Bioinspired Surface Design for Magnesium Alloys with Corrosion Resistance
Abstract
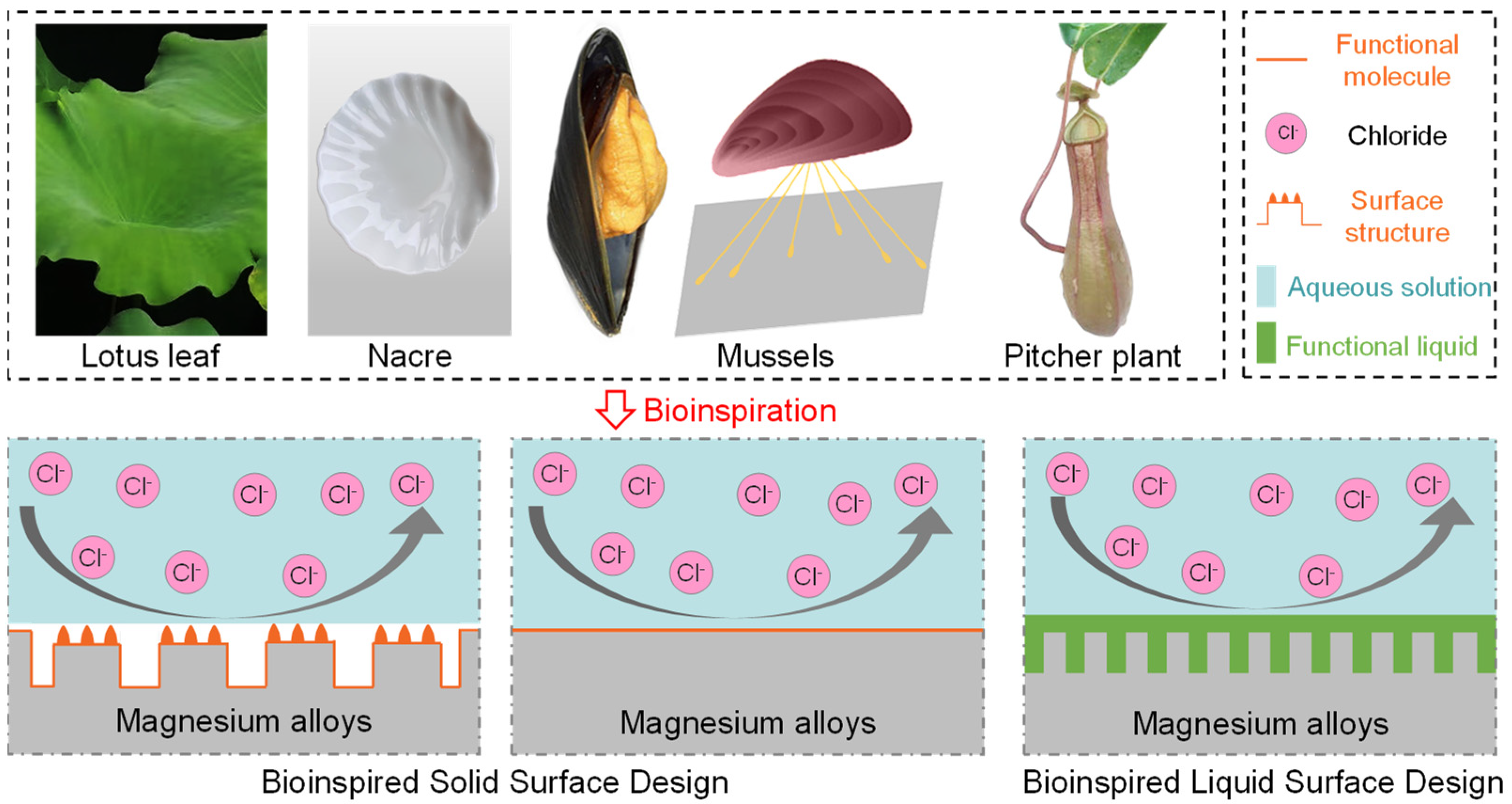
:1. Introduction
2. Corrosion Mechanism
3. Bioinspired Solid Surface Design
3.1. Bioinspired Surface Structure
3.2. Bioinspired Surface Modification
4. Bioinspired Liquid Surface Design
- The functional liquid must be stabilized on the magnesium alloys’ surfaces;
- The functional liquid must preferentially wet the surfaces of the magnesium alloys over an aqueous solution;
- The functional liquid and aqueous solution must be immiscible.
4.1. Bioinspired Surface Structure
4.2. Bioinspired Surface Modification
- The surface tension of functional liquids is much lower than that of water, leading to water cloaking and the gradual loss of functional liquid by entraining as the droplet falls off the surface. The cloak effect is common when there is inadequate functional liquid on the surface. The spreading coefficient provides the cloaking criterion [95]:where denotes the surface tension of the aqueous solution; the interfacial surface tension between the functional liquid and aqueous solution; the surface tension of the functional liquid, and the subscripts f and aq the functional liquid and aqueous solution, respectively.
- 2.
- The surface energy of the functional liquid is higher, so the aqueous solution can spread out on the functional liquid and cover the magnesium alloys’ surface:where denotes the interfacial surface tension between aqueous solution and functional liquid.
- 3.
- The functional liquid cannot completely infiltrate the magnesium alloys’ surface.
- 4.
- The aqueous solution shows a higher affinity for the magnesium alloys’ surface and can replace the functional liquid. Similarly, the spreading coefficient of functional liquid on magnesium alloys when in contact with air or aqueous solution can be calculated:where denotes the surface energy of magnesium alloys, the interfacial surface tension between the functional liquid and magnesium alloys, the interfacial surface tension between the aqueous solution and magnesium alloys, and the subscripts f, aq, and m the functional liquid, aqueous solution, and magnesium alloys, respectively. According to the three bioinspired liquid surface criteria, the magnesium alloys are closer to the functional liquid when , where φ represents the fraction of the magnesium alloys’ surface area filled by the functional liquid.
4.3. Functional Liquid
- Low surface tension, allowing it to spread readily and penetrate roughness.
- 2.
- Low vapor pressure (<1 Pa), so as not to evaporate fast.
- 3.
- Chemical inertness, in that it is not quickly destroyed when exposed to other chemicals.
- 4.
- Wide range of viscosity, with <100 cSt being the most typical; this value is neither too low to delay functional liquid depletion nor too high to hasten functional liquid infusion.
4.4. Application
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research—A review. J. Magnes. Alloy. 2021, 10, 1–61. [Google Scholar] [CrossRef]
- Park, J.E.; Jang, Y.S.; Choi, J.B.; Bae, T.S.; Park, I.S.; Lee, M.H. Evaluation of Corrosion Behavior and In Vitro of Strontium-Doped Calcium Phosphate Coating on Magnesium. Materials 2021, 14, 6625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Wei, Y.; Zeng, T.; Cao, B.; Liang, J. Preparation and Characterization of Hydroxyapatite Coating on AZ31 Magnesium Alloy Induced by Carboxymethyl Cellulose-Dopamine. Materials 2021, 14, 1849. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhai, C.; Gu, Q.; Zhu, W.; Li, Q. Experimental study and thermodynamic evaluation of Mg–La–Zn system. J. Alloys Compd. 2020, 814, 152297. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Hou, L.; Li, Z.; Pan, Y.; Du, L.; Li, X.; Zheng, Y.; Li, L. In vitro and in vivo studies on biodegradable magnesium alloy. Prog. Nat. Sci. Mater. Int. 2014, 24, 466–471. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel magnesium alloys developed for biomedical application: A review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Jamel, M.; Lopez, H.; Schultz, B.; Otieno, W. The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium. Metals 2021, 11, 1264. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Hou, J.; Du, W. A review on thermal conductivity of magnesium and its alloys. J. Magnes. Alloy. 2020, 8, 78–90. [Google Scholar] [CrossRef]
- Mordike, B.; Ebert, T. Magnesium: Properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Liu, J. Comparative study on yield behavior and non-associated yield criteria of AZ31B and ZK61 M magnesium alloys. Mater. Sci. Eng. A 2019, 759, 329–345. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A comprehensive review on surface modifications of biodegradable magnesium-based implant alloy: Polymer coatings opportunities and challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Duygulu, O.; Kaya, R.A.; Oktay, G.; Kaya, A.A. In Investigation on the potential of magnesium alloy AZ31 as a bone implant. Mater. Sci. Forum 2007, 546, 421–424. [Google Scholar] [CrossRef]
- Denkena, B.; Witte, F.; Podolsky, C.; Lucas, A. In Degradable implants made of magnesium alloys. In Proceedings of the 5th EUSPEN International Conference, Montpellier, France, 8–11 May 2005. [Google Scholar]
- Rahim, M.I.; Ullah, S.; Mueller, P.P. Advances and challenges of biodegradable implant materials with a focus on magnesium-alloys and bacterial infections. Metals 2018, 8, 532. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable magnesium alloys developed as bone repair materials: A review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef]
- Herber, V.; Okutan, B.; Antonoglou, G.; Sommer, G.N.; Payer, M. Bioresorbable magnesium-based alloys as novel biomaterials in oral bone regeneration: General review and clinical perspectives. J. Clin. Med. 2021, 10, 1842. [Google Scholar] [CrossRef]
- Liu, C.; Xin, Y.; Tian, X.; Chu, P.K. Degradation susceptibility of surgical magnesium alloy in artificial biological fluid containing albumin. J. Mater. Res. 2007, 22, 1806–1814. [Google Scholar] [CrossRef]
- Hsu, C.S.; Nazari, M.H.; Li, Q.; Shi, X. Enhancing degradation and corrosion resistance of AZ31 magnesium alloy through hydrophobic coating. Mater. Chem. Phys. 2019, 225, 426–432. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef]
- Harandi, S.E.; Banerjee, P.C.; Easton, C.D.; Raman, R.S. Influence of bovine serum albumin in Hanks’ solution on the corrosion and stress corrosion cracking of a magnesium alloy. Mater. Sci. Eng. C 2017, 80, 335–345. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Chen, L. Influence of artificial biological fluid composition on the biocorrosion of potential orthopedic Mg–Ca, AZ31, AZ91 alloys. Biomed. Mater. 2009, 4, 065011. [Google Scholar] [CrossRef] [PubMed]
- Höche, D.; Blawert, C.; Lamaka, S.V.; Scharnagl, N.; Mendis, C.; Zheludkevich, M.L. The effect of iron re-deposition on the corrosion of impurity-containing magnesium. Phys. Chem. Chem. Phys. 2016, 18, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of magnesium hydroxide and aluminum hydroxide on the thermal stability, latent heat and flammability properties of Paraffin/HDPE phase change blends. Polymers 2020, 12, 180. [Google Scholar] [CrossRef]
- Chen, X.B.; Zhou, X.; Abbott, T.B.; Easton, M.A.; Birbilis, N. Double-layered manganese phosphate conversion coating on magnesium alloy AZ91D: Insights into coating formation, growth and corrosion resistance. Surf. Coat. Technol. 2013, 217, 147–155. [Google Scholar] [CrossRef]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Du, Y. Investigation on the corrosion resistance of the Mg-10Al-xMn alloys based on thermodynamic calculations. Corros. Sci. 2021, 189, 109631. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion electrochemistry of magnesium (Mg) and its alloys. Corros. Magnes. Alloy. 2011, 56, 3–65. [Google Scholar]
- Gerengi, H.; Cabrini, M.; Solomon, M.; Kaya, E. Understanding the Corrosion Behavior of the AZ91D Alloy in Simulated Body Fluid through the Use of Dynamic EIS. ACS Omega 2022, 7, 11929–11938. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B.; Vivier, V. An impedance investigation of the mechanism of pure magnesium corrosion in sodium sulfate solutions. J. Electrochem. Soc. 2006, 154, C108. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Abdalla, M.; Joplin, A.; Elahinia, M.; Ibrahim, H. Corrosion modeling of magnesium and its alloys for biomedical applications. Corros. Mater. Degrad. 2020, 1, 11. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Z.; Li, G.; Jiang, Q.; Lian, J. Electroless Ni-P/Ni-B duplex coatings for improving the hardness and the corrosion resistance of AZ91D magnesium alloy. Appl. Surf. Sci. 2008, 254, 4949–4955. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef]
- Fan, X.L.; Li, C.Y.; Wang, Y.B.; Huo, Y.F.; Li, S.Q.; Zeng, R.C. Corrosion resistance of an amino acid-bioinspired calcium phosphate coating on magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 49, 224–235. [Google Scholar] [CrossRef]
- Wang, L.; Aversa, R.; Houa, Z.; Tian, J.; Liang, S.; Ge, S.; Chen, Y.; Perrotta, V.; Apicella, A.; Apicella, D. Bioresorption Control and Biological Response of Magnesium Alloy AZ31 Coated with Poly-β-Hydroxybutyrate. Appl. Sci. 2021, 11, 5627. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Current status and future prospects of applying bioinspired superhydrophobic materials for conservation of stone artworks. Coatings 2020, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Song, W.; Li, Z.; Cong, Q. Fabrication of superhydrophobic AAO-Ag multilayer mimicking dragonfly wings. Chin. Sci. Bull. 2012, 57, 4635–4640. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Appl. Mater. Interfaces 2017, 9, 11247–11257. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Yu, X.; Lin, L.; Guo, X.; Liu, M.; Jiang, L. Corrosion-resistant superhydrophobic coatings on Mg alloy surfaces inspired by lotus seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar] [CrossRef]
- Ishizaki, T.; Masuda, Y.; Sakamoto, M. Corrosion resistance and durability of superhydrophobic surface formed on magnesium alloy coated with nanostructured cerium oxide film and fluoroalkylsilane molecules in corrosive NaCl aqueous solution. Langmuir 2011, 27, 4780–4788. [Google Scholar] [CrossRef]
- Chu, J.; Tong, L.; Wen, M.; Jiang, Z.; Wang, K.; Zhang, H. Graphene oxide film as a protective barrier for Mg alloy: Worse or better is dependent on a chemical reduction process. Carbon 2019, 145, 389–400. [Google Scholar] [CrossRef]
- Jiang, D.; Zhou, H.; Wan, S.; Cai, G.Y.; Dong, Z.H. Fabrication of superhydrophobic coating on magnesium alloy with improved corrosion resistance by combining micro-arc oxidation and cyclic assembly. Surf. Coat. Technol. 2018, 339, 155–166. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, L.; Ji, X.; Li, J.; Han, Z.; Li, S.; Zeng, R.; Zhang, F.; Wang, Z. Corrosion resistance and antibacterial properties of polysiloxane modified layer-by-layer assembled self-healing coating on magnesium alloy. J. Colloid Interface Sci. 2018, 526, 43–50. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Liu, H.; Liu, Y.; Guo, L.; Jia, D.; Ouyang, J.; Zhou, Y. The fabrication and hydrophobic property of micro-nano patterned surface on magnesium alloy using combined sparking sculpture and etching route. Appl. Surf. Sci. 2016, 389, 80–87. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Li, B.; Zhao, X.; Zhang, J. Long-term corrosion protection for magnesium alloy by two-layer self-healing superamphiphobic coatings based on shape memory polymers and attapulgite. J. Colloid Interface Sci. 2021, 594, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, X.; Zhang, J.; Wang, Y.; Han, Z.; Ren, L. Biomimetic hydrophobic surface fabricated by chemical etching method from hierarchically structured magnesium alloy substrate. Appl. Surf. Sci. 2013, 280, 845–849. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, H.; Hu, X. A rapid one-step process for the construction of corrosion-resistant bionic superhydrophobic surfaces. Prog. Org. Coat. 2016, 100, 56–62. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, M.; Zhai, J.; Wang, J.; Jiang, L. Bioinspired construction of Mg–Li alloys surfaces with stable superhydrophobicity and improved corrosion resistance. Appl. Phys. Lett. 2008, 92, 183103. [Google Scholar] [CrossRef]
- Liang, M.; Wei, Y.; Hou, L.; Wang, H.; Li, Y.; Guo, C. Fabrication of a super-hydrophobic surface on a magnesium alloy by a simple method. J. Alloys Compd. 2016, 656, 311–317. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical deposition of nanomaterials for electrochemical sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Chang, X.; Li, M.; Tang, S.; Shi, L.; Chen, X.; Niu, S.; Zhu, X.; Wang, D.; Sun, S. Superhydrophobic micro-nano structured PTFE/WO3 coating on low-temperature steel with outstanding anti-pollution, anti-icing, and anti-fouling performance. Surf. Coat. Technol. 2022, 434, 128214. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.C.; He, H.; Ouyang, L.; Yuan, S. A stearic Acid/CeO2 bilayer coating on AZ31B magnesium alloy with superhydrophobic and self-cleaning properties for corrosion inhibition. J. Alloys Compd. 2020, 834, 155210. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, Y.; Wang, J.; Shi, X. One-step hydrothermal process to fabricate superhydrophobic surface on magnesium alloy with enhanced corrosion resistance and self-cleaning performance. Appl. Surf. Sci. 2017, 422, 566–573. [Google Scholar] [CrossRef]
- Yu, D.; Qiu, H.; Mou, X.; Dou, Z.; Zhou, N.; Guo, Q.; Lyu, N.; Lu, L.; Yang, Z.; Huang, N. One-pot but two-step vapor-based amine-and fluorine-bearing dual-layer coating for improving anticorrosion and biocompatibility of magnesium alloy. ACS Biomater. Sci. Eng. 2019, 5, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Wang, H.Y.; Liu, Y.; Wei, D.S.; Zhao, Z.X. Large-scale fabrication of durable and robust super-hydrophobic spray coatings with excellent repairable and anti-corrosion performance. Chem. Eng. J. 2019, 367, 169–179. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, B.; Gu, Y.; Shen, C.; Wen, J.; Meng, Z.; Chen, S.; Ou, J.; Qin, A. New approach for improving anticorrosion and biocompatibility of magnesium alloys via polydopamine intermediate layer-induced hydroxyapatite coating. Surf. Interfaces 2020, 19, 100501. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Zhang, K.; He, Z.; Yang, P.; Zou, D.; Huang, N. Multifunctional coating based on hyaluronic acid and dopamine conjugate for potential application on surface modification of cardiovascular implanted devices. ACS Appl. Mater. Interfaces 2016, 8, 109–121. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Zhang, K.; He, Z.; Zou, D.; Luo, X.; Fan, Y.; Yang, P.; Zhao, A.; Huang, N. Controlling molecular weight of hyaluronic acid conjugated on amine-rich surface: Toward better multifunctional biomaterials for cardiovascular implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. [Google Scholar] [CrossRef]
- Wu, F. Bioinspired Design for Medical Applications. In Biomaterials and Materials for Medicine; CRC Press: Boca Raton, FL, USA, 2021; pp. 319–328. [Google Scholar]
- Sun, J.; Zhu, Y.; Meng, L.; Chen, P.; Shi, T.; Liu, X.; Zheng, Y. Electrophoretic deposition of colloidal particles on Mg with cytocompatibility, antibacterial performance, and corrosion resistance. Acta Biomater. 2016, 45, 387. [Google Scholar] [CrossRef]
- Shivakumar, M.; Dharmaprakash, M.S.; Manjappa, S.; Nagashree, K.L. Corrosion inhibition performance of lignin extracted from black liquor on mild steel in 0.5 m H2SO4 acidic media. Port. Electrochim. Acta 2017, 35, 351. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Talebibahmanbigloo, N.; Javidparvar, A.A.; Bahlakehd, G.; Ramezanzadeh, B. Studying the adsorption/inhibition impact of the cellulose and lignin compounds extracted from agricultural waste on the mild steel corrosion in HCl solution. J. Mol. Liq. 2020, 304, 112751. [Google Scholar] [CrossRef]
- Guedes, L.A.; Bacca, K.G.; Lopes, N.F.; Costa, E.M. Tannin of Acacia mearnsii as green corrosion inhibitor for AA7075-T6 alluminum alloy in acidic medium. Mater. Corros. 2019, 70, 1288. [Google Scholar] [CrossRef]
- Abdulmajid, A.; Hamidon, T.S.; Rahim, A.A.; Hussin, M.H. Tamarind shell tannin extracts as green corrosion inhibitors of mild steel in hydrochloric acid medium. Mater. Res. Express 2019, 6, 106579. [Google Scholar] [CrossRef]
- AhadiParsa, M.; Mohammadloo, H.E.; Mirabedini, S.M.; Roshanc, S. Bio-corrosion assessment and surface study of hydroxyapatite-coated AZ31 Mg alloy pre-treated with vinyl triethoxy silane. Mater. Chem. 2022, 287, 126147. [Google Scholar]
- Qian, B.; Zheng, Z.; Michailids, M.; Fleck, N.; Bilton, M.; Song, Y.; Li, G.; Shchukin, D. Mussel-inspired self-healing coatings based on polydopamine-coated nanocontainers for corrosion protection. ACS Appl. Mater. Interfaces 2019, 11, 10283–10291. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Zhu, W.; Fang, L.; Ren, F. Mussel-inspired nano-multilayered coating on magnesium alloys for enhanced corrosion resistance and antibacterial property. Colloids Surf. B Biointerfaces 2017, 157, 432–439. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Chang, J.; Jin, S.; Wu, D.; Yan, H.; Wang, X.; Guan, S. Mg-Zn-Y-Nd coated with citric acid and dopamine by layer-by-layer self-assembly to improve surface biocompatibility. Sci. China Technol. Sci. 2018, 61, 1228–1237. [Google Scholar] [CrossRef]
- Bahremand, F.; Shahrabi, T.; Ramezanzadeh, B. Development of a nanostructured film based on samarium (III)/polydopamine on the steel surface with superior anti-corrosion and water-repellency properties. J. Colloid Interface Sci. 2021, 582, 342. [Google Scholar] [CrossRef]
- Carangelo, A.; Acquesta, A.; Monetta, T. In-vitro corrosion of AZ31 magnesium alloys by using a polydopamine coating. Bioact. Mater. 2019, 4, 71. [Google Scholar] [CrossRef]
- Singer, F.; Schlesak, M.; Mebert, C.; Höhn, S.; Virtanen, S. Corrosion properties of polydopamine coatings formed in one-step immersion process on magnesium. ACS Appl. Mater. Interfaces 2015, 7, 26758. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Roohani-Esfahani, S.I.; Zreiqat, H.; Xiong, P.; Zhou, W.; Yan, J.; Cheng, Y.; Zheng, Y. Triple-bioinspired burying/crosslinking interfacial coassembly strategy for layer-by-layer construction of robust functional bioceramic self-coatings for osteointegration applications. ACS Appl. Mater. Interfaces 2019, 11, 4447–4469. [Google Scholar] [CrossRef]
- Bouville, F.; Maire, E.; Meille, S.; Moortèle, B.V.; Stevenson, A.J.; Deville, S. Strong, tough and stiff bioinspired ceramics from brittle constituents. Nat. Naterials 2014, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Tong, L.; Wang, W.; Jiang, Z.; Sun, G.; Zou, D.; Wang, K.; Zhang, H. Sequentially bridged biomimetic graphene-based coating via covalent bonding with an effective anti-corrosion/wear protection for Mg alloy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125707. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, R.; Li, L.; Li, M.; Yang, L.; Liang, Z.; Yu, H.; Zhang, H.; Luo, R.; Wang, Y. Bionic Tea Stain–Like, All-Nanoparticle Coating for Biocompatible Corrosion Protection. Adv. Mater. Interfaces 2019, 6, 1900899. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility. Bioact. Mater. 2020, 5, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Zheng, F.; Li, B.; Zhang, R.; Wei, Y.; Yu, Y.; Zhang, Y.; Ouyang, Y.; Qiu, R. Self-healing dual biomimetic liquid-infused slippery surface in a partition matrix: Fabrication and anti-corrosion capability for magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127585. [Google Scholar] [CrossRef]
- Li, H.; Feng, X.; Peng, Y.; Zeng, R. Durable lubricant-infused coating on a magnesium alloy substrate with anti-biofouling and anti-corrosion properties and excellent thermally assisted healing ability. Nanoscale 2020, 12, 7700–7711. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Zhang, M. Bionspired slippery surfaces by cluster-like ZnO@ Co3O4 and its anti-corrosion performance. Dig. J. Nanomater. Biostruct. (DJNB) 2021, 16, 1565–1573. [Google Scholar]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, X.; Lin, D.; Xu, F.; Li, Y.; Wang, H. A novel slippery surface with enhanced stability and corrosion resistance. Prog. Org. Coat. 2020, 142, 105563. [Google Scholar] [CrossRef]
- Wu, D.; Ma, L.; Zhang, F.; Qian, H.; Minhas, B.; Yang, Y.; Han, X.; Zhang, D. Durable deicing lubricant-infused surface with photothermally switchable hydrophobic/slippery property. Mater. Des. 2020, 185, 108236. [Google Scholar] [CrossRef]
- Pant, R.; Ujjain, S.K.; Nagarajan, A.K.; Khare, K. Enhanced slippery behavior and stability of lubricating fluid infused nanostructured surfaces. Eur. Phys. J. Appl. 2016, 75, 11301. [Google Scholar] [CrossRef]
- Redon, R.; Vázquez-Olmos, A.; Mata-Zamora, M.; Ordóñez-Medrano, A.; Rivera-Torres, F.; Saniger, J. Contact angle studies on anodic porous alumina. J. Colloid Interface Sci. 2005, 287, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.J.; Song, Y.; Lu, Z.; Antao, D.S.; Wang, E.N. Design of lubricant infused surfaces. ACS Appl. Mater. Interfaces 2017, 9, 42383–42392. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Paxson, A.T.; Dhiman, R.; Smith, J.D.; Varanasi, K.K. Enhanced condensation on lubricant-impregnated nanotextured surfaces. ACS Nano 2012, 6, 10122–10129. [Google Scholar] [CrossRef]
- Peppou-Chapman, S.; Hong, J.K.; Waterhouse, A.; Neto, C. Life and death of liquid-infused surfaces: A review on the choice, analysis and fate of the infused liquid layer. Chem. Soc. Rev. 2020, 49, 3688–3715. [Google Scholar] [CrossRef]
- Sett, S.; Yan, X.; Barac, G.; Bolton, L.W.; Miljkovic, N. Lubricant-infused surfaces for low-surface-tension fluids: Promise versus reality. ACS Appl. Mater. Interfaces 2017, 9, 36400–36408. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Li, B.; Li, L.; Seeger, S.; Wang, A. Evaporation-induced transition from nepenthes pitcher-inspired slippery surfaces to lotus leaf-inspired superoleophobic surfaces. Langmuir 2014, 30, 14292–14299. [Google Scholar] [CrossRef]
- Pfruender, H.; Jones, R.; Weuster-Botz, D. Water immiscible ionic liquids as solvents for whole cell biocatalysis. J. Biotechnol. 2006, 124, 182–190. [Google Scholar] [CrossRef]
- Vorobev, A. Dissolution dynamics of miscible liquid/liquid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 300–308. [Google Scholar] [CrossRef]
- Howell, C.; Vu, T.L.; Johnson, C.P.; Hou, X.; Ahanotu, O.; Alvarenga, J.; Leslie, D.C.; Uzun, O.; Waterhouse, A.; Kim, P. Stability of surface-immobilized lubricant interfaces under flow. Chem. Mater. 2015, 27, 1792–1800. [Google Scholar] [CrossRef]
- Kim, J.H.; Rothstein, J.P. Droplet impact dynamics on lubricant-infused superhydrophobic surfaces: The role of viscosity ratio. Langmuir 2016, 32, 10166–10176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, C.; Wang, L.; Zhang, J.; Tu, J. Ionic liquids-infused slippery surfaces for condensation and hot water repellency. Chem. Eng. J. 2018, 343, 561–571. [Google Scholar] [CrossRef]
- Goodband, S.J.; Armstrong, S.; Kusumaatmaja, H.; Voïtchovsky, K. Effect of ageing on the structure and properties of model liquid-infused surfaces. Langmuir 2020, 36, 3461–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Song, D.; Liu, P.; Gao, L.; Wang, J. Long-Term Stability of a Liquid-Infused Coating with Anti-Corrosion and Anti-Icing Potentials on Al Alloy. ChemElectroChem 2019, 6, 3911–3919. [Google Scholar] [CrossRef]
- Xiang, T.; Zhang, M.; Sadig, H.R.; Li, Z.; Zhang, M.; Dong, C.; Yang, L.; Chan, W.; Li, C. Slippery liquid-infused porous surface for corrosion protection with self-healing property. Chem. Eng. J. 2018, 345, 147–155. [Google Scholar] [CrossRef]
- Jiang, D.; Xia, X.; Hou, J.; Cai, G.; Zhang, X.; Dong, Z. A novel coating system with self-reparable slippery surface and active corrosion inhibition for reliable protection of Mg alloy. Chem. Eng. J. 2019, 373, 285–297. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Liu, Y.; Xu, J.; Pan, C. Bioinspired Surface Design for Magnesium Alloys with Corrosion Resistance. Metals 2022, 12, 1404. https://doi.org/10.3390/met12091404
Wu F, Liu Y, Xu J, Pan C. Bioinspired Surface Design for Magnesium Alloys with Corrosion Resistance. Metals. 2022; 12(9):1404. https://doi.org/10.3390/met12091404
Chicago/Turabian StyleWu, Feng, Yixuan Liu, Jing Xu, and Changjiang Pan. 2022. "Bioinspired Surface Design for Magnesium Alloys with Corrosion Resistance" Metals 12, no. 9: 1404. https://doi.org/10.3390/met12091404
APA StyleWu, F., Liu, Y., Xu, J., & Pan, C. (2022). Bioinspired Surface Design for Magnesium Alloys with Corrosion Resistance. Metals, 12(9), 1404. https://doi.org/10.3390/met12091404





