In-Situ Fabrication, Microstructure and Mechanical Performance of Nano Iron-Rich Precipitate Reinforced Cu and Cu Alloys
Abstract
:1. Introduction
2. Strengthening Method of Copper Alloy
2.1. Dispersion Strengthening Copper Alloys
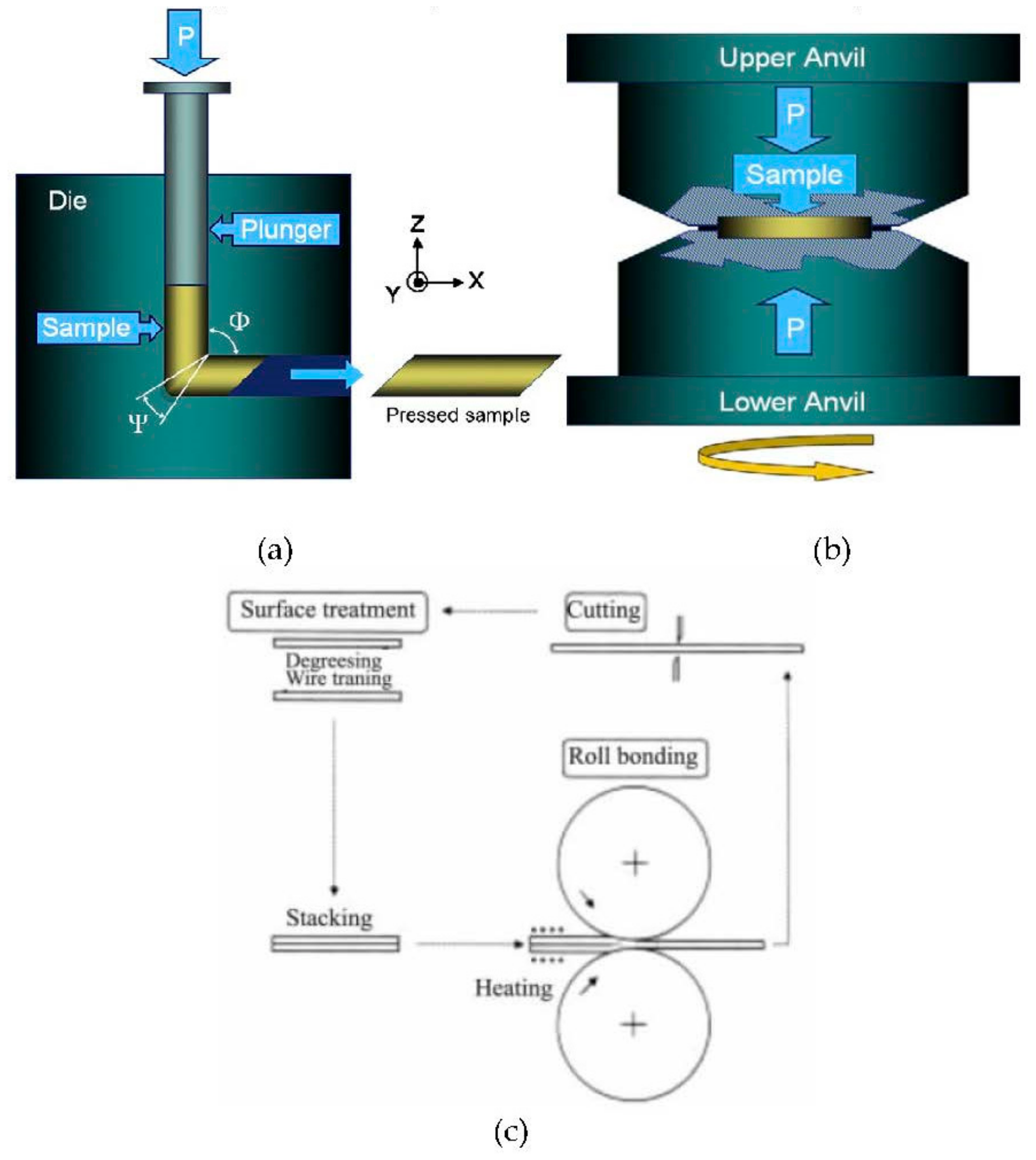
2.2. Ultrafine Grained Copper and Copper Alloys
2.3. Summary and Outlook
3. In-Situ Nano Precipitate Reinforced Copper and Copper Alloys
3.1. Design Idea and In-Situ Fabrication Route
3.2. Microstructural and Mechanical Characteristics
3.2.1. NPFG Structured tin Bronze Alloy
3.2.2. NPFG Structured Copper
3.3. Mechanism of Strengthening and Plasticizing
3.4. Mechanism of Microstructural Optimization
3.4.1. Mechanism of Grain Refining
3.4.2. Capture Mechanism of Iron-Rich Nanoparticles by Solidification Interface
3.4.3. Morphological Evolution of Iron-Rich Nanoparticles
3.4.4. Inhibition Mechanism on Tin Segregation
3.4.5. Section Summary
4. Conclusions, Gap Analysis and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, K. The future of metals. Science 2010, 328, 319–320. [Google Scholar] [PubMed]
- Hall, E.O. Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Lond. Ser. B 1951, 64, 747–753. [Google Scholar]
- Petch, N.J. The cleavage of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Huanga, Y.; Langdon, T.G. Advances in ultrafine-grained materials. Mater. Today 2013, 16, 85–93. [Google Scholar]
- Ogut, S.; Kaya, H.; Kentli, A. Comparison of the Effect of Equal Channel Angular Pressing, Expansion Equal Channel Angular Pressing, and Hybrid Equal Channel Angular Pressing on Mechanical Properties of AZ31 Mg Alloy. J. Mater. Eng. Perf. 2022, 31, 3341–3353. [Google Scholar]
- Pourhamid, R.; Shirazi, A. Microstructural evolution and mechanical behaviors of equal channel angular pressed copper. Proc. Inst. Mech. Part C J. Mech. Eng. Sci. 2020, 234, 171–179. [Google Scholar]
- Nuckowski, P.M.; Snopinski, P.; Wrobel, T. Influence of Plastic Strain Accumulation in Continuous Ingots during ECAP on Structure and Recrystallization Temperature of AlCu4MgSi Alloy. Materials 2020, 13, 576. [Google Scholar]
- Machackova, A. Decade of Twist Channel Angular Pressing: A Review. Materials 2020, 13, 1725. [Google Scholar]
- Gupta, A.; Chandrasekhar, B.; Saxena, K.K. Effect of Equal-channel angular pressing on mechanical Properties: An overview. Mater. Today 2021, 45, 5602–5607. [Google Scholar]
- Dong, W.Q.; Zhou, Z.; Zhang, M.D.; Ma, Y.M.; Yu, P.F.; Liaw, P.K.; Li, G. Applications of High-Pressure Technology for High-Entropy Alloys: A Review. Materials 2019, 9, 867. [Google Scholar]
- Figueiredo, R.B.; Horita, Z.; Langdon, T.G. Using High-Pressure Torsion to Achieve Superplasticity in an AZ91 Magnesium Alloy. Materials 2020, 10, 681. [Google Scholar]
- Kalahroudi, F.J.; Koohdar, H.; Sharafutdinov, A.; Jafarian, H.R.; Haung, Y.; Langdon, T.G.; Nili-Ahmadabadi, M. On the microstructure and mechanical properties of an Fe-10Ni-7Mn martensitic steel processed by high-pressure torsion. Mater. Sci. Eng. A 2019, 749, 27–34. [Google Scholar]
- Wang, H.F.; Tang, C.; An, O.G.; Lu, K.; Zhao, Y.H. Microstructure refinement mechanism of undercooled Cu(55)Ni(45)alloys. Mater. Sci. Pol. 2021, 39, 319–330. [Google Scholar]
- Xu, J.Q.; Chen, L.Y.; Choi, H.; Li, X.C. Theoretical study and pathways for nanoparticle capture during solidification of metal melt. J. Phys. Condens. Matter 2012, 24, 255304. [Google Scholar]
- Kushwaha, A.K.; Mishra, A.; Benson John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar]
- Schiøtz, J.; Jacobsen, K.W. A Maximum in the Strength of Nanocrystalline Copper. Science 2003, 301, 1357–1359. [Google Scholar]
- Huang, C.W.; Aoh, J.N. Friction Stir Processing of Copper-Coated SiC Particulate-Reinforced Aluminum Matrix Composite. Materials 2018, 11, 599. [Google Scholar]
- Zhang, G.H.; Mada, T.; Jiang, X.S.; Qiao, C.J.; Shao, Z.Y.; Zhu, D.G.; Zhu, M.H.; Valcarcel, V. Investigation of the Microstructure and Mechanical Properties of Copper-Graphite Composites Reinforced with Single-Crystal-Al2O3 Fibres by Hot Isostatic Pressing. Materials 2018, 11, 982. [Google Scholar]
- Lee, J.S.; Jung, J.Y.; Lee, E.S.; Park, W.J.; Ahn, S.; Kim, N.J. Microstructure and properties of titanium boride dispersed Cu alloys fabricated by spray forming. Mater. Sci. Eng. A 2000, 277, 274–283. [Google Scholar]
- Zhang, X.J.; Chen, Z.Q.; Liu, Z.H.; He, M.; Yang, Z.R.; Wang, Z.H. Microstructure and enhanced mechanical properties of ZrC/Zr composites added by in-situ Y2O3 reinforced particles. Vacuum 2022, 203, 111277. [Google Scholar]
- Wang, X.; Liu, J.; Zhang, Y.P.; Zhang, A.M.; Hou, H.P.; Zhuang, M.; Du, H.L. Microstructure and mechanical property of novel nanoparticles strengthened AlCrCuFeNi dual-phase high entropy alloy. Mater. Today 2022, 32, 104155. [Google Scholar]
- Li, Z.B.; Zhang, H.; Zhuang, Z.H.; Chou, C.H. Superior strength-ductility synergy in a novel tailored Zr-based particle-strengthened medium W content alloys. Compos. Part. B 2022, 236, 109817. [Google Scholar]
- Wang, Y.; Liu, Z.H.; Zhou, Y.Z.; Yang, X.S.; Tang, J.G.; Liu, X.; Li, J.F.; Le, G.M. Microstructure and mechanical properties of TiN particles strengthened 316L steel prepared by laser melting deposition process. Mater. Sci. Eng. A 2021, 814, 141220. [Google Scholar]
- Yang, X.N.; Cheng, G.G.; Wang, M.L.; Zhao, P. Precipitation and growth of titanium nitride during solidification of clean steel. J. Univ. Sci. Technol. B 2003, 10, 24–26. [Google Scholar]
- Tang, H.; Chen, X.H.; Chen, M.W.; Zuo, L.F.; Hou, B.; Wang, Z.D. Microstructure and mechanical property of in-situ nano-particle strengthened ferritic steel by novel internal oxidation. Mater. Sci. Eng. A 2014, 609, 293–299. [Google Scholar]
- Guan, C.; Chen, G.; Kai, X.Z.; Cao, R.; Miao, C.; Xu, Z.Z.; Zhao, Y.T. Evolution of microstructure and mechanical properties of graphene nanoplates and ZrB2 nanoparticles reinforced AA6111 composites during hot rolling deformation. J. Alloys Compd. 2022, 920, 165910. [Google Scholar]
- Rashid, M.S. High-strength, low-alloy steels. Science 1980, 208, 862–869. [Google Scholar] [PubMed]
- Wu, Z.Z.; Duan, B.H.; Wang, D.Z. Effects of shape and size of second phase on mechanical properties of sintered Mo-Y2O3 alloys. Trans. Nonferrous. Met. Soc. China 2022, 32, 1926–1934. [Google Scholar]
- Shi, G.D.; Chen, X.H.; Jiang, H.; Wang, Z.D.; Tang, H.; Fan, Y.Q. Strengthening mechanisms of Fe nanoparticles for single crystal Cu–Fe alloy. Mater. Sci. Eng. A 2015, 636, 43–47. [Google Scholar]
- Takata, N.; Ohtake, Y.; Kita, K.; Kitagawa, K.; Tsuji, N. Increasing the ductility of ultrafine-grained copper alloy by introducing fine precipitates. Scr. Mater. 2009, 60, 590–593. [Google Scholar]
- Wang, Z.D.; Wang, X.W.; Wang, Q.S.; Shih, I.; Xu, J.J. Fabrication of a nanocomposite from in-situ iron nanoparticle reinforced copper alloy. Nanotechnology 2009, 20, 075605. [Google Scholar] [PubMed]
- Chen, X.H.; Wang, Z.D.; Ding, D.; Tang, H.; Qiu, L.L.; Luo, X.; Shi, G.D. Strengthening and toughening strategies for tin bronze alloy through fabricating in-situ nanostructured grains. Mater. Des. 2015, 66, 60–66. [Google Scholar] [CrossRef]
- Chen, W.; Gao, G.J.; Meng, X.P.; Zhao, X.J.; Jiang, Y.B.; Wang, M.; Li, Z.; Xiao, L.R. Microstructure, properties and strengthening mechanism of Cu-TiB2-Al2O3 composite prepared by liquid phase in-situ reaction casting. J. Alloys Compd. 2022, 912, 165170. [Google Scholar]
- Qin, Y.Q.; Tian, Y.; Peng, Y.Q.; Luo, L.M.; Zan, X.; Xu, Q.; Wu, Y.C. Research status and development trend of preparation technology of ceramic particle dispersion strengthened copper-matrix composites. J. Alloys Compd. 2020, 848, 156475. [Google Scholar] [CrossRef]
- Yao, L.Y.; Gao, Y.M.; Li, Y.F.; Huang, X.Y.; Wang, Y.R.; Huang, Y.J. Preparation of nanostructural oxide dispersion strengthened (ODS) Mo alloy by mechanical alloying and spark plasma sintering, and its characterization. Int. J. Refract. Met. Hard Mater. 2022, 105, 105822. [Google Scholar]
- Rocky, B.P.; Weinberger, C.R.; Daniewicz, S.R.; Thompson, G.B. Carbide Nanoparticle Dispersion Techniques for Metal Powder Metallurgy. Metals 2021, 11, 871. [Google Scholar]
- Schneibel, J.H.; Liu, C.T.; Hoelzer, D.T.; Mills, M.J.; Hayashi, T.; Wendt, U.; Heyse, H. Development of porosity in an oxide dispersion-strengthened ferritic alloy containing nanoscale oxide particles. Scr. Mater. 2007, 57, 1040–1043. [Google Scholar] [CrossRef]
- Lu, K.; Lu, L.; Suresh, S. Strengthening Materials by Engineering Coherent Internal Boundaries at the Nanoscale. Science 2009, 324, 349–352. [Google Scholar]
- Liu, Y.G.; Zhang, J.Q.; Tan, Q.Y.; Liu, S.Y.; Li, M.; Zhang, M.X. Additive manufacturing of high strength copper alloy with heterogeneous grain structure through laser powder bed fusion. Acta Mater. 2021, 220, 117311. [Google Scholar]
- Pan, S.W.; Zhou, X.L.; Chen, X.L.; Yang, M.; Cao, Y.D.; Chen, X.H.; Wang, Z.D. In-Situ Nanoparticles: A New Strengthening Method for Metallic Structural Material. Appl. Sci. 2018, 8, 2479. [Google Scholar]
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Acta Mater. 2002, 48, 1–29. [Google Scholar]
- Chen, K.X.; Chen, X.H.; Ding, D.; Wang, Z.D. Crystallographic features of iron-rich nanoparticles in cast Cu–10Sn–2Zn–1.5Fe–0.5Co alloy. Mater. Charact. 2016, 113, 34–42. [Google Scholar]
- Wang, Q.S.; Song, Z.F.; Feng, Z.Q.; Wang, Z.D. Microstructure and properties of ZCuSn3Zn8Pb6NiFeCo alloy. Adv. Mater. Res. 2012, 430–432, 609–618. [Google Scholar]
- Chen, K.X.; Chen, X.H.; Wang, Z.D.; Mao, H.H.; Sandström, R. Optimization of deformation properties in as-cast copper by microstructural engineering. Part I. microstructure. J. Alloys Compd. 2018, 763, 592–605. [Google Scholar]
- Chen, K.X.; Pa, S.W.; Zhu, Y.Z.; Cheng, Y.J.; Chen, X.H.; Wang, Z.D. In situ observations of crack propagation in as-cast Cu-1.5Fe-0.5Co (wt%) alloy. Mater. Sci. Eng. A 2017, 706, 211–216. [Google Scholar]
- Chen, K.X.; Pan, S.H.; Chen, X.H.; Wang, Z.D.; Sandström, R. Optimisation of deformation properties in as-cast copper by microstructural engineering. Part II. Mechanical properties. J. Alloys Compd. 2020, 812, 151910. [Google Scholar]
- Chen, K.X.; Korzhavyi, P.A.; Demange, G.; Zapolsky, H.; Patte, R.; Boisse, J.; Wang, Z.D. Morphological instability of iron-rich precipitates in Cu-Fe-Co alloys. Acta Mater. 2019, 163, 55–67. [Google Scholar]
- Chen, K.X.; Chen, X.H.; Ding, D.; Shi, G.D.; Wang, Z.D. Formation mechanism of in-situ nanostructured grain in cast Cu–10Sn–2Zn–1.5Fe–0.5Co (wt.%) alloy. Mater. Des. 2016, 94, 338–344. [Google Scholar]
- Chen, K.X.; Chen, X.H.; Ding, D.; Shi, G.D.; Wang, Z.D. Heterogeneous nucleation effect of in situ iron-rich nanoparticles on grain refinement of copper alloy. Mater. Lett. 2016, 168, 188–191. [Google Scholar]
- Chen, K.X.; Zhang, J.W.; Chen, Y.J.; Chen, X.H.; Wang, Z.D.; Sandström, R. Slow strain rate tensile tests on notched specimens of as-cast pure Cu and Cu-Fe-Co alloys. J. Alloys Compd. 2020, 822, 153647. [Google Scholar]
- Neikov, O.D.; Naboychenko, S.S.; Murashova, I.B. Handbook of Non-Ferrous Metal Powders; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 331–368. ISBN 978-1-85617-422-0. [Google Scholar]
- Zhao, M.; Xu, B.Y.; Zhang, P.; Xu, J.J.; Jiang, Y.; Liu, F.; Yan, Y.W. Microstructure development of Y-Ti-O dispersion strengthened Cu alloys fabricated by mechanical alloying. Mater. Charact. 2022, 186, 111808. [Google Scholar] [CrossRef]
- Shen, K.; Wang, M.P.; Li, S.M. Study on the properties and microstructure of dispersion strengthened copper alloy deformed at high temperatures. J. Alloys Compd. 2009, 479, 401–408. [Google Scholar] [CrossRef]
- Geng, Y.F.; Ban, Y.J.; Song, K.X.; Zhang, Y.; Tian, B.H.; Liu, Y. A review of microstructure and texture evolution with nanoscale precipitates for copper alloys. J. Mater. Res. Technol. 2020, 9, 11918–11934. [Google Scholar] [CrossRef]
- Danninger, H.; Mayer, C.G. Advances in Powder Metallurgy; Woodhead Publishing: Cambridge, UK; Sawston, UK, 2013; pp. 149–201. ISBN 978-0-85709-420-9. [Google Scholar]
- Hu, Y.S.; Hu, Z.Y.; Fan, G.L.; Tan, Z.Q.; Zhou, Z.D.; Zhang, H.; Li, Z.Q. Simultaneous enhancement of strength and ductility with nano dispersoids in nano and ultrafine grain metals: A brief review. Rev. Adv. Mater. Sci. 2020, 59, 352–360. [Google Scholar] [CrossRef]
- Chinh, N.Q.; Kovacs, Z. Unique microstructural and mechanical properties of Al-Zn alloys processed by high-pressure torsion. Mater. Sci. Eng. 2019, 613, 012028. [Google Scholar] [CrossRef]
- Lowe, T.C.; Zhu, Y.T.; Semiatin, S.L.; Berg, D.R. Overview and outlook for materials processed by severe plastic deformation. In Investigations and Applications of Severe Plastic Deformation; Springer: Berlin/Heidelberg, Germany, 2000; p. 347. [Google Scholar]
- Wu, B.; Fu, H.; Qian, L.; Luo, J.S.; Lee, W.B.; Yang, X.S. Severe plastic deformation-produced gradient nanostructured copper with a strengthening-softening transition. Mater. Sci. Eng. A 2021, 819, 141495. [Google Scholar] [CrossRef]
- Segal, V.M. Materials processing by simple shear. Mater. Sci. Eng. A 1995, 197, 157–164. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Jiang, S.H.; Wang, H.; Wu, Y.; Liu, X.J.; Chen, H.H.; Yao, M.J.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Tong, Y.; Jiao, Z.B.; Wei, J.; Cai, J.X.; Han, X.D.; Chen, D.; Hu, A.; Kai, J.J.; et al. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 2018, 362, 933–937. [Google Scholar] [CrossRef]
- Peng, S.; Wei, Y.; Gao, H. Nanoscale precipitates as sustainable dislocation sources for enhanced ductility and high strength. Proc. Natl. Acad. Sci. USA 2020, 117, 5204–5209. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Shen, L.; Cao, F.; Jia, Y.; Wang, M. Study of the coarsening and hardening behaviors of coherent α-Fe particles in Cu-2.1Fe alloy. Acta Metall. Sin. 2014, 50, 1224–1230. [Google Scholar]
- Guo, C.J.; Wan, J.; Chen, J.H.; Xiao, X.P.; Huang, H.; Liu, J.P. Inhibition of discontinuous precipitation and enhanced properties of Cu-15Ni-8Sn alloy with Fe addition. Mater. Sci. Eng. A 2020, 795, 139917. [Google Scholar] [CrossRef]
- Chen, K.X.; Chen, X.H.; Wang, Z.D. Precipitates-interaction capture of nano-sized iron-rich precipitates during copper solidification. Mater. Sci. Tech. Lond. 2019, 35, 1743–2847. [Google Scholar] [CrossRef]
- Chen, K.X.; Chen, X.H.; Ding, D.; Wang, Z.D. Effect of in-situ nanoparticle wall on inhibiting segregation of tin bronze alloy. Mater. Lett. 2016, 175, 148–151. [Google Scholar] [CrossRef]
- Chen, K.X.; Zhang, J.W.; Chen, X.H.; Ding, D.; Wang, Z.D.; Shi, R.J.; Zhang, A.J. The effect of iron on the microstructure and mechanical properties of a cast Cu–12Sn-1.5Ni (wt. %) alloy. Mater. Sci. Eng. A 2020, 785, 139330. [Google Scholar] [CrossRef]
- Chen, Z.N.; Kang, H.J.; Fan, G.H.; Li, J.H.; Lu, Y.P.; Jie, J.C.; Zhang, Y.B.; Li, T.J.; Jian, X.G.; Wang, T.M. Grain refinement of hypoeutectic Al-Si alloys with B. Acta Mater. 2016, 120, 168–178. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, S.R.; Shi, J.Q.; Shen, Z.; Shi, P.Z.; Zheng, T.X.; Ding, B.; Guo, Y.F.; Zhong, Y.B. Grain refinement and mechanical properties enhancement of Cu-10 wt%Fe alloys via Zr addition. Mater. Sci. Eng. A 2022, 864, 143309. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, T.; Yang, H.B.; Hu, K.Q.; Liu, G.L.; Sun, Q.Q.; Nie, J.F.; Liu, X.F. Enhanced grain refinement and mechanical properties of a high–strength Al–Zn–Mg–Cu–Zr alloy induced by TiC nano–particles. Mater. Sci. Eng. A 2021, 806, 140852. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Qiu, D.; Gibson, M.A.; Zheng, Y.F.; Fraser, H.L. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef]
- Maxwell, I.; Hellawell, A. An analysis of the peritectic reaction with particular reference to Al-Ti alloys. Acta Metall. 1975, 23, 229–237. [Google Scholar] [CrossRef]
- GREER, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of Aluminium by Al-Ti-B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Zhao, L.J.; Park, N.; Liu, R.; Zhang, P.; Zhang, Z.J.; Shibata, A.; Zhang, Z.F.; Tsuji, N. Revealing the deformation mechanisms of Cu–Al alloys with high strength and good ductility. Acta Mater. 2016, 110, 61–72. [Google Scholar] [CrossRef]
- Qin, S.Y.; Chen, C.R.; Zhang, G.D.; Wang, W.L.; Wang, Z.G. The effect of particle shape on ductility of SiCp reinforced 6061 Al matrix composites. Mater. Sci. Eng. A 1999, 272, 363–370. [Google Scholar] [CrossRef]
- Han, S.Z.; Kim, K.H.; Kang, J.; Joh, H.; Kim, S.M.; Ahn, J.H.; Lee, J.; Lim, S.H.; Han, B. Design of exceptionally strong and conductive Cu alloys beyond the conventional speculation via the interfacial energy-controlled dispersion of γ-Al2O3 nanoparticles. Sci. Rep. 2015, 5, 17364. [Google Scholar]
- Kumar, N.; Choudhuri, D.; Banerjee, R.; Mishra, R.S. Strength and ductility optimization of Mg–Y–Nd–Zr alloy by microstructural design. Int. J. Plast. 2015, 68, 77–97. [Google Scholar] [CrossRef]
- Yuan, S.P.; Liu, G.; Wang, R.H.; Zhang, G.J.; Pu, X.; Sun, J.; Chen, K.H. Aging-dependent coupling effect of multiple precipitates on the ductile fracture of heat-treatable aluminum alloys. Mater. Sci. Eng. A 2009, 499, 387–395. [Google Scholar] [CrossRef]
- Guo, Z.K.; Jie, G.C.; Liu, J.M.; Yue, S.P.; Liu, S.C.; Li, T.J. Effect of cold rolling on aging precipitation behavior and mechanical properties of Cu-15Ni-8Sn alloy. J. Alloys Compd. 2020, 848, 156275. [Google Scholar] [CrossRef]
- Martin, J.W. Precipitation Hardening, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1998; pp. 1–125. [Google Scholar]
- Ardell, A.J. Precipitation Hardening. Metall. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]
- Gazizov, M.; Kaibyshev, R. Precipitation structure and strengthening mechanisms in an Al-Cu-Mg-Ag alloy. Mater. Sci. Eng. A 2017, 702, 29–40. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Tang, B.B.; Yu, F.X.; Shen, B. Evaluation of nanoscaled precipitates in a Cu–Ni–Si–Cr alloy during aging. J. Alloys Compd. 2014, 614, 189–195. [Google Scholar] [CrossRef]
- Rajkovic, V.; Bozic, D.; Stasic, J.; Wang, H.W.; Jovanovic, M.T. Processing, characterization and properties of copper-based composites strengthened by low amount of alumina particles. Powder Technol. 2014, 268, 392–400. [Google Scholar] [CrossRef]
- Zander, J.; Sandström, R.; Vitos, L. Modelling mechanical properties for non-hardenable aluminium alloys. Comp. Mater. Sci. 2007, 41, 86–95. [Google Scholar] [CrossRef]
- Correia, J.B.; Davies, H.A.; Sellars, C.M. Strengthening in rapidly solidified age hardened Cu-Cr and Cu-Cr-Zr alloys. Acta Mater. 1997, 45, 177–190. [Google Scholar] [CrossRef]
- Koike, J.; Kobayashi, T.; Mukai, T.; Watanabe, H.; Suzuki, M.; Maruyama, K.; Higashi, K. The activity of non-basal slip systems and dynamic recovery at room temperature in fine-grained AZ31B magnesium alloys. Acta Mater. 2003, 51, 2055–2065. [Google Scholar] [CrossRef]
- Ng, K.L.; Sasaki, H.; Kimura, H.; Yoshikawa, T.; Maeda, M. Heterogeneous Nucleation of Graphite on Rare Earth Compounds during Solidification of Cast Iron. ISIJ Int. 2018, 58, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Shu, S.P.; Wells, P.B.; Almirall, N.; Odette, G.R.; Morgan, D.D. Thermodynamics and kinetics of core-shell versus appendage co-precipitation morphologies: An example in the Fe-Cu-Mn-Ni-Si system. Acta Mater. 2018, 157, 298–306. [Google Scholar] [CrossRef]
- Qian, M. Heterogeneous nucleation on potent spherical substrates during solidification. Acta Mater. 2007, 55, 943–953. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Qian, M. Crystallography of grain refinement in Mg–Al based alloys. Acta Mater. 2005, 53, 3261–3270. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F.; Wang, Y.; Men, H.; Zhou, L. Effect of solutes on grain refinement. Prog. Mater. Sci. 2022, 123, 100809. [Google Scholar] [CrossRef]
- Park, S.B. Heterogeneous nucleation models to predict grain size in solidification. Prog. Mater. Sci. 2022, 123, 100822. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Chen, X.R.; Jia, Y.H.; Le, Q.C.; Ning, S.C.; Li, X.Q.; Yu, F.X. The interaction between in situ grain refiner and ultrasonic treatment and its influence on the mechanical properties of Mg-Sm-Al magnesium alloy. J. Mater. Res. Technol. 2020, 9, 9262–9270. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xia, M.; Arumuganathar, S. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing. Acta Mater. 2009, 57, 4891–4901. [Google Scholar] [CrossRef]
- Turnbull, D.; Vonnegut, B. Nucleation catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, G.H. Analysis Methods in Materials Science: X-ray Diffraction and Electron Microscopy IN Materials Science; Harbin Institute of Technology Press: Harbin, China, 2007. [Google Scholar]
- Kim, W.T.; Cantor, B. An adsorption model of the heterogeneous nucleation of solidification. Acta Metall. Mater. 1994, 42, 3115–3127. [Google Scholar] [CrossRef]
- Egerton, R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope, 3rd ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Böhm, H.J.; Rasool, A. Effects of particle shape on the thermoelastoplastic behavior of particle reinforced composites. Int. J. Solids Struct. 2016, 87, 90–101. [Google Scholar] [CrossRef]
- Shan, L.Y.; Li, Y.; Wang, Y.P. Improving the high temperature mechanical performance of Cu-Cr alloy induced by residual nano-sized Cr precipitates. Mater. Sci. Eng. A 2022, 845, 143250. [Google Scholar] [CrossRef]
- Xu, R.F.; Geng, Z.W.; Wu, Y.Y.; Chen, C.; Ni, M.; Li, D.; Zhang, T.M.; Huang, T.M. Microstructure and mechanical properties of in-situ oxide-dispersion-strengthened NiCrFeY alloy produced by laser powder bed fusion. Adv. Pow. Mater. 2022, 1, 100056. [Google Scholar] [CrossRef]
- Yusuke, S.; Masataka, M.; Kenta, Y.; Yasuyoshi, N.; Toyokiho, J.K. Microstructural changes of oxide dispersion strengthened copper powders fabricated by mechanical alloying. Fus. Eng. Des. 2021, 173, 112804. [Google Scholar]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, K.; Chen, X.; Zhu, Y.; Chen, M.; Wang, Y.; Shen, J.; Shi, J.; Wang, Z. In-Situ Fabrication, Microstructure and Mechanical Performance of Nano Iron-Rich Precipitate Reinforced Cu and Cu Alloys. Metals 2022, 12, 1453. https://doi.org/10.3390/met12091453
Li Z, Chen K, Chen X, Zhu Y, Chen M, Wang Y, Shen J, Shi J, Wang Z. In-Situ Fabrication, Microstructure and Mechanical Performance of Nano Iron-Rich Precipitate Reinforced Cu and Cu Alloys. Metals. 2022; 12(9):1453. https://doi.org/10.3390/met12091453
Chicago/Turabian StyleLi, Zongxuan, Kaixuan Chen, Xiaohua Chen, Yuzhi Zhu, Mingwen Chen, Yanlin Wang, Jiangxu Shen, Jiayun Shi, and Zidong Wang. 2022. "In-Situ Fabrication, Microstructure and Mechanical Performance of Nano Iron-Rich Precipitate Reinforced Cu and Cu Alloys" Metals 12, no. 9: 1453. https://doi.org/10.3390/met12091453




