Utilization of Metal Oxides Nanoparticles in Modulating Polyvinyl Chloride Films to Resist Ultraviolet Light
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials and Apparatuses
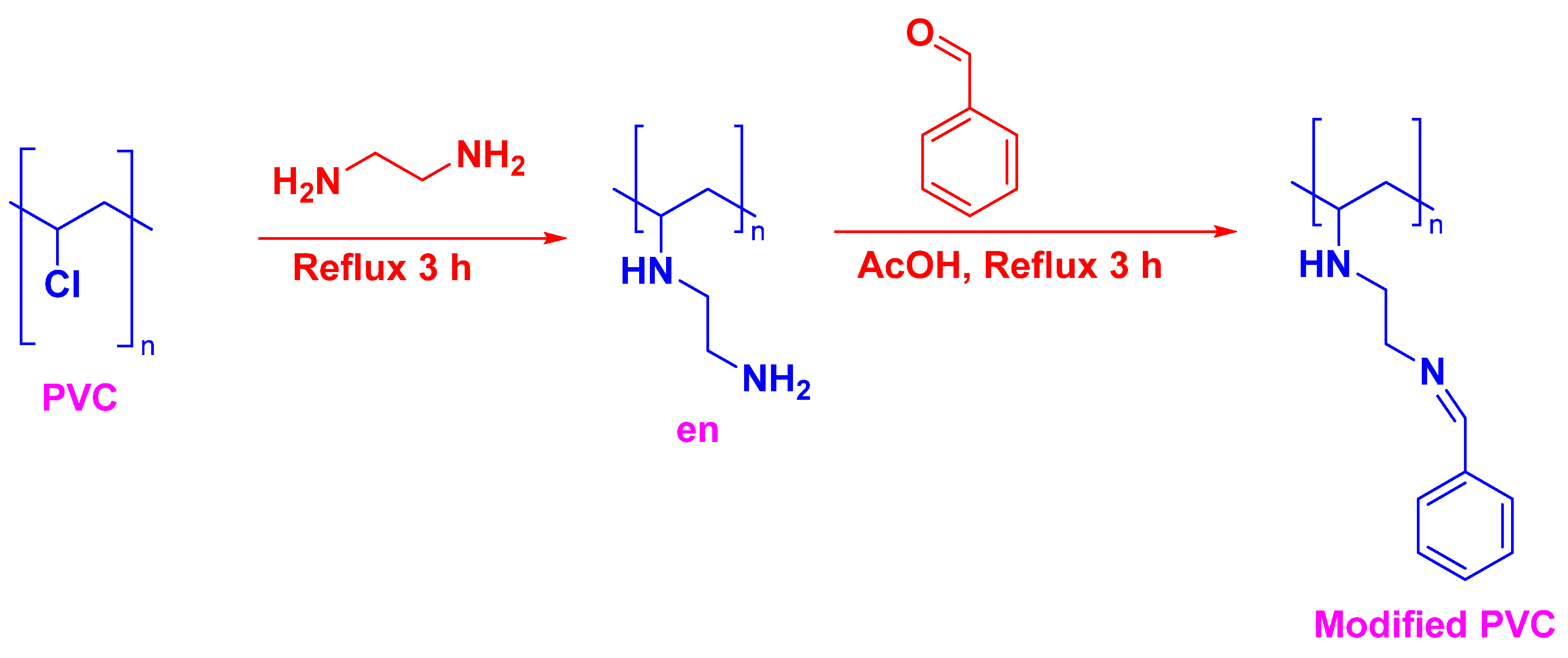
2.2. Preparation of Modified PVC Films
2.2.1. Preparation of PVC (Blank) Film
2.2.2. Preparation of Modified PVC Film
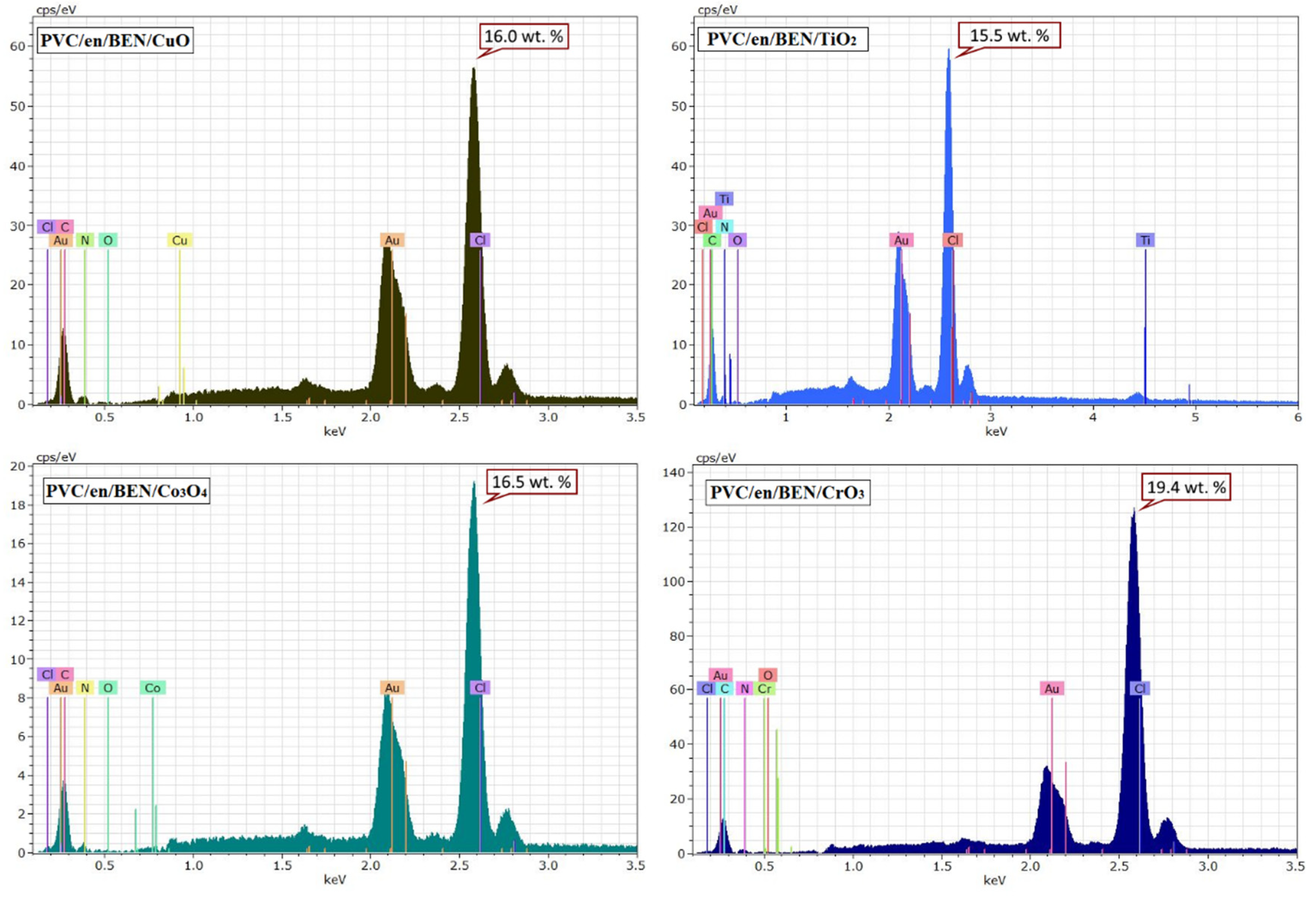
2.2.3. Preparation of Modified PVC Film Doped with Different Nanoparticles Metals
2.3. Films Irradiation by UV Radiation
3. Results and Discussion
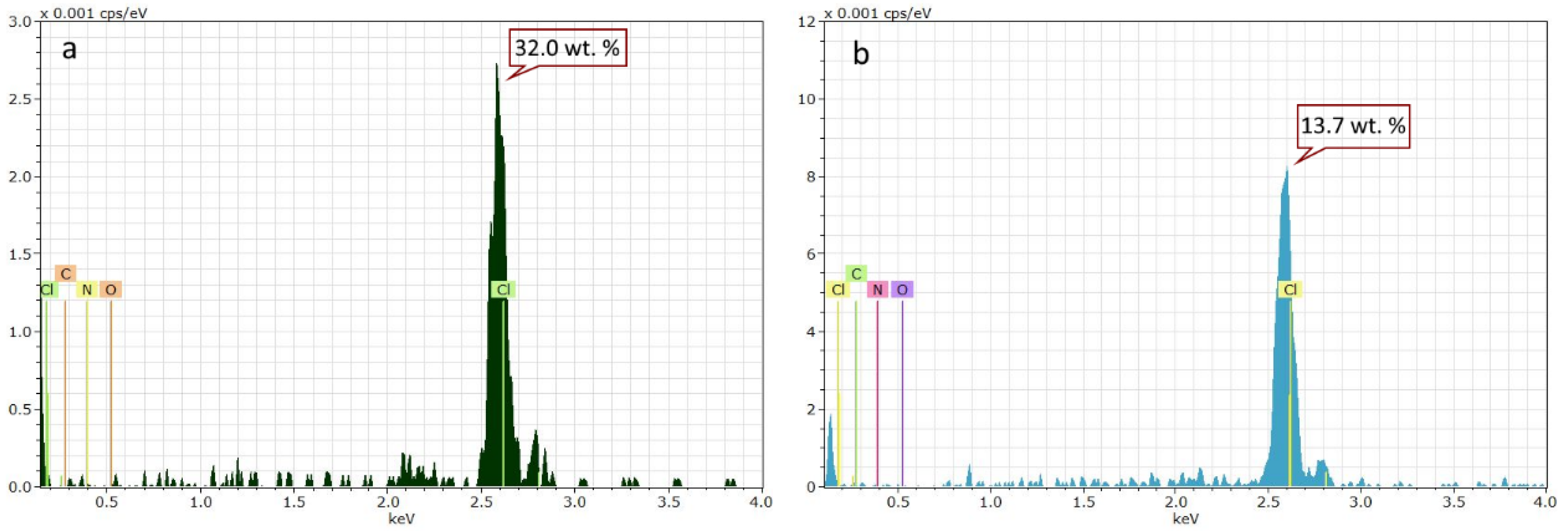
3.1. Characterization of PVC Films by NMR Spectrophotometer
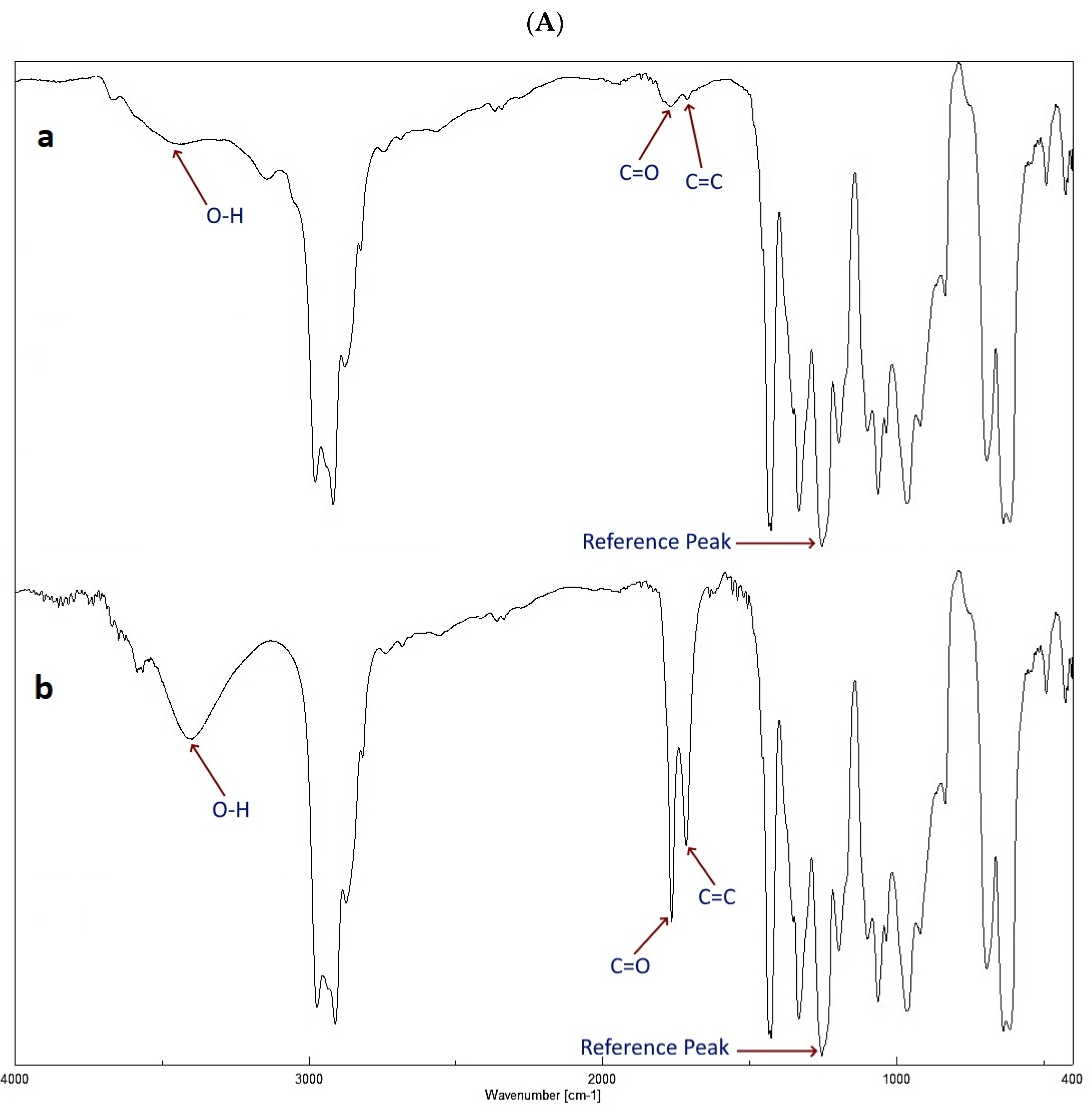
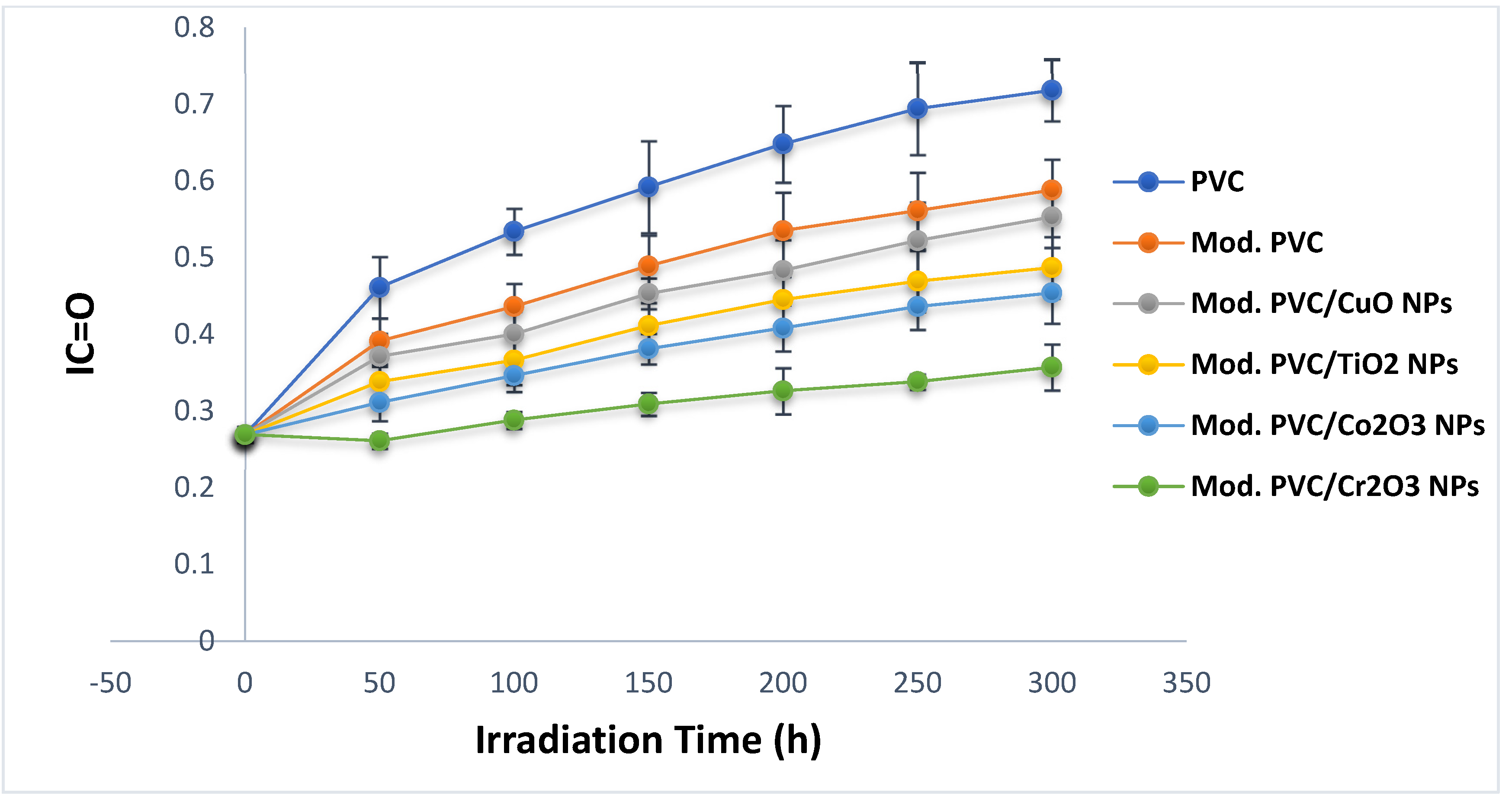
3.2. Photodegradation Rate Observation by FTIR Spectrophotometer
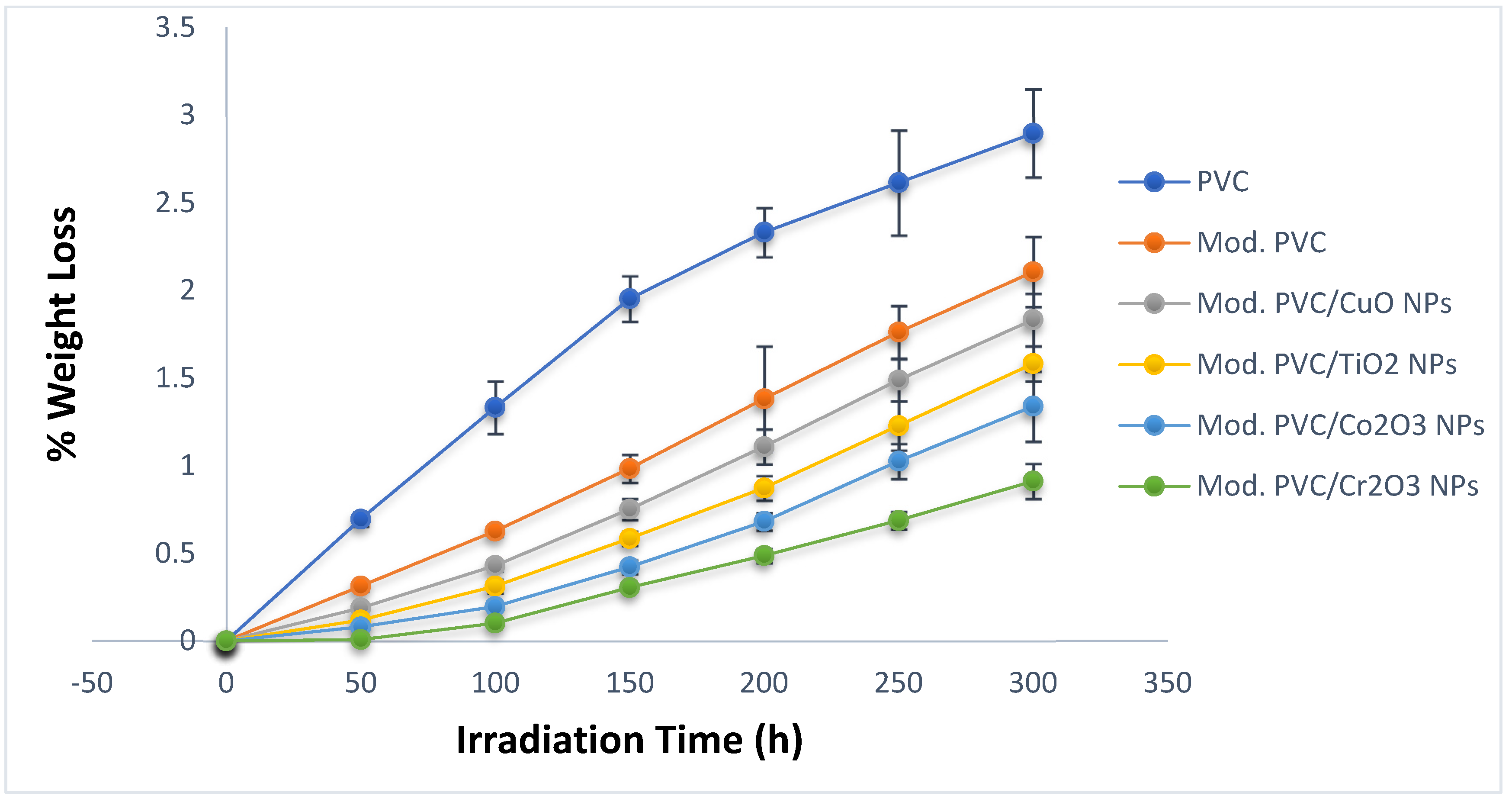
3.3. Photodegradation Rate Assessment by Weight Loss
3.4. Surface Analysis
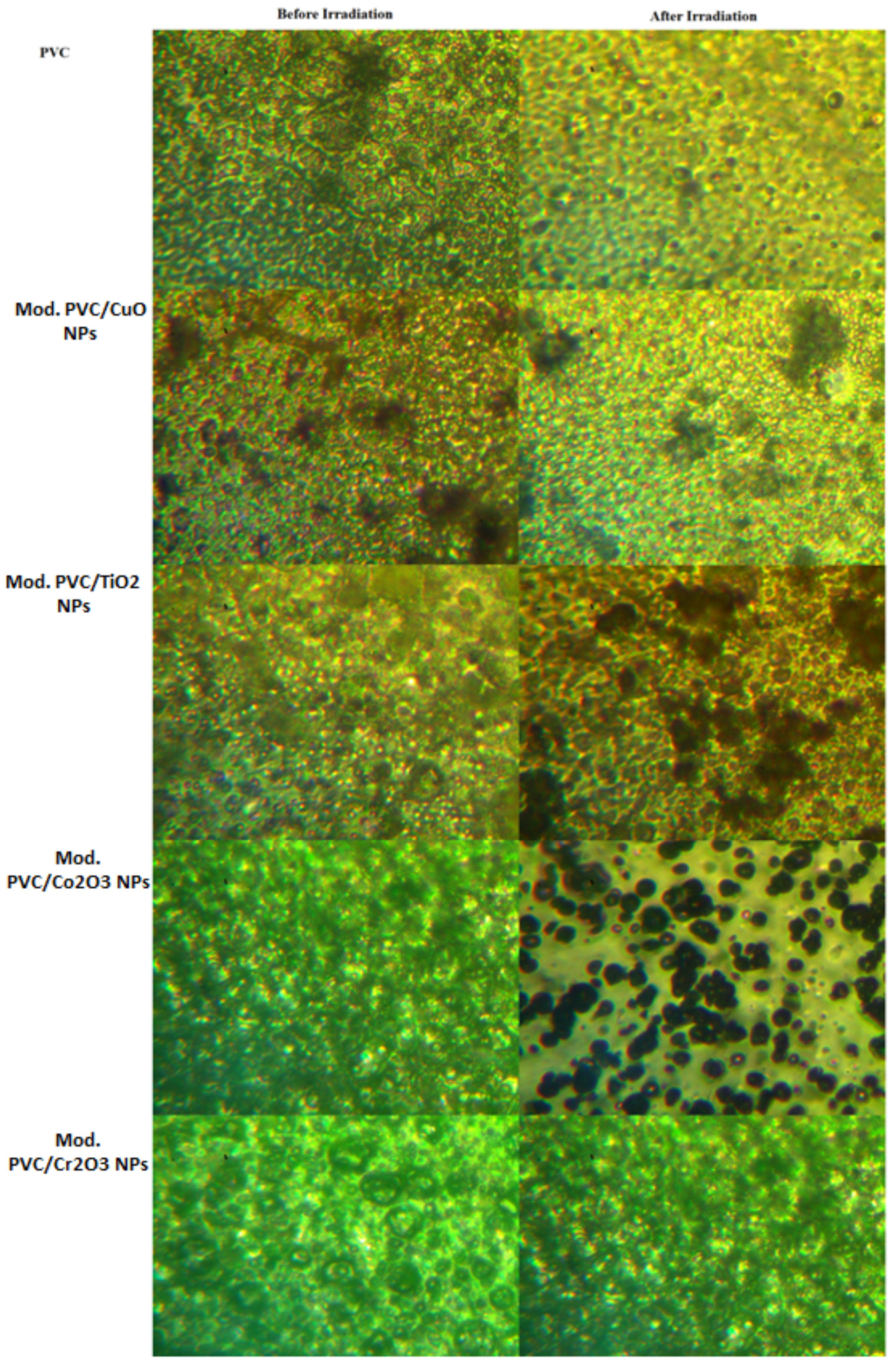
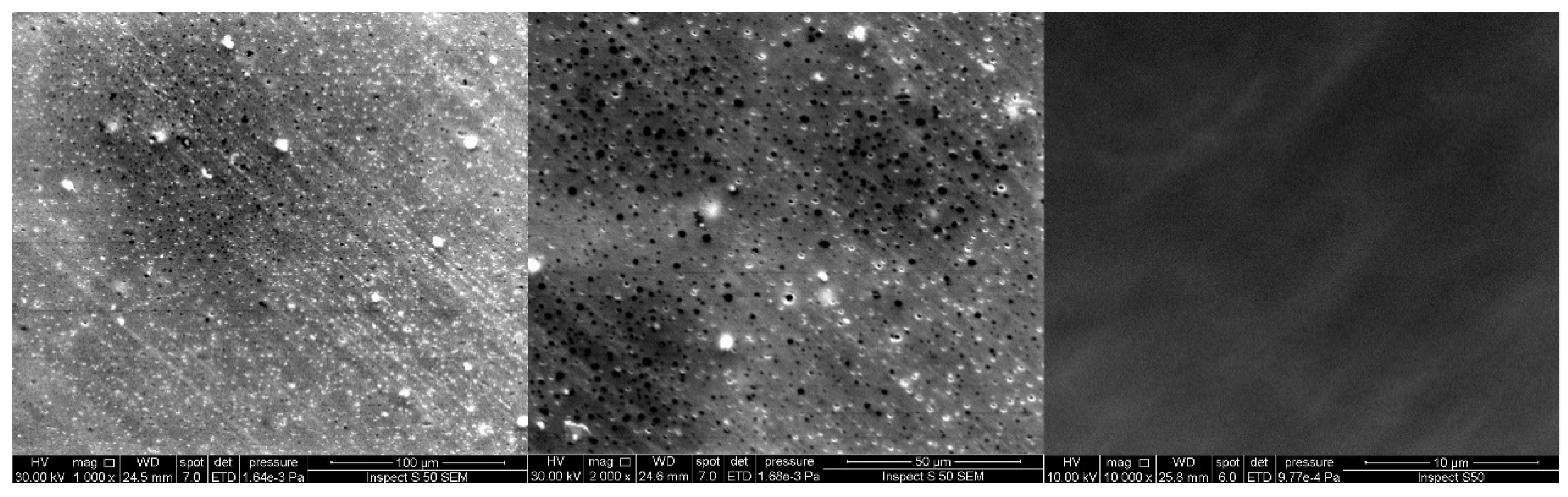
3.4.1. Optical Microscope
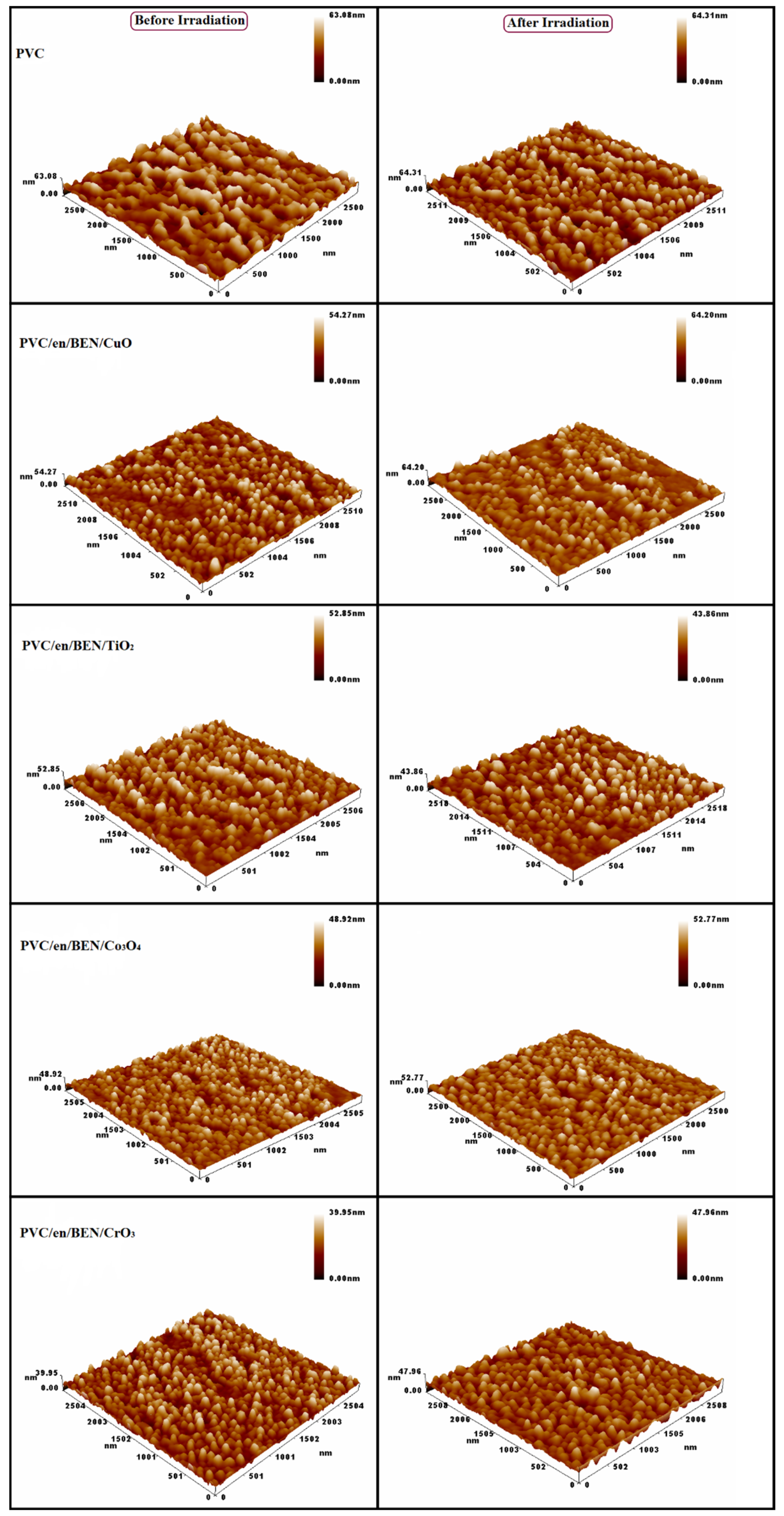
3.4.2. Atomic Force Microscope (AFM)
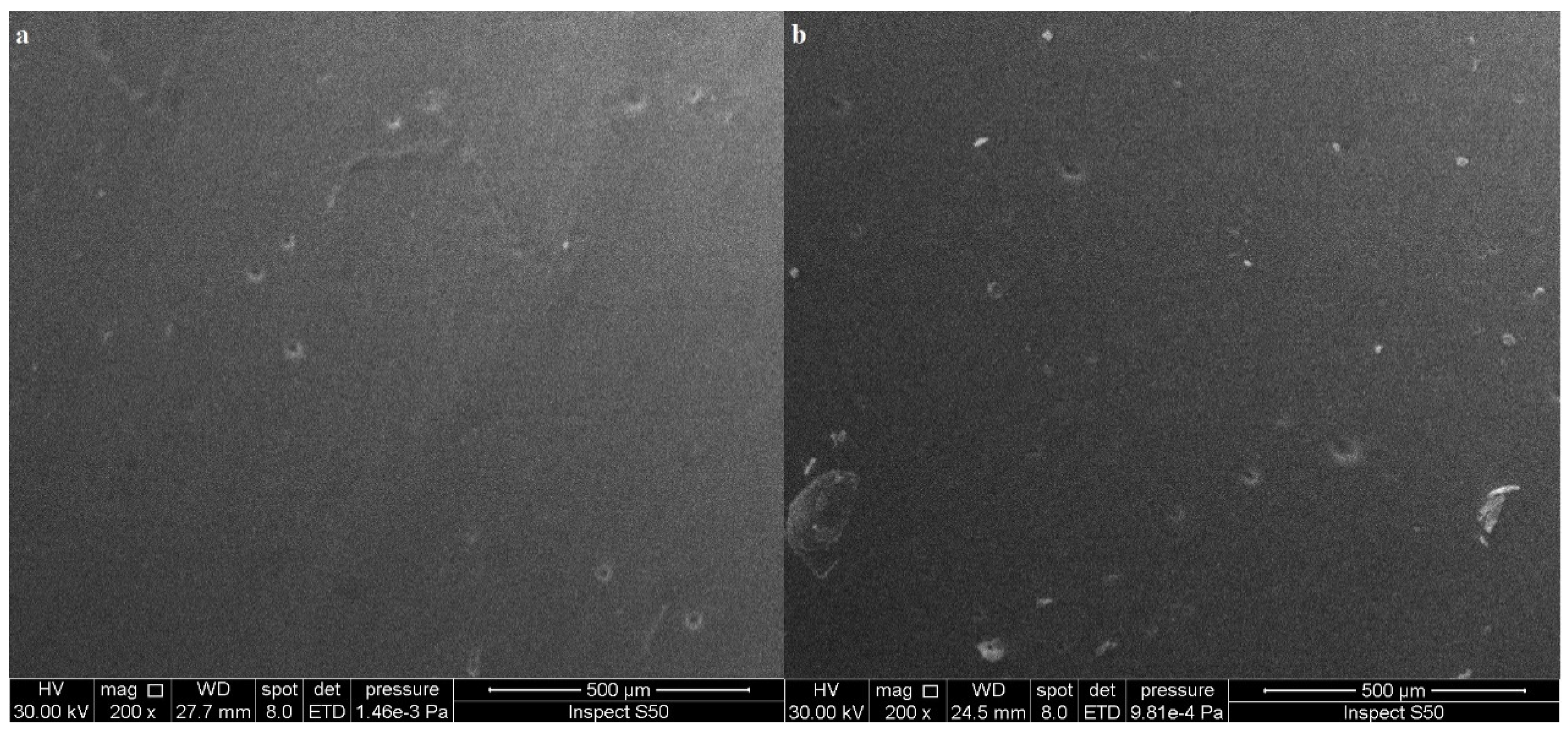
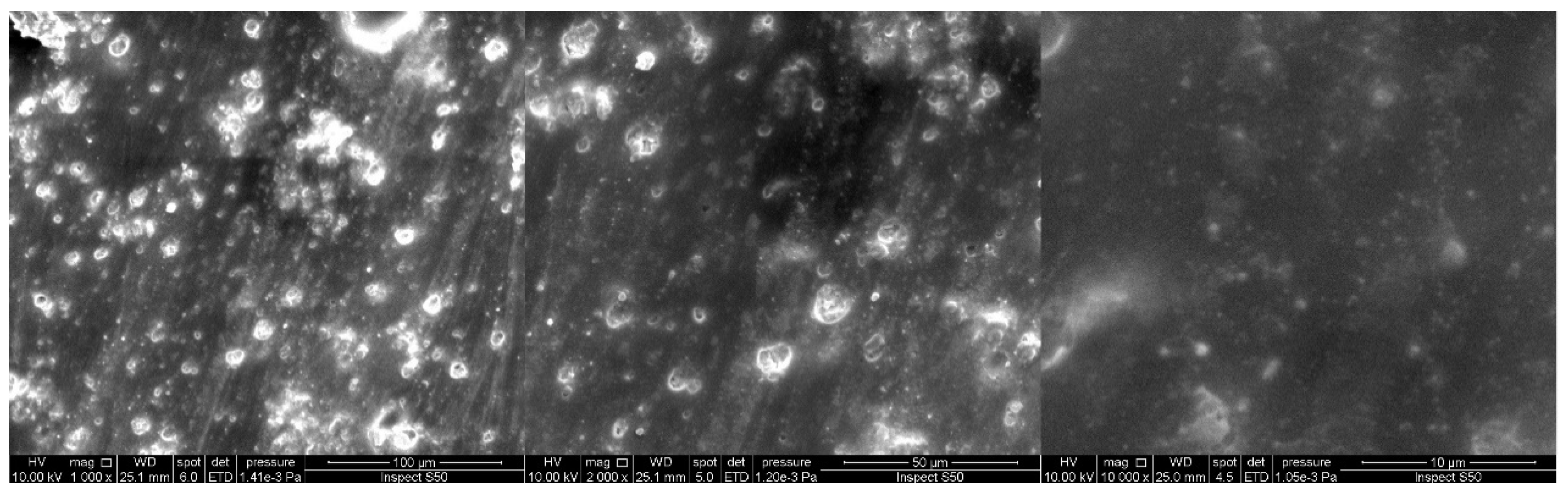
3.4.3. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Ahmed, D.; Zainulabdeen, K.; Jawad, A. Photo-physical and Morphological Study of Polymers: A Review. Phys. Chem. Res. 2023, 11, 409–424. [Google Scholar]
- The State of Plastics: World Environment Day Outlook 2018. Available online: https://www.unep.org/resources/report/stateplastics-world-environment-day-outlook-2018 (accessed on 5 November 2021).
- Plastics—The Facts 2020. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://issuu.com/plasticseuropeebook/docs/plastics_the_facts-web-dec2020 (accessed on 2 November 2021).
- Martins, J.N.; Freire, E.; Hemadipou, H. Applications and market of PVC for piping industry. Polímeros 2009, 19, 58–62. [Google Scholar] [CrossRef]
- Wheeler, R.N., Jr. Poly (vinyl chloride) processes and products. Environ. Health Perspect. 1981, 41, 123–128. [Google Scholar] [CrossRef]
- Tian, Y.; Shu, Y.; Zhang, X.; Mahmud, S.; Zhu, J.; Su, S. Electrospun PVDF-Ag@ AgCl porous fiber membrane: Stable antifoul and antibacterial surface. Surf. Innov. 2020, 9, 156–165. [Google Scholar] [CrossRef]
- Lin, P.Y.; Wu, I.H.; Tsai, C.Y.; Kirankumar, R.; Hsieh, S. Detecting the release of plastic particles in packaged drinking water under simulated light irradiation using surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2022, 1198, 339516. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Kumagai, H.; Tashiro, T.; Kobayashi, T. Formation of conjugated carbon bonds on poly (vinyl chloride) films by microwavedischarge oxygen-plasma treatments. J. Appl. Polym. Sci. 2005, 96, 589–594. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Vohlídal, J. Polymer degradation: A short review. Chem. Teach. Int. 2020, 3, 213–220. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer additives. Phys. Sci. Rev. 2017, 2, 20160130. [Google Scholar]
- Brostow, W.; Lu, X.; Gencel, O.; Osmanson, A.T. Effects of UV stabilizers on polypropylene outdoors. Materials 2020, 13, 1626. [Google Scholar] [CrossRef] [PubMed]
- Noukakis, D.; Suppan, P. Mechanism of protection of polymers by photostabilizers. J. Photochem. Photobiol. A 1991, 58, 393–396. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Torikai, A.; Kobatake, T.; Okisaki, F.; Shuyama, H. Photodegradation of polystyrene containing flame-retardants: Wavelength sensitivity and efficiency of degradation. Polym. Degrad. Stab. 1995, 50, 261–267. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. Simultaneous enhancements of UV-shielding properties and photostability of poly (vinyl alcohol) via incorporation of sepia eumelanin. ACS Sustain. Chem. Eng. 2016, 4, 2252–2258. [Google Scholar] [CrossRef]
- Abed, R.N.; Kadhom, M.; Ahmed, D.S.; Hadawey, A.; Yousif, E. Enhancing Optical Properties of Modified PVC and Cr2O3 Nanocomposite. Trans. Electr. Electron. Mater. 2021, 22, 317–327. [Google Scholar] [CrossRef]
- Ghani, H.; Kadhom, M.; Husain, A.A.; Jawad, A.; Yousif, E. The effect of high UV radiation exposure environment on the novel PVC polymers. Prog. Color Colorants Coat. (PCCC) 2022, 15, 319–326. [Google Scholar]
- Ahmed, D.S.; Abdallh, M.; Alsayed, R.; Ahmed, A.; Yousif, E. Graft modification of PVC with 1,2,4-triazole ring system for preparing flexible PVC materials. AIP Conf. Proc. 2020, 2213, 020129. [Google Scholar]
- Kilduff, J.E.; Mattaraj, S.; Pieracci, J.P.; Belfort, G. Photochemical modification of poly (ethersulfone) and sulfonated poly(sulfone) nanofiltration membranes for control of fouling by natural organic matter. Desalination 2000, 132, 133–142. [Google Scholar] [CrossRef]
- Omer, R.M.; Yousif, E.; Al-Tikrity, E.T.B.; Ahmed, D.S.; Ali, A.A.; Abed, R.N. A detailed examination of UV radiation effects on the structural and morphological properties of polyvinyl butyral films containing different nanoparticles. Prog. Color Colorants Coat. (PCCC) 2021, 14, 209–219. [Google Scholar]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S. Polyphosphates as inhibitors for poly (vinyl chloride) photodegradation. Molecules 2017, 22, 1849. [Google Scholar] [CrossRef]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly (vinyl chloride) by organotin (IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [PubMed]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly (vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, D.S.; Mohammed, A.; Husain, A.A.; El-Hiti, G.A.; Kadhom, M.; Kariuki, B.M.; Yousif, E. Fabrication of highly photostable polystyrene films embedded with organometallic complexes. Polymers 2022, 14, 1024. [Google Scholar] [CrossRef]
- Salam, B.; El-Hiti, G.A.; Bufaroosha, M.; Ahmed, D.S.; Ahmed, A.; Alotaibi, M.H.; Yousif, E. Tin complexes containing an atenolol moiety as photostabilizers for poly (vinyl chloride). Polymers 2020, 12, 2923. [Google Scholar] [CrossRef]
- Krehula, L.K.; Papić, A.; Krehula, S.; Gilja, V.; Foglar, L.; Hrnjak-Murgić, Z. Properties of UV protective films of poly(vinyl-chloride)/TiO2 nanocomposites for food packaging. Polym. Bull. 2017, 74, 1387–1404. [Google Scholar] [CrossRef]
- Dona, E.D.; Sivakumar, A.; Dhas, S.S.J.; Sivaprakash, P.; Arumugam, S.; Dhas, S.M.B. Sustainability of corundum-type Cr2O3 nanoparticles at shock wave loaded conditions. Solid State Sci. 2021, 119, 106701. [Google Scholar] [CrossRef]





| 1H NMR (500 MHz: DMSO-d6, δ) | ||||
|---|---|---|---|---|
| Film | N-H | H-C-H | H-C-Cl | Ar-H |
| PVC | - | 2.504–2.512 | 3.428 | - |
| Modified PVC | 1.039–1.074 | 2.509 | 3.398–3.878 | 7.257–8.344 |
| 13C NMR (δ, ppm) | ||||
|---|---|---|---|---|
| Film | C-Cl | C-C | C-N | C=C |
| PVC | - | 39.27–40.52 | - | - |
| Modified PVC | 19.02 | 39.31–40.56 | 56.49 | 129.12 |
| PVC Films | Rq (Roughness Average) (nm) |
|---|---|
| PVC | 368.3 ± 41.6 |
| Modified PVC | 76.1 ± 11.7 |
| Modified PVC/CuO NPs | 62.6 ± 8.3 |
| Modified PVC/TiO2 NPs | 53.2 ± 7.8 |
| Modified PVC/Co2O3 NPs | 45.8 ± 6.1 |
| Modified PVC/Cr2O3 NPs | 33.8 ± 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujbil, H.H.; Al Jebur, L.A.; Yousif, E.; Kadhom, M.; Mohammed, A.; Ahmed, D.S.; Ali, M.; Hashim, H. Utilization of Metal Oxides Nanoparticles in Modulating Polyvinyl Chloride Films to Resist Ultraviolet Light. Metals 2022, 12, 1413. https://doi.org/10.3390/met12091413
Mujbil HH, Al Jebur LA, Yousif E, Kadhom M, Mohammed A, Ahmed DS, Ali M, Hashim H. Utilization of Metal Oxides Nanoparticles in Modulating Polyvinyl Chloride Films to Resist Ultraviolet Light. Metals. 2022; 12(9):1413. https://doi.org/10.3390/met12091413
Chicago/Turabian StyleMujbil, Hussein H., Layla A. Al Jebur, Emad Yousif, Mohammed Kadhom, Alaa Mohammed, Dina S. Ahmed, Muataz Ali, and Hassan Hashim. 2022. "Utilization of Metal Oxides Nanoparticles in Modulating Polyvinyl Chloride Films to Resist Ultraviolet Light" Metals 12, no. 9: 1413. https://doi.org/10.3390/met12091413








