Wear and Corrosion Resistance of FeCoCrxNiAl High-Entropy Alloy Coatings Fabricated by Laser Cladding on Q345 Welded Joint
Abstract
:1. Introduction
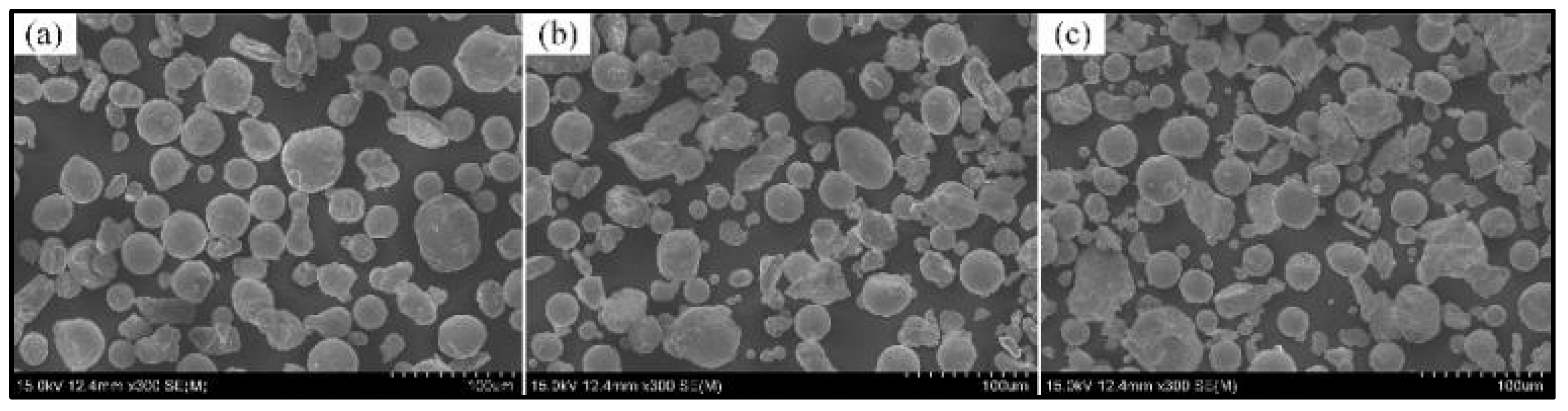
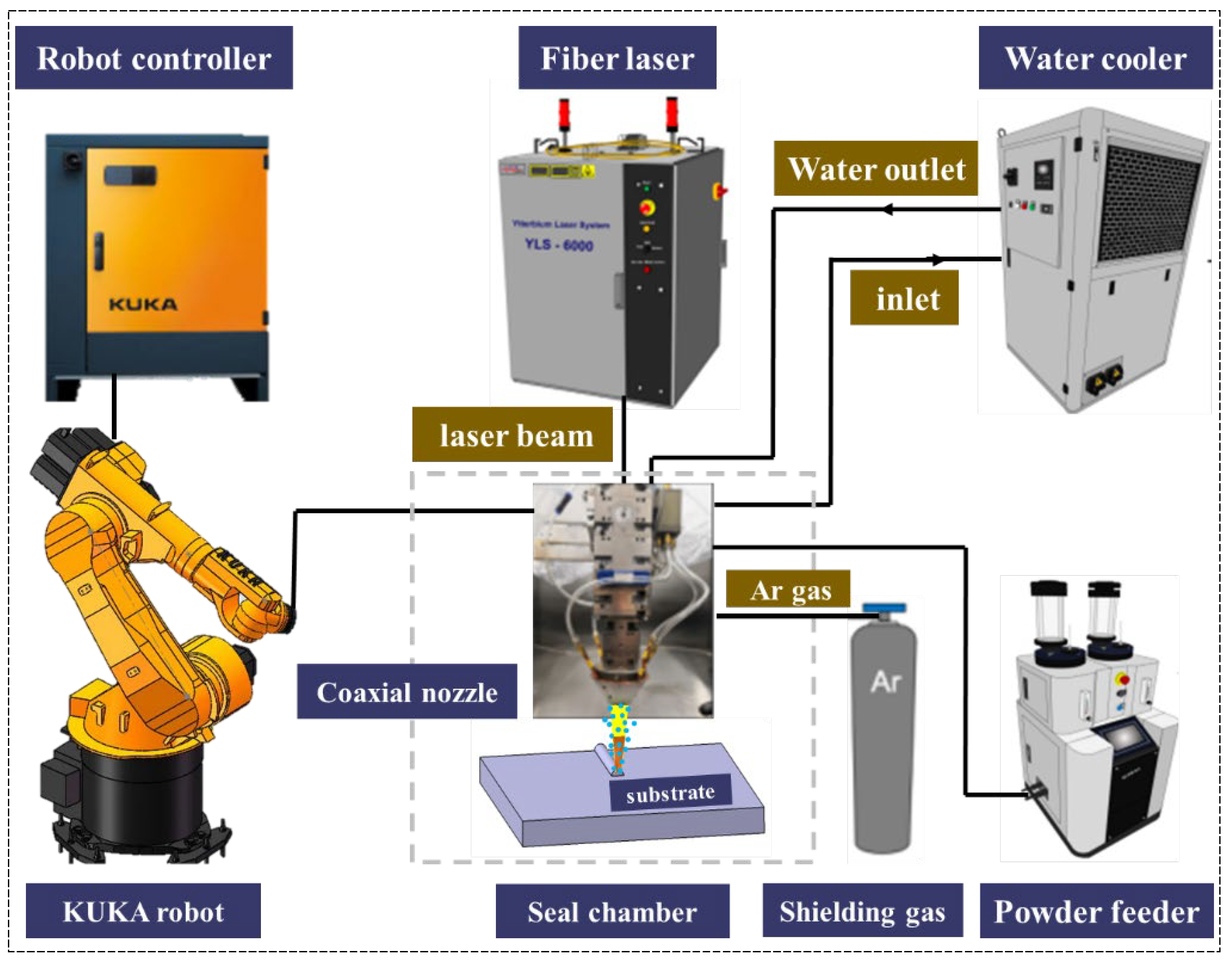
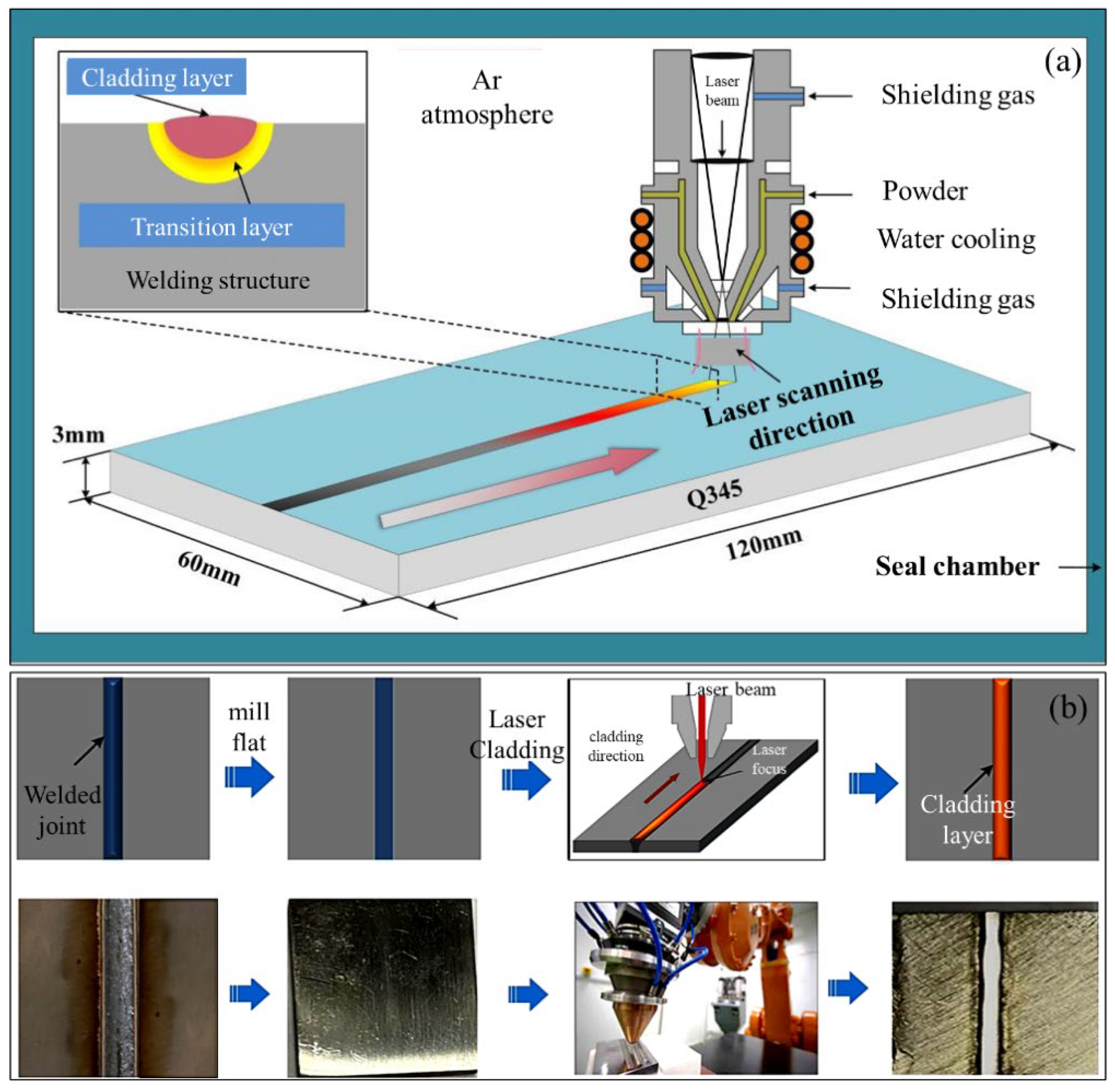
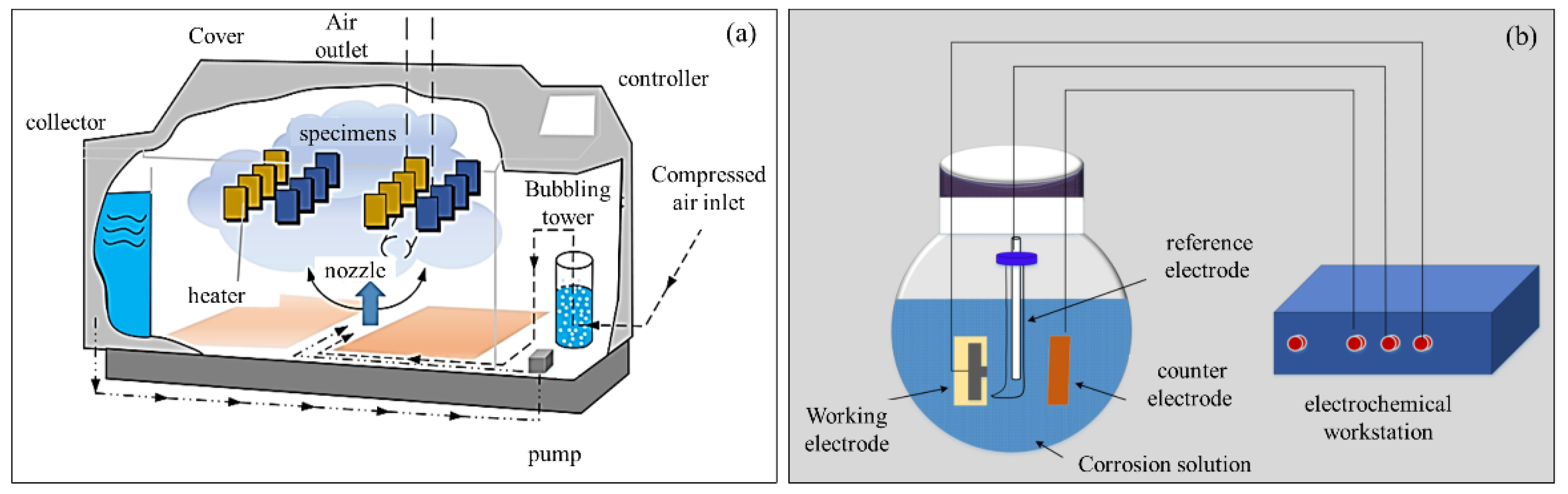
2. Materials and Methods
3. Results
3.1. Effect of Cr Contents on the Corrosion Resistance of FeCoCrxNiAl HEA Coatings
3.1.1. Macro Morphology
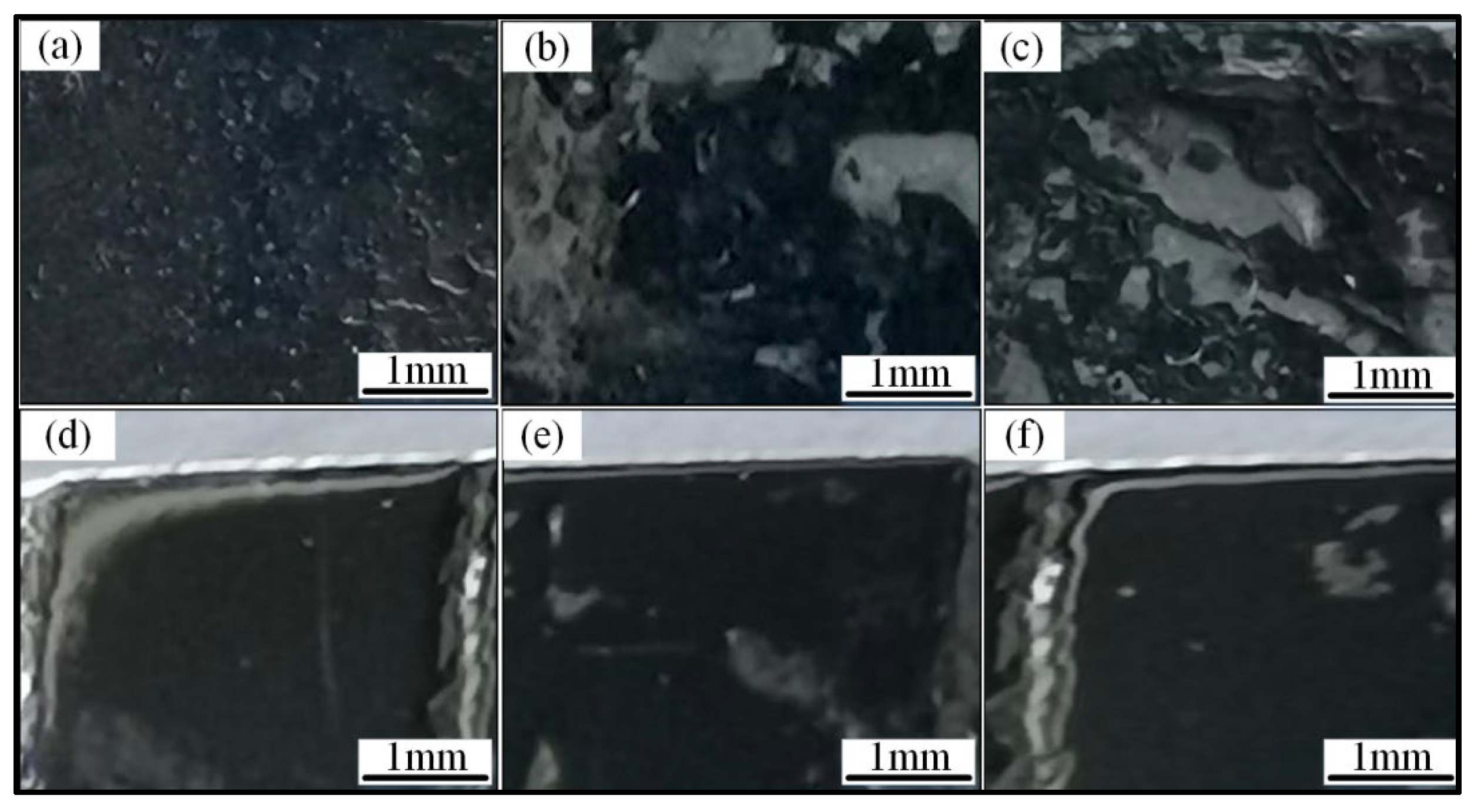
3.1.2. Microstructure
3.1.3. Phase Transformation
3.2. Effect of Cr Content on Mechanical Properties of HEA Coatings
3.2.1. Microhardness
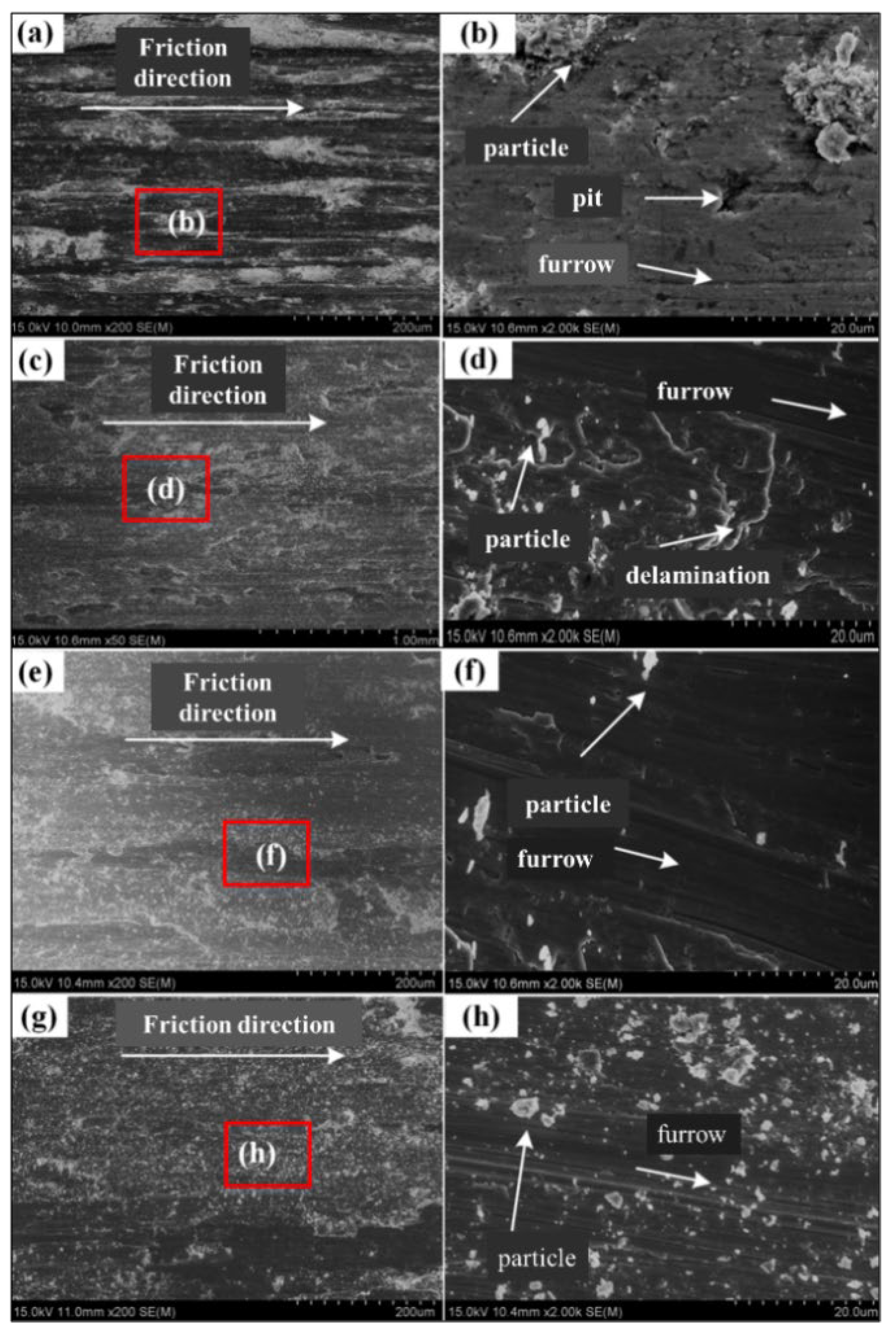
3.2.2. Wear Resistance
3.3. Effect of Cr Content on Corrosion Resistance of HEA Coatings
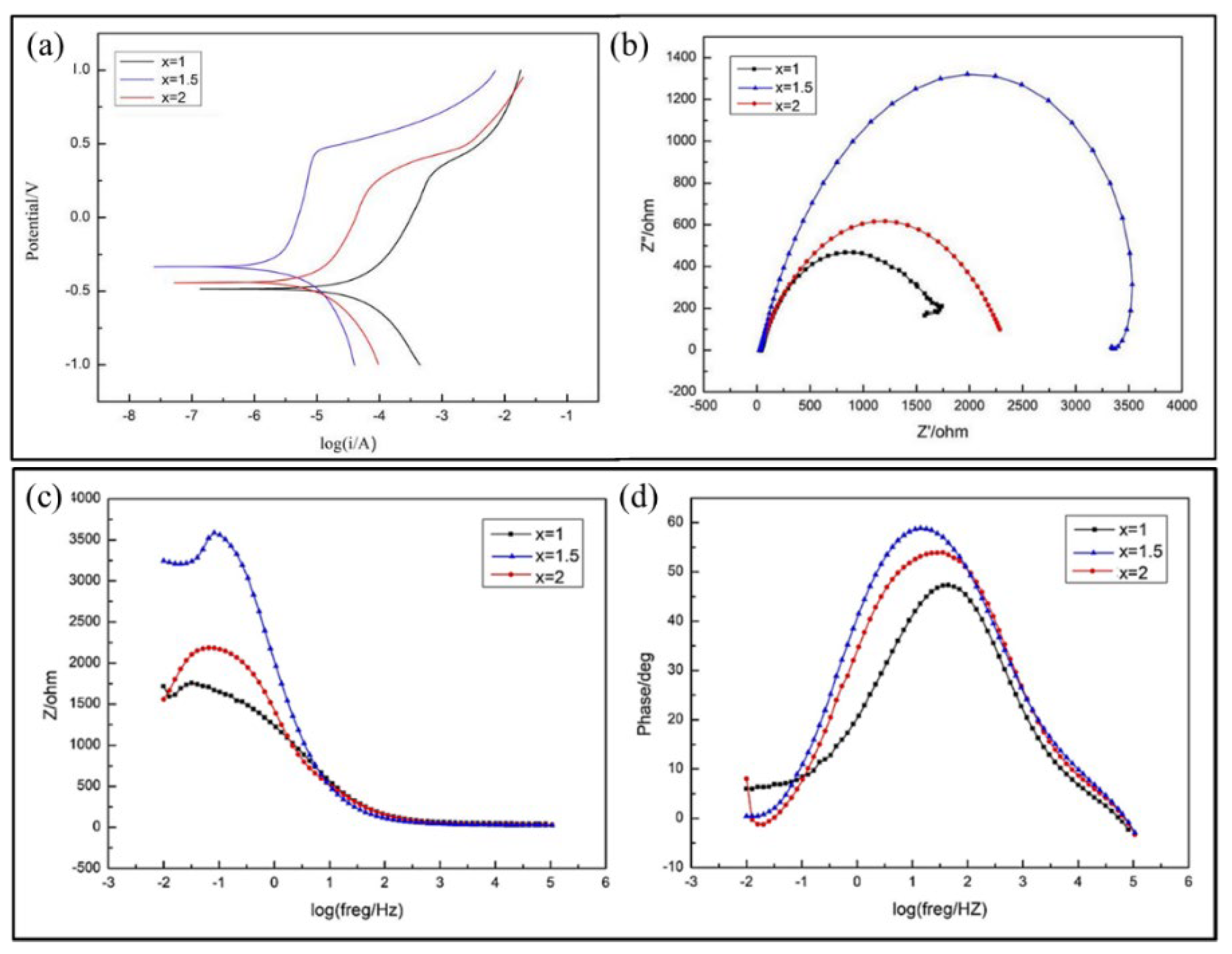
3.3.1. Electrochemical Corrosion Resistance
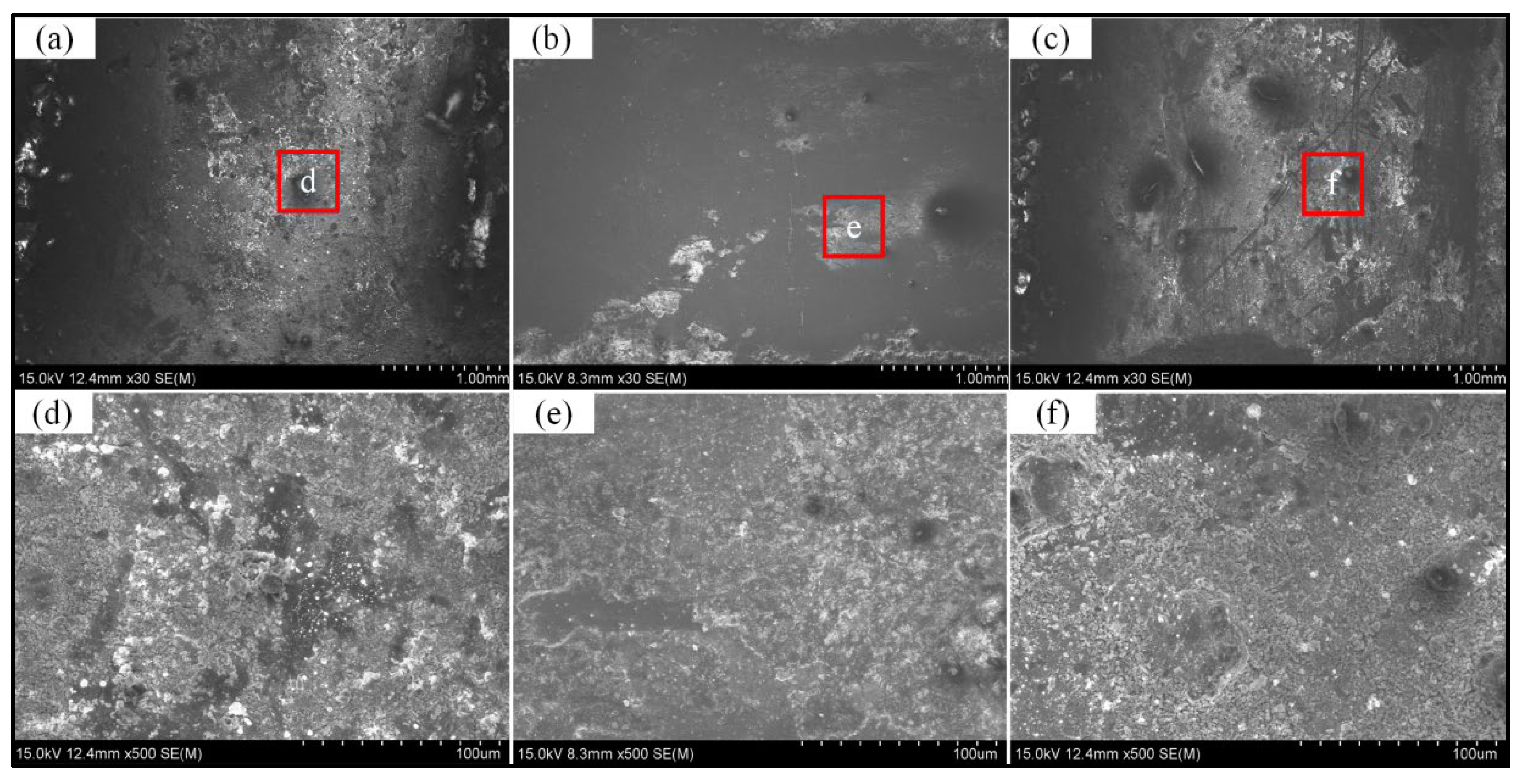
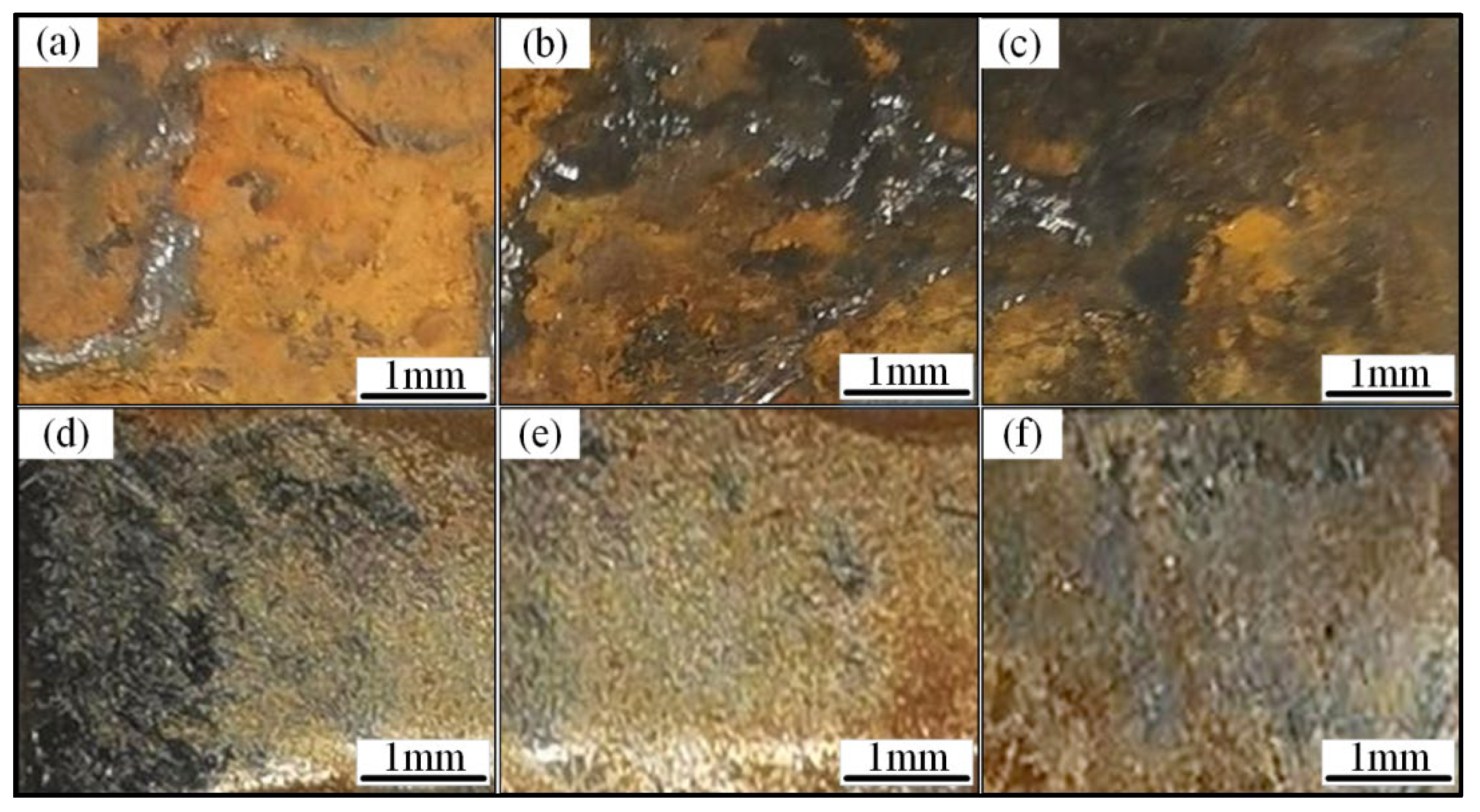
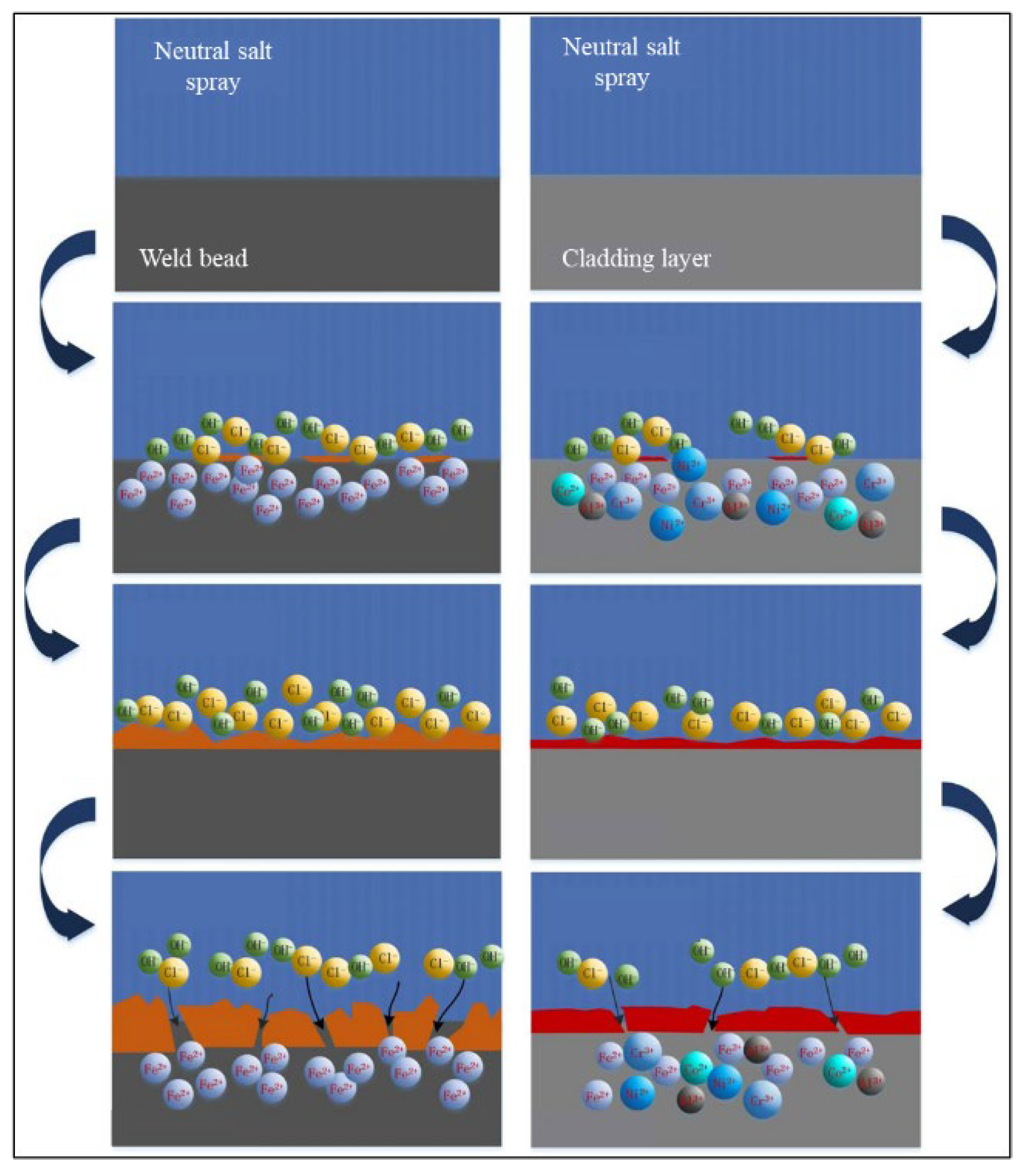
3.3.2. Corrosion Resistance of HEA Layers in Simulated Marine Atmospheric Environment
3.4. Comparative Study on Corrosion Resistance Improvement in Different Areas of Welded Joint
4. Conclusions
- (1)
- The layer has the good metallurgical bonding with plate, with no obvious defects in the cross section of the cladding layer. The increase of Cr element can promote the formation of BCC phase in the HEA layer contributing to the improvement of hardness and wear resistance.
- (2)
- In the electrochemical corrosion test, the corrosion potential of the cladding layers increases at first and then decreases with the increase of Cr content in the powder, while the self-corrosion current displays the opposite trend. According to the results of neutral salt spray experiment, the corrosion resistance shows the same tendency as the above-mentioned with the increase of Cr content, and the corrosion degree is the lightest when x = 1.5. Due to the different dilution rate, the actual Cr content in the layers is different from the theoretical value. With the increase of the actual Cr content, the corrosion resistance is enhanced.
- (3)
- Laser cladding of FeCoCrxNiAl HEA on the surfaces of welded joint and the electrochemical corrosion test are carried out to explore the enhancing effect of cladding layers on different regions of welded joint. The protective efficiencies on the WB, HAZ and BM are 99.1, 98.4 and 96.6%, respectively. In the neutral salt spray test, compared with the layers, the corrosion degree of welded joint is more serious, and the corrosion products are basically iron oxides. While the corrosion products of the cladding layers are iron oxides and a small amount of Co, Cr, Ni and Al oxides.
- (1)
- In order to further study the corrosion resistance of high entropy alloy repair layer, the corrosion behavior in corrosive environment at different time can be considered in the future study.
- (2)
- In this paper, the composition ratio of high-entropy alloy powder is designed to study the corrosion resistance of the repair layer. Subsequently, the effects of different laser power, scanning speed and different powder feeding amount on the corrosion resistance of the repair layer can be considered.
- (3)
- This paper mainly uses experimental methods to study the corrosion resistance of high-entropy alloy repair layer. The following work can be focused on the software used to simulate the corrosion behavior of repair layer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Chen, W.P.; Fang, S.C.; Zhang, D.Y.; Xiao, H.Q.; Zhu, D.Z. Alloying behavior and deformation twinning in a CoNiFeCrAl0.6Ti0.4 high entropy alloy processed by spark plasma sintering. J. Alloy. Compd. 2013, 553, 316–323. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Dobbelstein, H.; Gurevich, E.L.; George, E.P.; Ostendorf, A.; Laplanche, G. Laser metal deposition of a refractory TiZrNbHfTa high-entropy alloy. Addit. Manuf. 2018, 24, 386–390. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.; Hao, J. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Chen, P.J.; Yang, H.F.; Hao, J.B.; Tian, X.H. Microstructure and Properties of AlCoCrFeNiTi High-Entropy Alloy Coating on AISI1045 Steel Fabricated by Laser Cladding. J. Mater. Eng. Perform. 2019, 28, 1544–1552. [Google Scholar] [CrossRef]
- Anupam, A.; Kottada, R.S.; Kashyap, S.; Meghwal, A.; Murty, B.S.; Berndt, C.C.; Ang, A.S.M. Understanding the microstructural evolution of high entropy alloy coatings manufactured by atmospheric plasma spray processing. Appl. Surf. Sci. 2020, 505, 144117. [Google Scholar] [CrossRef]
- Wang, W.R.; Qi, W.; Xie, L.; Yang, X.; Li, J.T.; Zhang, Y. Microstructure and Corrosion Behavior of (CoCrFeNi)(95)Nb-5 High-Entropy Alloy Coating Fabricated by Plasma Spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef] [Green Version]
- Dou, B.; Zhang, H.; Zhu, J.H.; Xu, B.Q.; Zhou, Z.Y.; Wu, J.L. Uniformly Dispersed Carbide Reinforcements in the Medium-Entropy High-Speed Steel Coatings by Wide-Band Laser Cladding. Acta Metall. Sin.-Engl. Lett. 2020, 33, 1145–1150. [Google Scholar] [CrossRef]
- Gao, J.L.; Wu, C.Z.; Hao, Y.B.; Xu, X.C.; Guo, L.J. Numerical simulation and experimental investigation on three-dimensional modelling of single-track geometry and temperature evolution by laser cladding. Opt. Laser Technol. 2020, 129, 106287. [Google Scholar] [CrossRef]
- Savrai, R.A.; Soboleva, N.N.; Malygina, I.Y.; Osintseva, A.L. The structural characteristics and contact loading behavior of gas powder laser clad CoNiCrW coating. Opt. Laser Technol. 2020, 126, 106079. [Google Scholar] [CrossRef]
- Deng, X.K.; Zhang, G.J.; Wang, T.; Ren, S.; Bai, Z.L.; Cao, Q. Investigations on microstructure and wear resistance of Fe-Mo alloy coating fabricated by plasma transferred arc cladding. Surf. Coat. Technol. 2018, 350, 480–487. [Google Scholar] [CrossRef]
- Gao, L.B.; Song, J.; Jiao, Z.B.; Liao, W.B.; Luan, J.H.; Surjadi, J.U.; Li, J.Y.; Zhang, H.T.; Sun, D.; Liu, C.T.; et al. High-Entropy Alloy (HEA)-Coated Nanolattice Structures and Their Mechanical Properties. Adv. Eng. Mater. 2018, 20, 1700625. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Hirota, M.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. CoCrFeNiTi-based high-entropy alloy with superior tensile strength and corrosion resistance achieved by a combination of additive manufacturing using selective electron beam melting and solution treatment. Mater. Lett. 2017, 189, 148–151. [Google Scholar] [CrossRef]
- Varallyay, Z.; Szabo, A.; Rosales, A.; Gonzales, E.; Tobioka, H.; Headley, C. Accurate modeling of cladding pumped, star-shaped, Yb-doped fiber amplifiers. Opt. Fiber Technol. 2015, 21, 180–186. [Google Scholar] [CrossRef]
- Pinkerton, A.J. Advances in the modeling of laser direct metal deposition. J. Laser Appl. 2015, 27, S15001. [Google Scholar] [CrossRef]
- Sanecki, P.T.; Gibala, K.; Skital, P.M. The Application of Metal Deposition in Optical Sensor Technique. The Microscale Cu Deposition as “Electrochemical Welding”. IEEE Sens. J. 2015, 15, 1275–1279. [Google Scholar] [CrossRef]
- Jheng, D.Y.; Hsu, K.Y.; Liang, Y.C.; Huang, S.L. Broadly Tunable and Low-Threshold Cr4+:YAG Crystal Fiber Laser. IEEE J. Sel. Top. Quantum Electron. 2015, 21, 16–23. [Google Scholar] [CrossRef]
- Wang, B.A.; Zhang, W.G.; Bai, Z.Y.; Wang, L.; Zhang, L.Y.; Zhou, Q.; Chen, L.; Yan, T.Y. CO2-Laser-Induced Long Period Fiber Gratings in Few Mode Fibers. IEEE Photonics Technol. Lett. 2015, 27, 145–148. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z.D.; Zhao, W. Comparative study on continuous and pulsed wave fiber laser cladding in-situ titanium-vanadium carbides reinforced Fe-based composite layer. Mater. Lett. 2015, 139, 255–257. [Google Scholar] [CrossRef]
- Chaves, I.A.; Melchers, R.E. Pitting corrosion in pipeline steel weld zones. Corros. Sci. 2011, 53, 4026–4032. [Google Scholar] [CrossRef]
- Fan, E.; Zhao, Q.; Wang, S.; Liu, B.; Yang, Y.; Huang, Y.; Luo, H.; Li, X. Investigation of the Hydrogen-Induced Cracking of E690 Steel Welded Joint in Simulated Seawater. Mater. Sci. Eng. A 2022, 143802. [Google Scholar] [CrossRef]
- Lu, Y.X.; Jing, H.Y.; Han, Y.D.; Xu, L.Y. Effect of Welding Heat Input on the Corrosion Resistance of Carbon Steel Weld Metal. J. Mater. Eng. Perform. 2016, 25, 565–576. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Zhang, Y.-P.; He, L.; Liu, C.-g. Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloy. Compd. 2013, 549, 195–199. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Chen, J.; Dong, S.Y. Phase evolution characteristics and corrosion behavior of FeCoCrAlCu-X0.5 coatings on cp Cu by laser high-entropy alloying. Opt. Laser Technol. 2017, 94, 68–71. [Google Scholar] [CrossRef]
- Qiu, X.-w.; Wu, M.-j.; Liu, C.-g.; Zhang, Y.-p.; Huang, C.-x. Corrosion performance of Al2CrFeCoxCuNiTi high-entropy alloy coatings in acid liquids. J. Alloy. Compd. 2017, 708, 353–357. [Google Scholar] [CrossRef]
- Bao, Y.; Ji, X.; Ji, C.; Zhao, J.; Cheng, J.; Xu, L. Corrosion and slurry erosion properties of FeCrNiCoCuAl_x high-entropy alloy coatings prepared by laser cladding. J. Mater. Eng. 2019, 47, 141–147. [Google Scholar]
- Gu, Z.; Mao, P.; Gou, Y.; Chao, Y.; Xi, S. Microstructure and properties of MgMoNbFeTi2Yx high entropy alloy coatings by laser cladding. Surf. Coat. Technol. 2020, 402, 126303. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Ma, Q.; Wu, T.; Liu, M.; Zhang, M.; Li, Z.; Yin, F. Selected Corrosion of X80 Pipeline Steel Welded Joints Induced by Desulfovibrio Desulfuricans. Corros. Sci. 2022, 202, 110313. [Google Scholar] [CrossRef]
- Shen, K.; Jiang, W.; Sun, C.; Zhao, W.; Sun, J. Unlocking the Influence of Microstructural Evolution on Hardness and Pitting Corrosion in Duplex Stainless Welded Joints. Corros. Sci. 2022, 206, 110532. [Google Scholar] [CrossRef]
- Zong, L.; Shi, G.; Wang, Y. Experimental Investigation on Fatigue Crack Behavior of Bridge Steel Q345qD Base Metal and Butt Weld. Mater. Des. 2015, 66, 196–208. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; He, L.; Ye, X.; Ouyang, W.; Xu, Z.; Zhang, W.; Zhou, X. Microstructure and Elevated Temperature Wear Behavior of Laser-Cladded AlCrFeMnNi High-Entropy Alloy Coating. Opt. Laser Technol. 2022, 149, 107845. [Google Scholar] [CrossRef]
- Kołodziejczak, P.; Golanski, D.; Chmielewski, T.; Chmielewski, M. Microstructure of Rhenium Doped Ni-Cr Deposits Produced by Laser Cladding. Materials 2021, 14, 2745. [Google Scholar] [CrossRef]
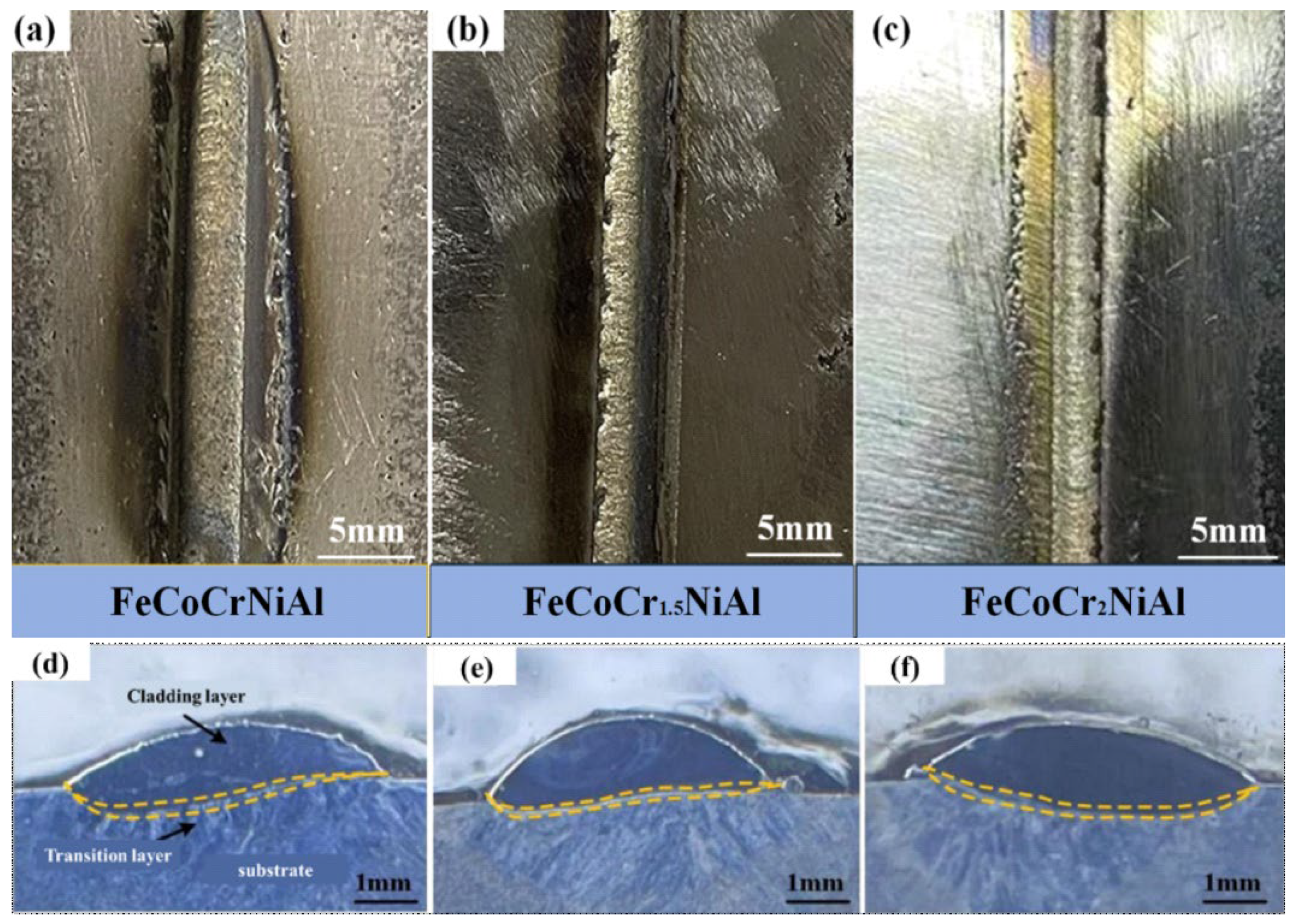
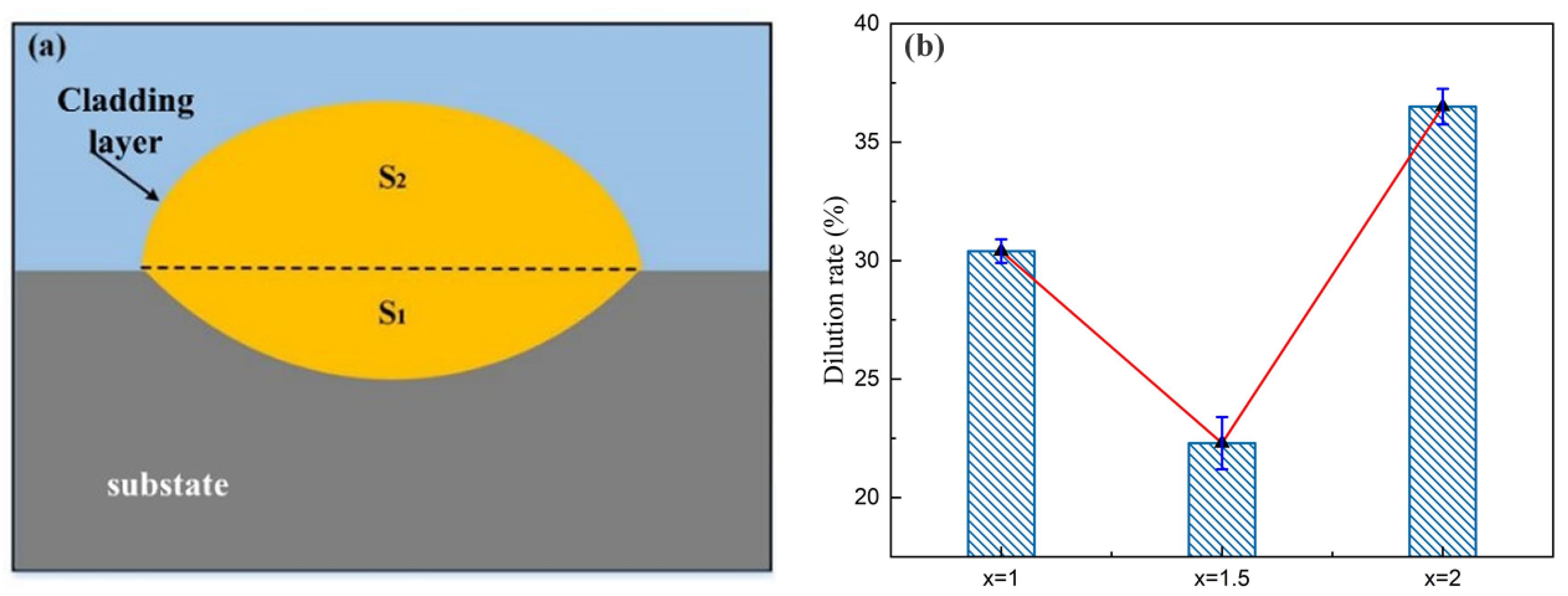
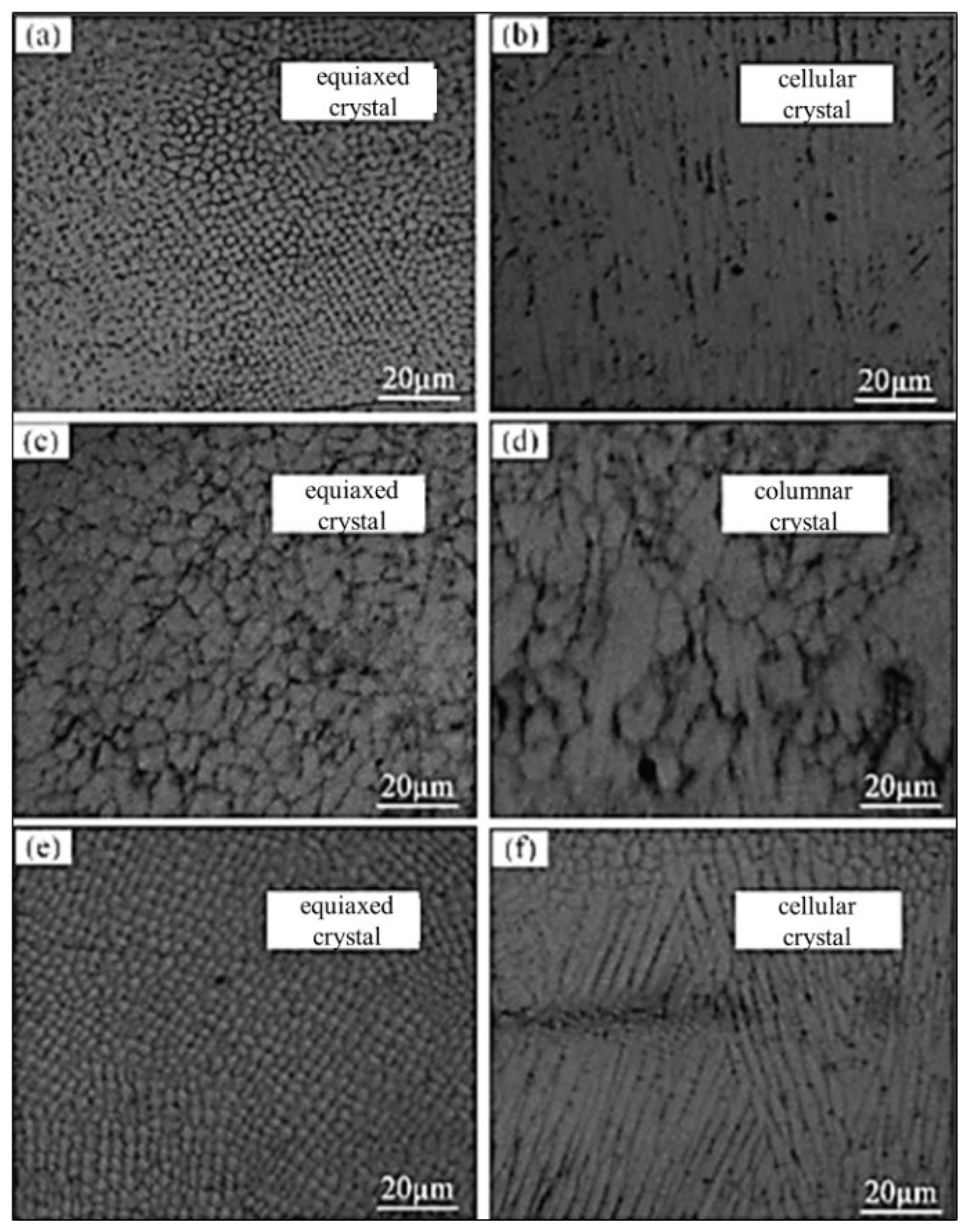

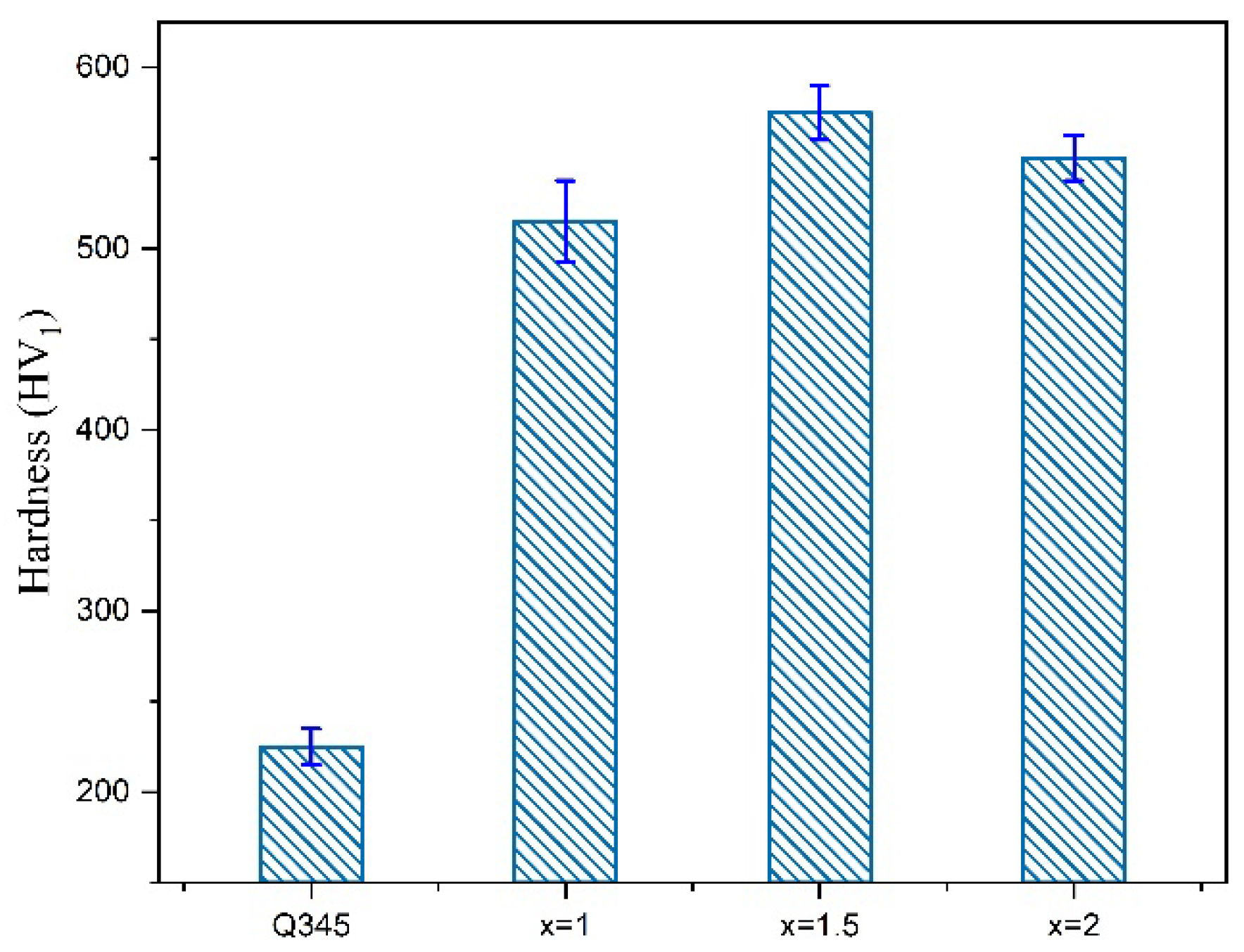



| Heat Source | Current (a) | Voltage (V) | Welding Gap (mm) | Wire Extension (mm) | Protective Gas Flow Rate (L/min) |
|---|---|---|---|---|---|
| MIG | 205 | 23 | 1 | 12 | 20 |
| No. | Composition | Fe | Co | Cr | Ni | Al |
|---|---|---|---|---|---|---|
| 1 | FeCoCrNiAl | 20 | 20 | 20 | 20 | 20 |
| 2 | FeCoCr1.5NiAl | 18.2 | 18.2 | 27.2 | 18.2 | 18.2 |
| 3 | FeCoCr2NiAl | 16.7 | 16.7 | 33.2 | 16.7 | 16.7 |
| Heat Source | Laser Power (KW) | Scanning Speed (mm/s) | Powder Feeding (r/min) | Protective Gas Flow Rate (L/min) |
|---|---|---|---|---|
| Laser | 1000 | 4 | 0.4 | 20 |
| Cr Content | Fe | Co | Cr | Ni | Al |
|---|---|---|---|---|---|
| x = 1 | 44.34 | 13.68 | 17.67 | 15.14 | 9.17 |
| x = 1.5 | 28.49 | 17.90 | 24.24 | 17.54 | 11.83 |
| x = 2 | 52.94 | 10.18 | 20.83 | 9.35 | 6.70 |
| Cr Content | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|
| x = 1 | −0.485 | 1.297 × 10−5 |
| x = 1.5 | −0.297 | 2.567 × 10−6 |
| x = 2 | −0.423 | 6.983 × 10−6 |
| Cr Content | Fe | Co | Cr | Ni | Al | O | Na | Cl |
|---|---|---|---|---|---|---|---|---|
| x = 1 | 43.78 | 3.58 | 4.9 | 3.59 | 2.75 | 38.67 | 1.59 | 1.14 |
| x = 1.5 | 33.03 | 3.65 | 8.91 | 3.81 | 3.92 | 37.48 | 4.51 | 4.69 |
| x = 2 | 40.93 | 2.81 | 5.05 | 2.83 | 2.77 | 38.54 | 3.76 | 3.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben, Q.; Zhang, Y.; Sun, L.; Wang, L.; Wang, Y.; Zhan, X. Wear and Corrosion Resistance of FeCoCrxNiAl High-Entropy Alloy Coatings Fabricated by Laser Cladding on Q345 Welded Joint. Metals 2022, 12, 1428. https://doi.org/10.3390/met12091428
Ben Q, Zhang Y, Sun L, Wang L, Wang Y, Zhan X. Wear and Corrosion Resistance of FeCoCrxNiAl High-Entropy Alloy Coatings Fabricated by Laser Cladding on Q345 Welded Joint. Metals. 2022; 12(9):1428. https://doi.org/10.3390/met12091428
Chicago/Turabian StyleBen, Qiang, Yumeng Zhang, Longxiang Sun, Leilei Wang, Yanni Wang, and Xiaohong Zhan. 2022. "Wear and Corrosion Resistance of FeCoCrxNiAl High-Entropy Alloy Coatings Fabricated by Laser Cladding on Q345 Welded Joint" Metals 12, no. 9: 1428. https://doi.org/10.3390/met12091428
APA StyleBen, Q., Zhang, Y., Sun, L., Wang, L., Wang, Y., & Zhan, X. (2022). Wear and Corrosion Resistance of FeCoCrxNiAl High-Entropy Alloy Coatings Fabricated by Laser Cladding on Q345 Welded Joint. Metals, 12(9), 1428. https://doi.org/10.3390/met12091428








