma, Z.-J.; Guo, Y.-C.; Yang, Z.; Wang, P.; Li, J.-P. Glass-forming ability and corrosion performance of Mn-doped Mg–Zn–Ca amorphous alloys for biomedical applications. Rare Met. 2018, 37, 579–586. [Google Scholar] [CrossRef]





| Tested Alloy | Fe | Mn | Cu | Ca | Zn |
|---|---|---|---|---|---|
| Zn-2%Fe | 1.9 | 0.005 | 0.006 | 0.007 | Balance |
| Zn-2%Fe-0.3%Mn | 1.7 | 0.3 | 0.004 | 0.01 | Balance |
| Zn-2%F-0.6%Mn | 1.7 | 0.6 | 0.007 | 0.007 | Balance |
| Zn-2%Fe-0.8%Mn | 1.8 | 0.8 | 0.003 | 0.004 | Balance |
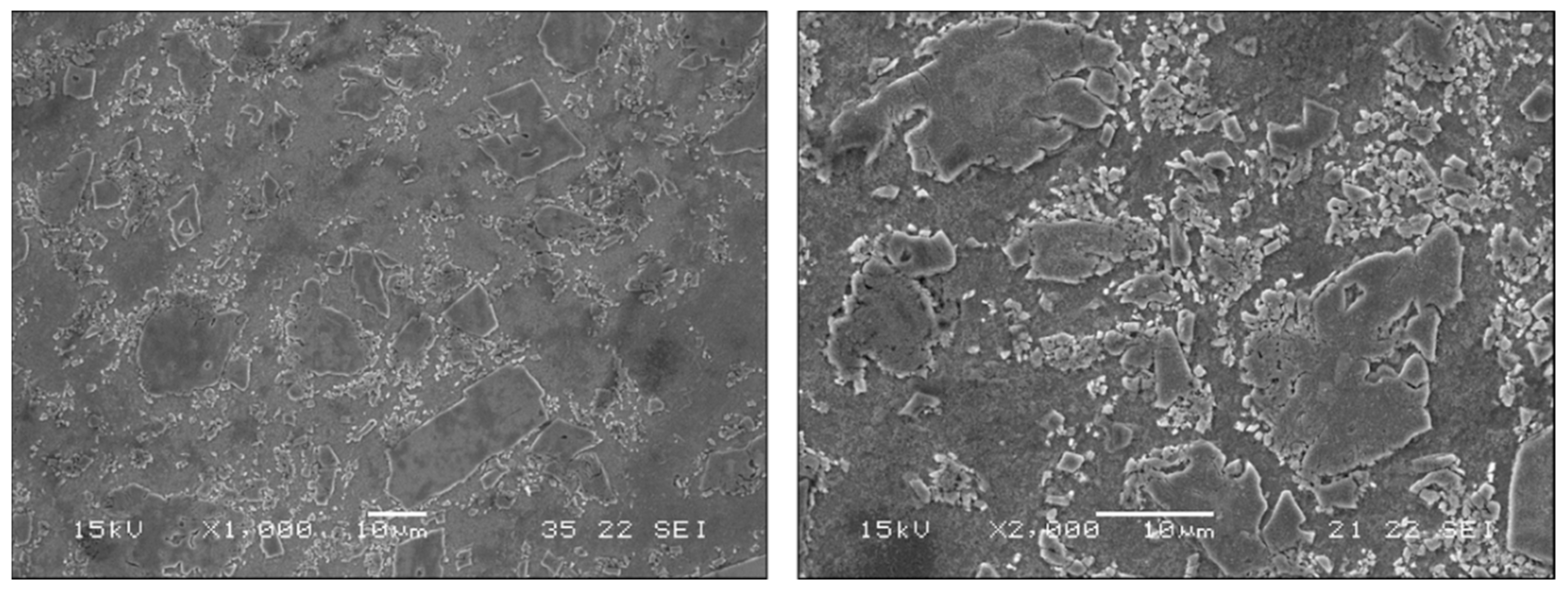
| Point | Zn | Fe | Mn | Dominant Phases |
|---|---|---|---|---|
| 1 | 98.7 ± 0.3 | 0.4 ± 0.2 | 0.9 ± 0.1 | Zn base matrix |
| 2 | 91.3 ± 1 | 7.5 ± 0.8 | 1.2 ± 0.2 | Zn11Fe |
| 3 | 96.3 ± 0.9 | 2.2 ± 0.7 | 1.5 ± 0.3 | (Fe, Mn)Zn13 |
| Tested Alloy | Y.P (MPa) | UTS (MPa) | Elongation (%) |
|---|---|---|---|
| Zn-2%Fe | 69 ± 4 | 119 ± 9 | 13 ± 1 |
| Zn-2%Fe-0.3%Mn | 136 ± 8 | 199 ± 12 | 12 ± 2 |
| Zn-2%Fe-0.6%Mn | 177 ± 10 | 221 ± 13 | 8.5 ± 1 |
| Zn-2%Fe-0.8%Mn | 191 ± 7 | 224 ± 9 | 8.1 ± 2 |
| Corrosion Parameter | Zn-2%Fe | Zn-2%Fe-0.3%Mn | Zn-2%Fe-0.6%Mn | Zn-2%Fe-0.8%Mn |
|---|---|---|---|---|
| Ecorr (V) | −1.182 ± 0.09 | 1.177 ± 0.02 | −1.171 ± 0.01 | −1.192 ± 0.01 |
| I- corrosion current density (µA/cm2) | 1.353 ± 0.1 | 1.478 ± 0.3 | 1.61 ± 0.2 | 2.314 ± 0.3 |
| C.R (mmpy) | 0.072 ± 0.006 | 0.079 ± 0.01 | 0.086 ± 0.001 | 0.124± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Tzion-Mottye, L.; Ron, T.; Eliezer, D.; Aghion, E. The Effect of Mn on the Mechanical Properties and In Vitro Behavior of Biodegradable Zn-2%Fe Alloy. Metals 2022, 12, 1291. https://doi.org/10.3390/met12081291
Ben Tzion-Mottye L, Ron T, Eliezer D, Aghion E. The Effect of Mn on the Mechanical Properties and In Vitro Behavior of Biodegradable Zn-2%Fe Alloy. Metals. 2022; 12(8):1291. https://doi.org/10.3390/met12081291
Chicago/Turabian StyleBen Tzion-Mottye, Lital, Tomer Ron, Dan Eliezer, and Eli Aghion. 2022. "The Effect of Mn on the Mechanical Properties and In Vitro Behavior of Biodegradable Zn-2%Fe Alloy" Metals 12, no. 8: 1291. https://doi.org/10.3390/met12081291







