Abstract
A condensation reaction of 2,3,5,6-tetraamino-1,4-benzoquinone 1 with 4,5-Dichloro-3,6-dihydroxy-phthalonitrile 2 produced p-benzoquinone [2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazine)-6,7-dinitrile] 3. Utilizing acetic acid with lithium/pentanol, the tetra-nitrile monomer was cyclo-tetramerized, yielding the matching network polymer, tetra p-benzoquinone[2,3-b:2,3-b]. bis[(5,8-dihydroxybenzopyrazino) porphyrazine (2H-Pz) 4a. The equivalent tetra p-benzoquinone[2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazino) metallic porphyrazine networks (M-Pz) M = Zn 4b or Ni 4c, were obtained by cyclo-tetramerizing the tetra-nitril monomer 3 using metal salt and quinoline. The synthesized molecules’ elemental analytical results, as well as their IR and NMR spectral data, are consistent with their assigned structures. The prepared compounds have large molecular weights and metal content, indicating that reactions of tetramerization, polymerization, and chelation were all productive. The synthesized porphyrazines were proved to be excellent substrates for oxidizing thiophenol and benzyl thiol to their respective disulfides in atmospheric oxygen. The maximal production of the corresponding disulfides after 15 min was 96 percent for thiophenol and 93 percent for benzyl thiol, respectively.
1. Introduction
Porphyrin derivatives, tetra benzoporphyrazine derivatives, and associated aza counterparts are interesting macro heterocycle systems that have been the subject of substantial research and practical applications in science, industry, and medicine. As an outgrowth of phthalocyanines, Sir R. Patrick Linstead produced tetra azaporphyrins, commonly known as porphyrazines, which are tetrapyrrole macrocycles connected to porphyrins and phthalocyanines [1]. Porphyrazine compounds are distinguished from porphyrin compounds by the presence of meso nitrogen instead of carbon atoms and they are distinguished from phthalocyanines by the interchangeability of their pyrrole sites. Both porphyrins and phthalocyanines have physical features that distinguish them from one another [2]. The cyclotetramerization reaction following the Linstead approach led to novel symmetrical magnesium(II) aminoporphyrazines with methyl(6-bromo-3-pyridylmethyl) and methyl(4-bromobenzyl) substituents [3]. Porphyrazines are a new category of tetrapyrroles that have high absorption and retention qualities in situ, making them ideal for photodynamic treatment of cancer (PDT) [4].
In the breakdown of hydrogen peroxide under heterogeneous conditions, the catalytic characteristics of network poly tetra pyrazinoporphyrazine complexes with Cu2+, Co2+, and Fe2+ were investigated [5]. In a one-pot manner, the aromatic replacement processes of pyrrole and aldehydes were employed to fabricate stable porphyrin-based permeable macromolecular chains, PPN-23 and PPN-24 [6]. Cyclotetramerization of dinitrile derivative with magnesium butoxide in hot butanol affords the crosswise phthalocyanine derivatives or naphthalocyanine counterparts, notably tetra pyrazino-porphyrazines and tetra-6,7-quinoxalino-porphyrazines [7]. The pyrazino-porphyrazine compound (without metal, zinc, and copper variants) were afforded by tetramerizing 2,3-dicyanopyrazine [8].
Beginning with 3,3-dimethyl-2-butanone, selenium dioxide, 2,3-diaminomaleonitrile, salts, and urea, manufacture of cobalt combination of tetra-2,3-(5-tert-butyl-pyrazino) porphyrazine as porphyrazine analogues with six-membered pyrazines was evaluated [9]. Substituted porphyrazine macrocycles were described and prepared from easily functionalized diaminomaleonitrile (DAMN) [10]. Chloranil was used to make novel cyanine dyes by combining it with mercapto-, or imino-pyrazole derivative [11].
Organic components have been increasingly popular in the creation of microporous materials in recent years. It is evident that accurate prediction over the chemical characteristics of the obtainable surface, as well as the incorporation of catalytic sites, can be accomplished by carefully choosing organic materials, enabling for chemo adsorption on the surface and the layout of high-performance heterogeneous catalysts. Diffusion failures are associated with insoluble resins, while the optimal catalysts are soluble linear polymer ligands. The use of cobalt (II) phthalocyanines to accelerate thiol autoxidation has proved to be quite advantageous [12,13,14].
Palladium (II) has recently been supported by a structured quinone polymer possessing sulphonic acid moieties, providing a catalyst capable of converting ethylene to acetaldehyde [15]. Novel 2H- and metal-pyrazino-porphyrazine derivatives, as well as 2H- and metal-phthalocyanines, were investigated for conductivity [16]. The pyrazinoporphyrazine networks supported metal (II)-based catalysts were produced, evaluated, and spectroscopically examined [17]. Two novel kinds of neutral porphyrazine compounds have recently been discovered [18]. Porphyrazine combinations of Y, La, and Lu were studied in terms of optoelectronic and architectural aspects [19].
The diazepine rings were created by employing ultrasonic energy as a friendly form of energy to 1,4-cycloaddition operations of chalcones with 2,3-diaminomaleonitrile, resulting in unique Cu-porphyrazines annulated with diazepine moieties [20]. The fundamental macrocyclic pyrazinoporphyrazine combination of In(III) complex was produced [21]. New zinc(II) porphyrazine derivatives were revealed [22]. The goal of this work, which is a continuation of our previous work [23], is to synthesize novel porphyrazine-based network polymers with a quinone moiety for use as supported metal (II)-based catalysts.
2. Materials and Methods
2.1. Ingredients and Procedures
All substances were provided by different sources and utilized without being purified. Zhengzhou Alfa Chemical Co., Ltd., Jiaozuo, China, provided the 2,3,5,6-tetraamino-1,4-benzoquinone (purity: 97 percent min). Dayang Chem (Hangzhou) Co., Ltd., Hangzhou, China, provided 4,5-dichloro-3,6-dihydroxy-phthalonitrile (purity: 98.0 percent). Ethyl alcohol, N,N-dimethylformamide (anhydrous, purity: 99.8 percent), 1-pentanol (purity: 99 percent), quinoline (purity: 98 percent), petroleum ether (AR, bp 40–60 °C), acetone (purity: 99.5 percent), lithium metal (purity: 99 percent), zinc chloride (purity: 98 percent), nickel(II) chloride (purity: 97%), and copper(II) chloride (purity: 98 percent), pyridine (anhydrous, purity: 99.8 percent), dichloromethane (anhydrous, purity: 99.8 percent), methanol (anhydrous, purity: 99.8 percent), acetonitrile (anhydrous, purity: 99.8 percent), chloroform (anhydrous, purity: 99.8 percent), anhydrous, (purity: 99 percent), sodium thiosulfate pentahydrate (purity: 99 percent), n-hexane, benzyl thiol (purity: 99 percent), and thiophenol (purity: 99 percent) were acquired from Merck Ltd., Shanghai, China. Electrothermal melting point equipment (Electrothermal Engineering) was used to determine uncorrected melting points in open capillaries.
2.2. Characterization
Infrared analyzer Shimadzu 8101 M including Fourier transform (Shimadzu, Tokyo, Japan) was used to take infrared spectral studies. Ultraviolet spectra were obtained using the spectrometer (Pye Unicam SP6-550 UV/Vis, 5651 CA, Eindhoven, the Netherlands). A Varian VXR 400S NMR device operated in deuterochloroform at 400 MHz (1HNMR) and 100 MHz (13CNMR) using tetramethyl silane as a standard procedure. Spectrometry of plasma-optical emission with a flame ionization detector ICP-OES (Horiba Instruments, Shanghai, China) was used to assess the composition of metals in polymers in a more practical way.
PerkinElmer 2400 CHN (International Equipment Trading Ltd., Illinois, IL, USA) was used to acquire the elemental evaluations. Thin-layer chromatography (TLC) technique examining the rates of reactions utilized pre-coated (Merck, Darmstadt, Germany) silica gel 60F-254 plate with a chloroform/acetone (8:2 v/v) solvent system. The apparent molecular masses (Mn: number average molecular weight, Mw: weight average molecular weight) were calculated using gel permeation chromatography (GPC) with polymeric references and mild polymer concentrations (10 mg/5 mL) in tetrahydrofuran (THF) at ambient temperature at a level of approximately 1 mL/min (Polymer laboratories, PL-GPC 20)). Utilizing a Micromeritics ASAP 2000 surface detector, BET surface values were estimated from nitrogen isotherm models at 77 K. ′
2.3. Synthesis of p-Benzoquinone [2,3-b:2′,3′-b′]bis[(5,8-dihydroxybenzopyrazine)-6,7-dinitrile] 3
For 6 h, a combination of 2,3,5,6-Tetraamino-1,4-benzoquinone 1 (0.01 mol) and bi-molar ratio of 4,5-Dichloro-3,6-dihydroxy-phthalonitrile 2 (0.02 mol) was refluxed in ethyl alcohol (50 mL)/pyridine (20 mL) mixture. At the end of the refluxing, the mixture had turned a deep blue color. It was filtered while heating, precipitated with cold water, washed multiple times with distilled water, dried, and crystallized using ethyl alcohol. Yield: 94%. MP: 246 °C. FT-IR ν (cm–1): 1623 (C=C), 1688 (C=O), 2232 (CN), 3358 (NH), 3422 (OH); 1H-NMR (CDCl3): δ (ppm) = 6.32 (s, H, NH), 5.62 (s, H, OH); 13C-NMR (CDCl3) δ = 106.4, 111.5, 134.7, 162.6, 164.8, 188.6. Anal. calcd. for C22H8N8O6 (MWt: 480.34) requires C, 55.01; H, 1.68; N, 23.33%. Found: C, 55.08; H, 1.76; N, 23.39%.
2.4. Synthesis of Tetra p-Benzoquinone [2,3-b:2′,3′-b′]bis[(5,8-dihydroxybenzopyrazino) Porphyrazine-Based Network (2H-Pz) 4a
We heat mixed 3 (0.5 mmol) in a blend of pentanol (100 mL) and lithium metal (20 mg, 2.8 mmol) for 16 h. Upon cooling, 2 mL acetic acid was added into the mixture and extraction was used to collect the result. Column chromatography (eluent: chloroform/dichloromethane 90:10) was used to refine the light blue substance, which was then precipitated from methyl alcohol.
FT-IR ν (cm–1): 1518 (C=N), 1624 (C=C), 1694 (C=O). 3342, 3368 (NH), 3414 (OH). UV-Vis (CH2Cl2), λmax (nm): 310, 544, 632. 1H-NMR (CDCl3): δ (ppm) = 6,45, 1.82 (s, H, NH), 5.45 (s, H, OH). 13C-NMR (CDCl3) δ = 103.4, 133.5, 151.7, 152.1, 152.8, 161.4, 163.7, 189.2. Anal. Calcd. for the polymeric repetition unit predicted C84H42N28O24 (MWt = 2118.1937) requires C, 61.24; H, 1.99; N, 18.51%. Found: C, 61.32; H, 2.06; N, 18.59%.
2.5. Synthesis of Porphyrazinato-Metal II-Networks (M-Pz), M = Zn 4b or M = Ni 4c
The corresponding porphyrazine network was formed by reacting the tetra-nitrile precursor 3 (0.5 mmol) with quinoline (100 mL) at 200 °C for 16 h with 0.75 mmol of metal salt (Zn Cl2, or NiCl2). The mixture was milted in acetone and the metals that had not been entrapped dropped out of the solution and were retrieved. A large amount of cold methanol was added to the resulting acetone solution, resulting in particles that can be refined by chromatographic separation (eluent: chloroform/methylene chloride 90:10). Overnight, the porphyrazines were vacuum dried.
4b: FT-IR ν (cm–1): 1512 (C=N), 1608 (C=C), 1690 (C=O), 3354, 3372 (NH). UV-Vis (CH2Cl2), λmax (nm): 360, 478 (shoulder), 628, 726. 1H-NMR (CDCl3): δ (ppm) = 6.48 (s, H, NH), 5.33 (s, H, OH). 13C-NMR (CDCl3) δ = 104.6, 113.8, 134.2, 154.6, 160.2, 161.5, 168.5, 189.8. Anal. Calcd. for the polymeric repetition unit predicted C108H40N28O24Zn (MWt = 2181.5737) requires C, 59.46; H, 1.84; N, 17.97; Zn, 2.99%. Found: C, 59.54; H, 1.91; N, 18.07; Zn, 3.05%.
4c: FT-IR ν (cm–1): 1510 (C=N), 1606 (C=C), 1695 (C=O), 3348, 3368 (NH). UV-Vis (CH2Cl2), λmax (nm): 352, 432 (shoulder), 594, 704. 1H-NMR (CDCl3): δ (ppm) = 6.53 (s, H, NH), 5.38 (s, H, OH). 13C-NMR (CDCl3) δ = 103.5, 114.9, 134.8, 155.8, 160.4, 162.2, 167.9, 185.8. Anal. Calcd. for the polymeric repetition unit predicted C108H40N28O24Ni (MWt = 2174.89) requires C, 59.64; H, 1.85; N, 24.44; Ni, 18.03%. Found: C, 59.69; H, 1.93; N, 24.52; Ni, 18.12%.
2.6. Catalytic Process
An appropriate flask was loaded with 10 mmol thiol compound, 30 mL acetonitrile, and Porphyrazine catalyst (1 g). For 15 min, the resultant mix was blended under heating. After the experiment is completed, we added 30 mL of a 1 percent aqueous sodium thiosulfate solution and the mixture was agitated for 10 min. The catalyst was extracted by simple filtration after diluting the solution with chloroform. Using n-hexane as an extraction solvent, the sample was refined through chromatographic separation on a short silica gel column. A considerable yield of disulfide compound was produced. Colorless crystals of pure diphenyl disulfide were generated, with an average yield of 92 percent for all used porphyrazines, MP: 59 °C [24], 60 °C); IR (KBr): ν cm–1: 3058, 1565, 1476, 1434, 736, 694, 457. Pure dibenzyl disulfide was produced as a white solid, with an average yield of 93 percent for all used porphyrazines, MP: 68 °C [25], 67 °C).
3. Results and Discussion
3.1. Synthesis
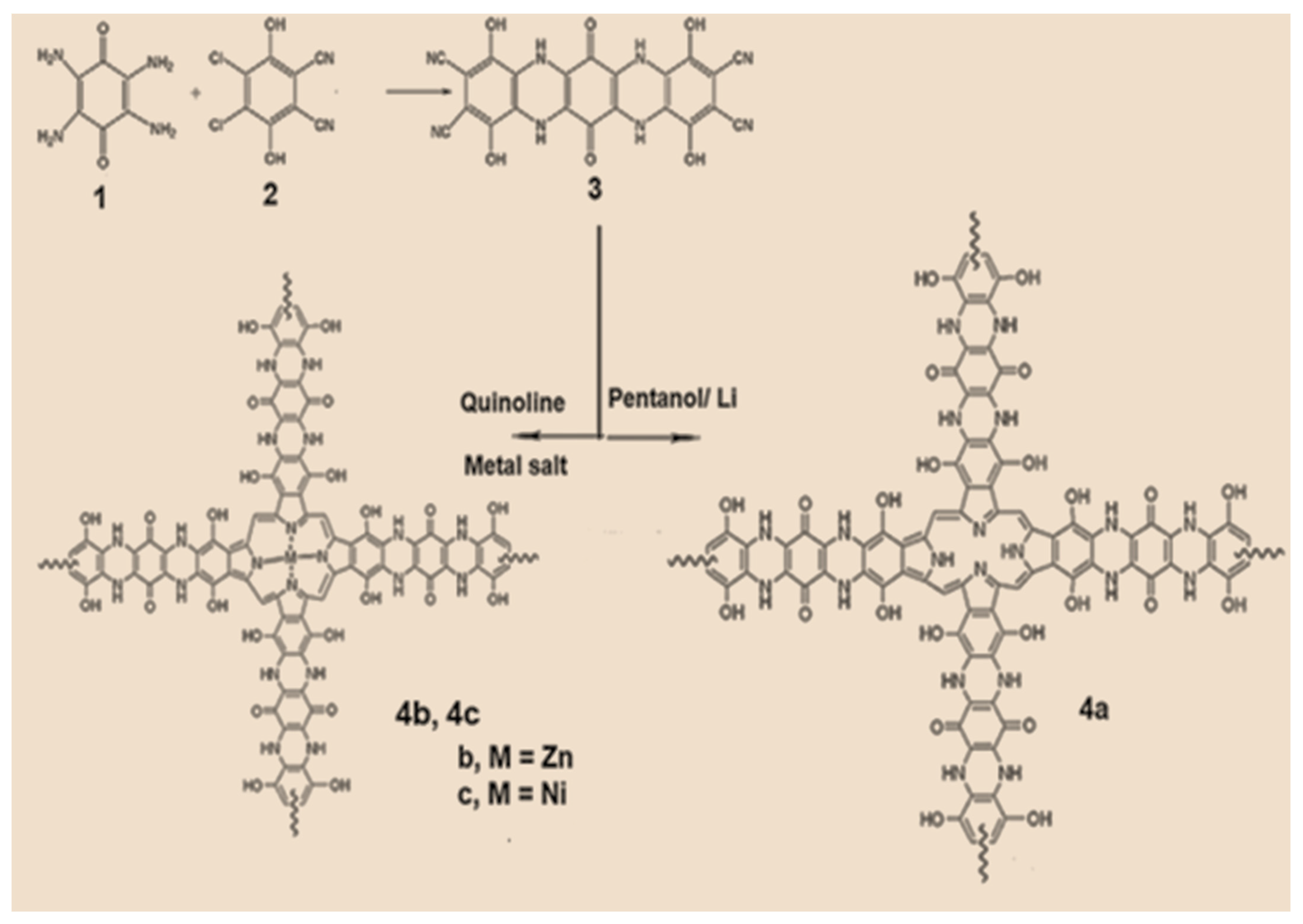
The condensation reaction of 2,3,5,6-tetraamino-1,4-benzoquinone 1 with 4,5-Dichloro-3,6-dihydroxy-phthalonitrile 2 yielded the tetranitrile derivative, p-benzoquinone [2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazine)-6,7-dinitrile] 3. utilizing a mixture of lithium, pentanol, and acetic acid, the tetra-nitrile monomer was cyclo-tetramerized, producing the corresponding network polymer, tetra p-benzoquinone [2,3-b:2,3-b]. bis[(5,8-dihydroxybenzopyrazino) porphyrazine (2H-Pz) 4a. The matching tetra p-benzoquinone [2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazino) porphyrazinate metal (II)-based networks (M-Pz), M = Zn 4b or Ni 4c, were obtained by cyclo-tetramerizing the tetra-nitril monomer 3 utilizing metal salt and quinoline (Scheme 1). The various obtained porphyrazine chemicals, such as THF, diethyl ether, ethyl acetate, acetone, dichloromethane, chloroform, and dimethyl sulfoxide, can dissolve polymers.
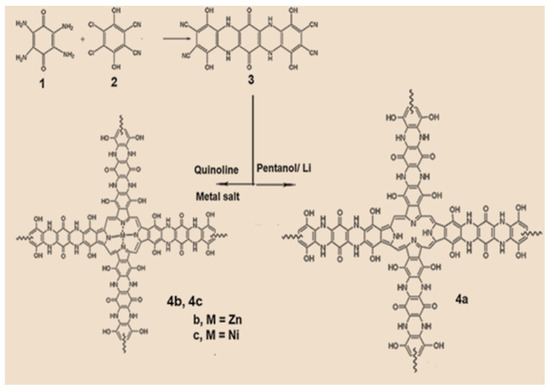
Scheme 1.
Metal-free and metal porphyrazines derived from tetra-nitrile derivative.
3.2. Infrared Spectra
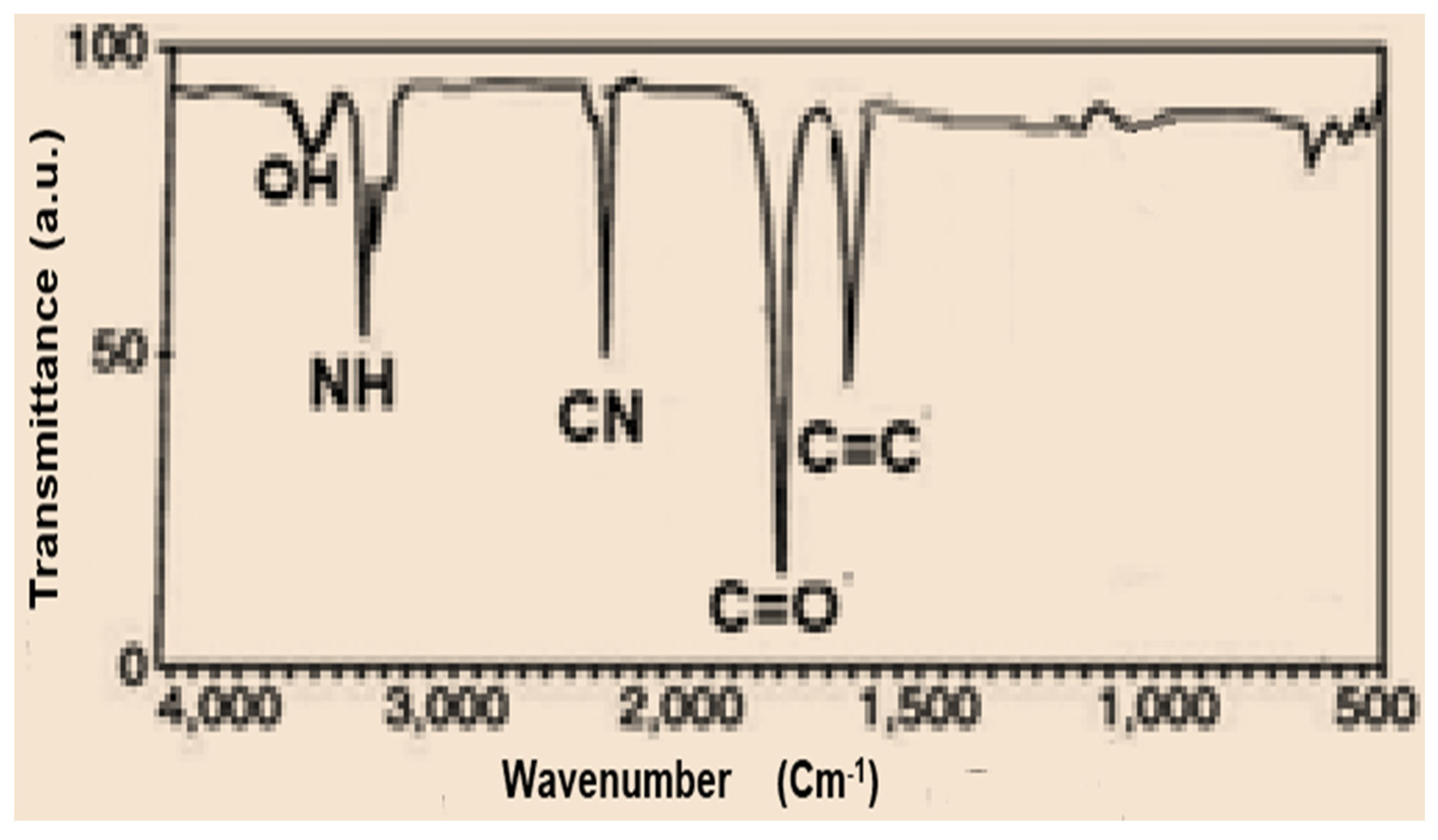
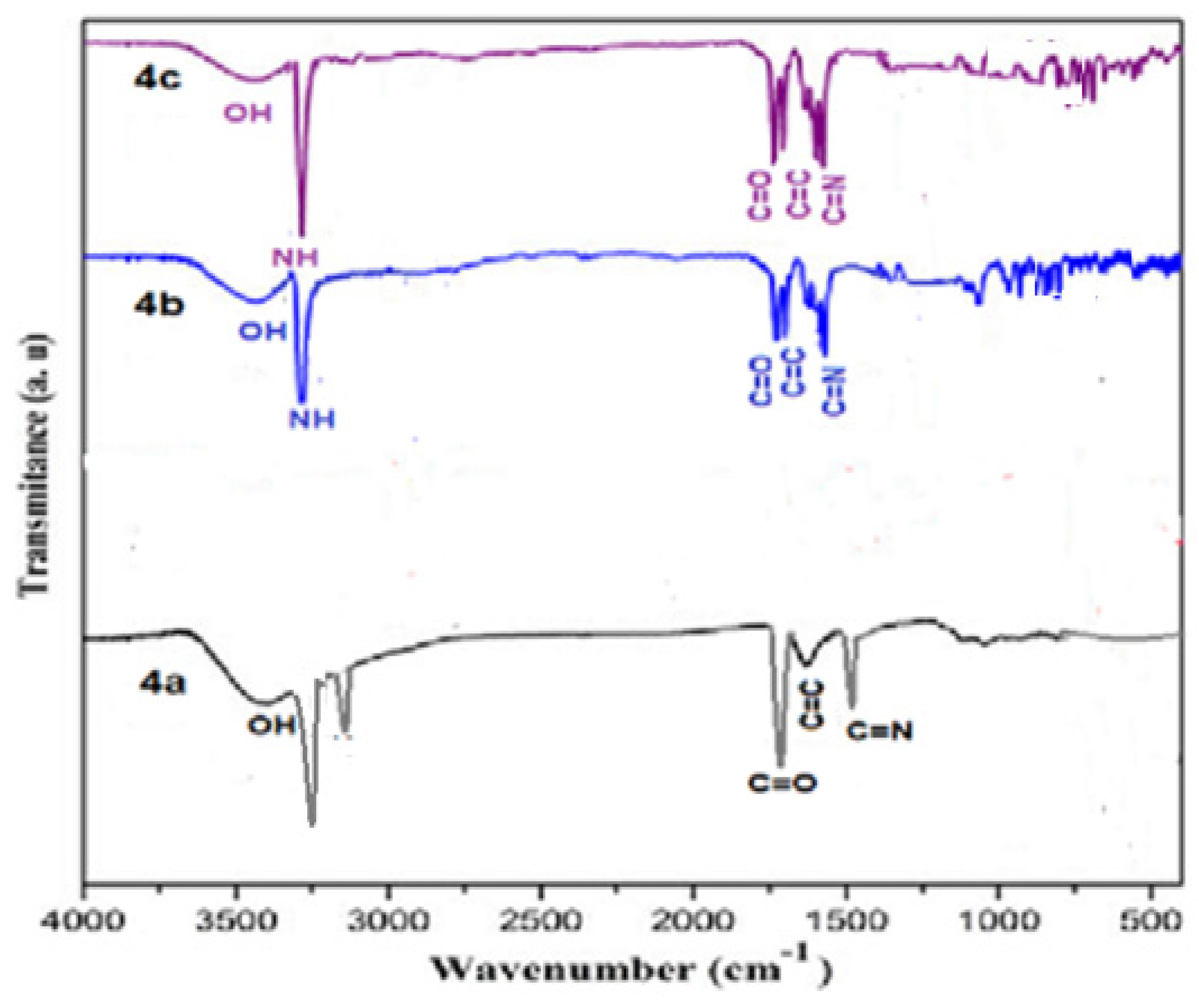
IR spectrum data of compound 3 indicate powerful peaks at 1623, 1688, 2226, and 3362 cm−1 attributed to C=C, C=O, C≡N and NH groups, correspondingly (Figure 1). The spectra of the prepared porphyrazine 2H-Pz 4a revealed a wide peak at 1518 cm−1, which is related to the absorption band of the C=N moiety. The absorbance ratios of the C=N vibrating at 1512, 1510, and 1515 cm−1 [26] (see Experimental section), for Zn-Pz 4b and Ni-Pz 4c, correspondingly, are about 310 cm−1 fewer than that for product 2H-Pz 4a, showing that the nitrogen atom (azomethine) is ligand bonded to metal ions in the products. Absorbance band of the NH bond in product porphyrazine 2H-Pz 4a is responsible for two distinct signal bands at 3356 and 3370 cm−1. Because the absorption band of the NH bond is not present in the spectrum of metallic porphyrazines 4b and 4c, the NH nitrogen is implicated in ligation to metal (Figure 2).
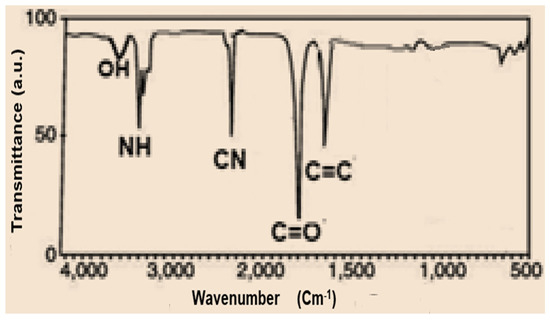
Figure 1.
FT-IR spectrum of tetranitrile 3.
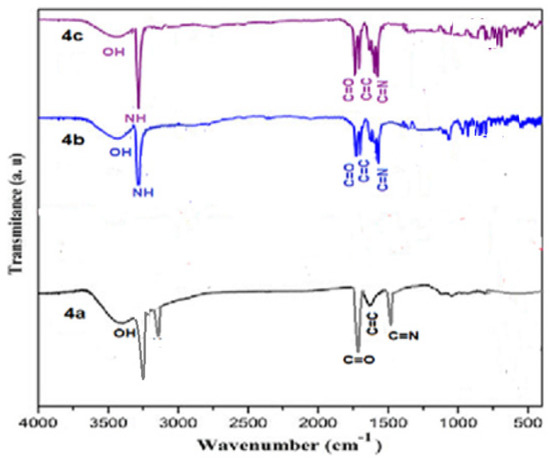
Figure 2.
FT-IR spectrum of Porphyrazines 4a–4c.
3.3. Measurements of NMR and Molecular Masses
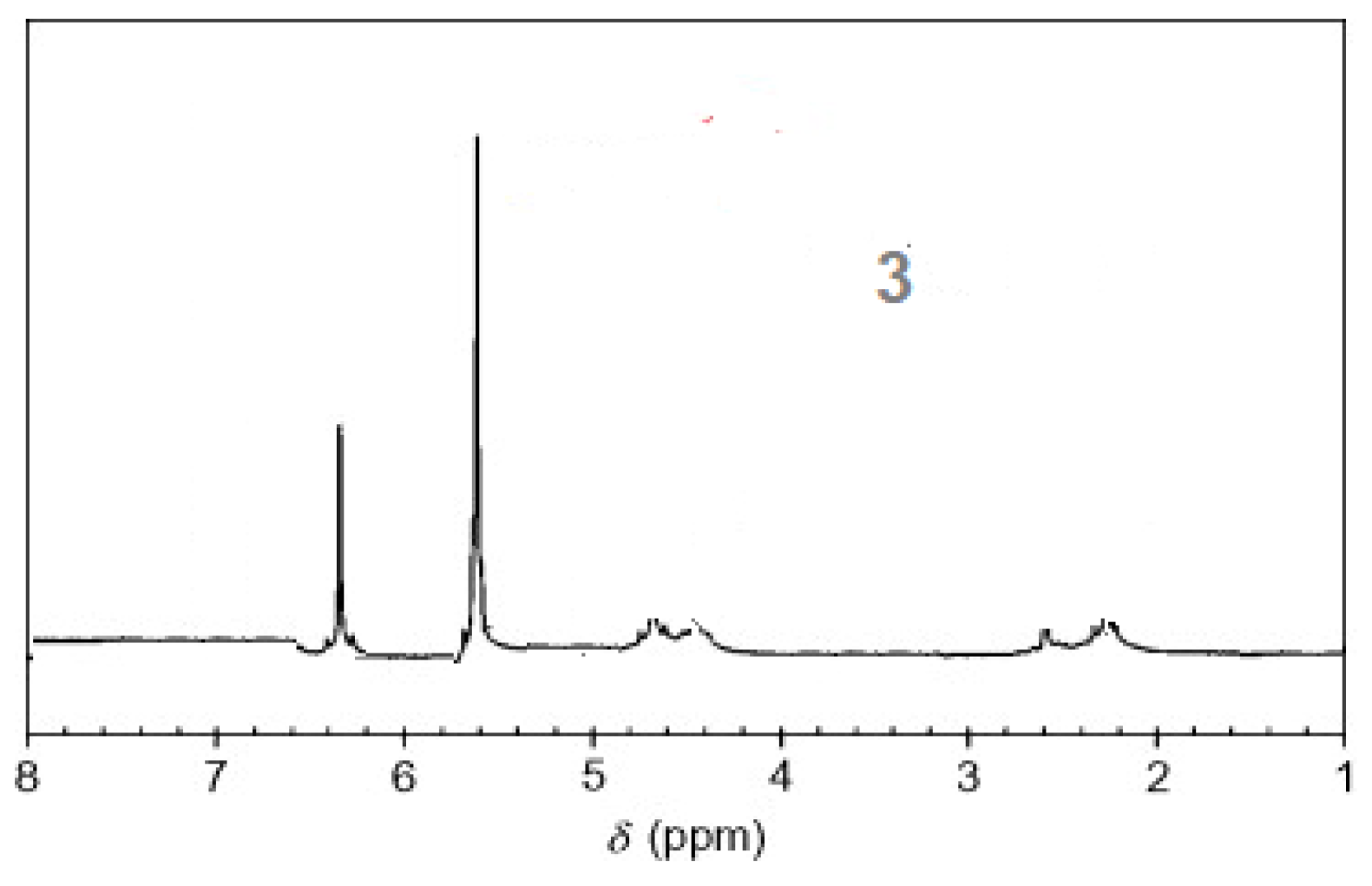
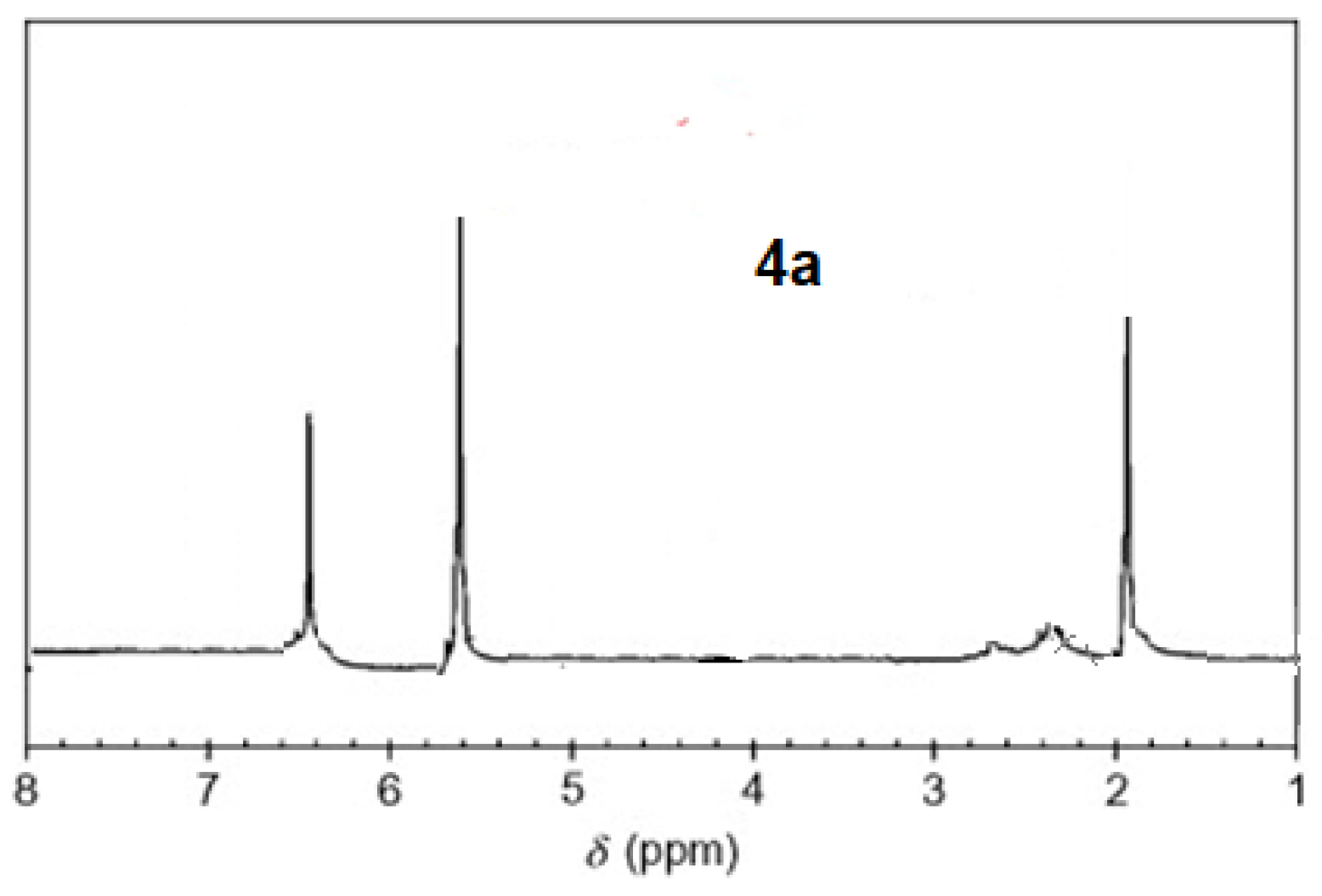
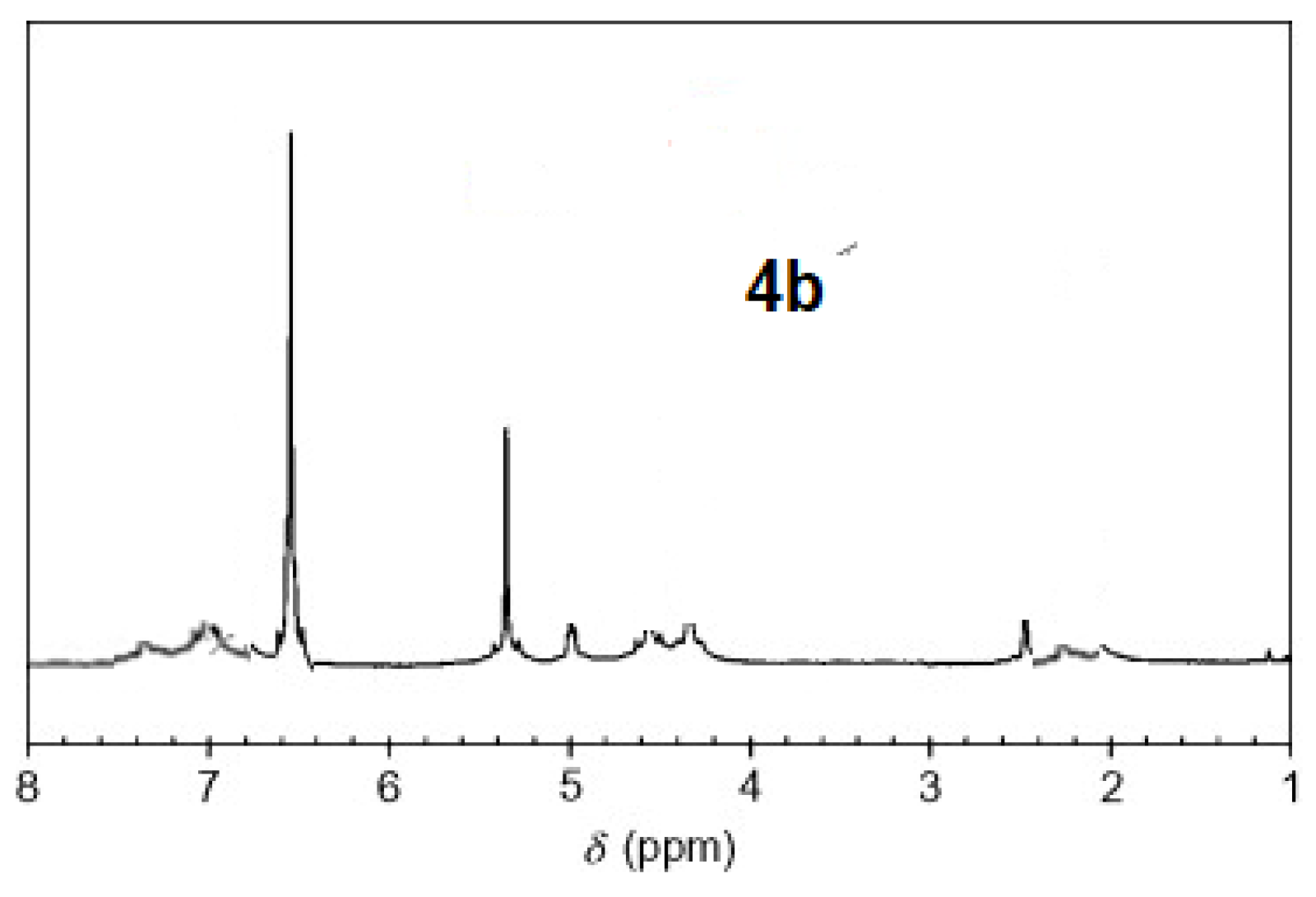
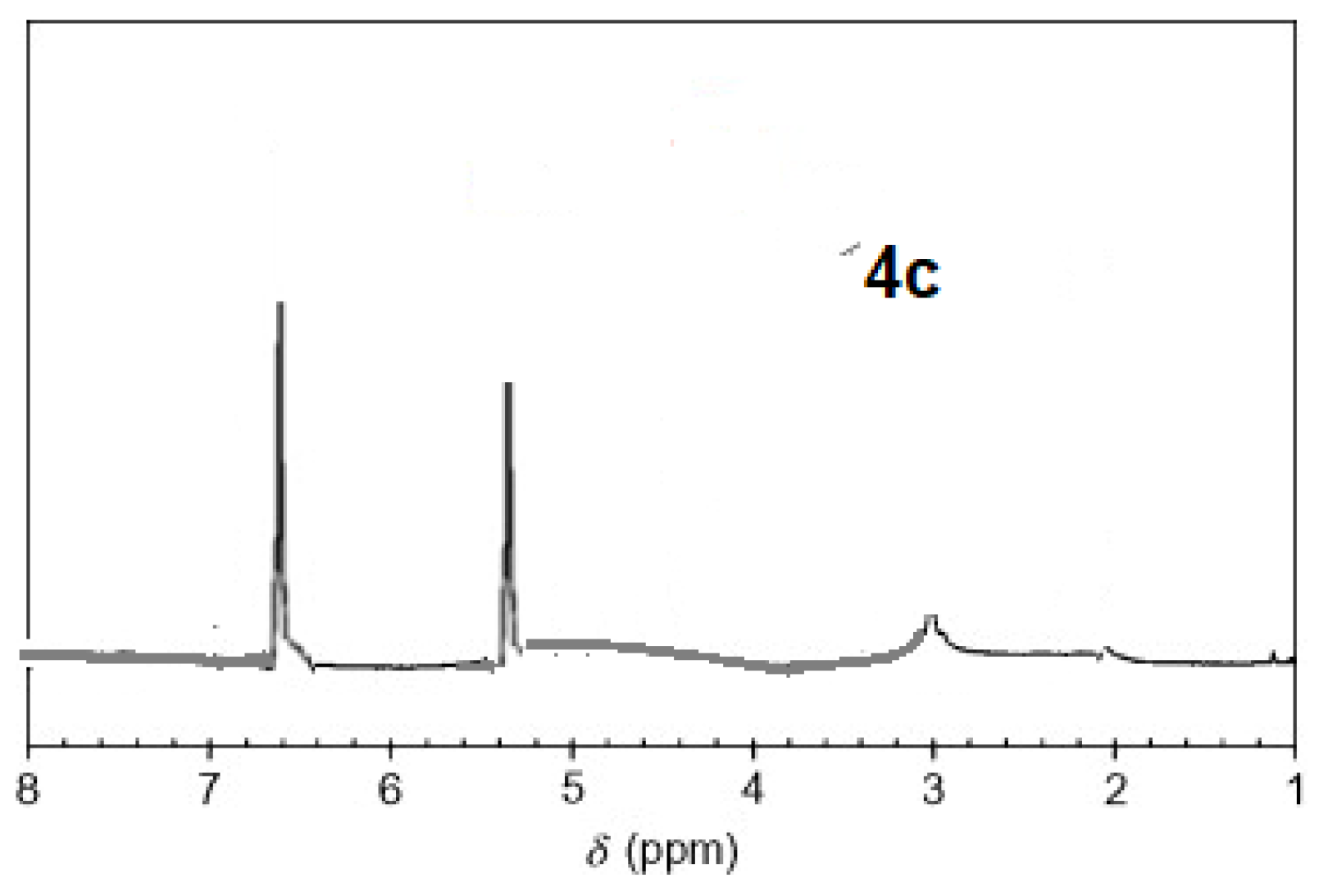
The 1H-NMR spectra of compound 3 revealed a singlet at δ = 2.93 that was assigned to the NH proton, which was consistent with the structure (Figure 3). The assigned formulation matches the novel tetra-nitrile 3′s elemental analyses results and 13C-NMR spectroscopic information (see Experimental section, Scheme 1, and Figure 4). The 1H-NMR spectroscopy of 2H-Pz 4a, a free-metal porphyrazine-based network polymer, exhibits two NH protons at = δ 1.82 and 6.45 [27] (Figure 5). The existence of a peak in the 1H-NMR spectra of 2H-Pz 4a that corresponds to the hydrogen atom in the NH portion (deshelled by tetracyclic structure) assures verification of asymmetric tetradentate ligand. The disappearance of the pyrrole ring-NH signal in metal-porphyrazine network polymers’ 1H-NMR spectra supports the complexation process (Figure 5 and Figure 6).
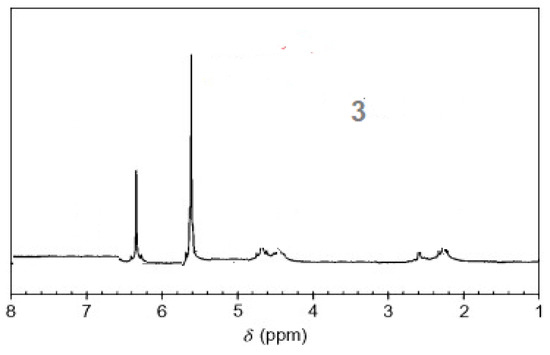
Figure 3.
1H-NMR spectra of product 3.
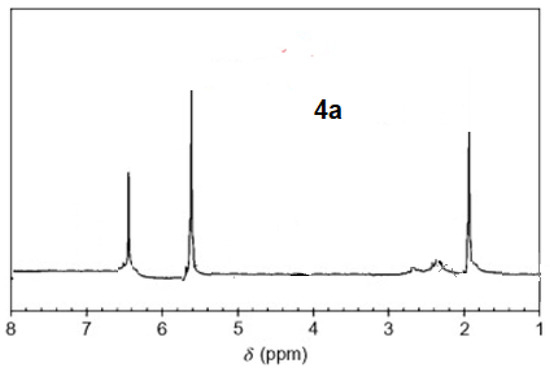
Figure 4.
1H-NMR spectra of product 4a.
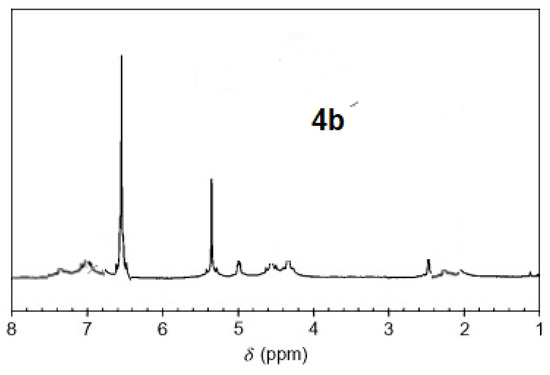
Figure 5.
1H-NMR spectra of product 4b.
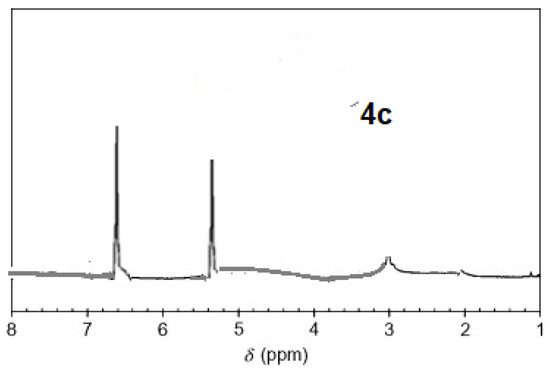
Figure 6.
1H-NMR spectra of product 4c.
The carbon content in the porphyrazine networks’ repeat units is consistent with the assumed structures shown in Scheme 1, according to elemental examinations of the produced polymers. Furthermore, the observed metal concentration values (see Experimental) of the synthesized porphyrazines match those anticipated for the predicted network backbone unit.
Additionally, the large molecular weights and metal content of the produced polymers formed further support the effectiveness of the tetramerization polymerization and complex formation processes. As demonstrated by the broad molecular weight distribution (Table 1), the polymerization has a one-step kinetic reaction and it is not living and regio/stero-regular polymerization in all materials, with Mw/Mn = 2.72–3.06. These porphyrazines’ large molar mass and strong solubility allow them to be processed using traditional solution-based polymer processing procedures.

Table 1.
Features of the porphyrazine networks that were constructed.
3.4. Ultraviolet-Visible Spectra and Optical Properties
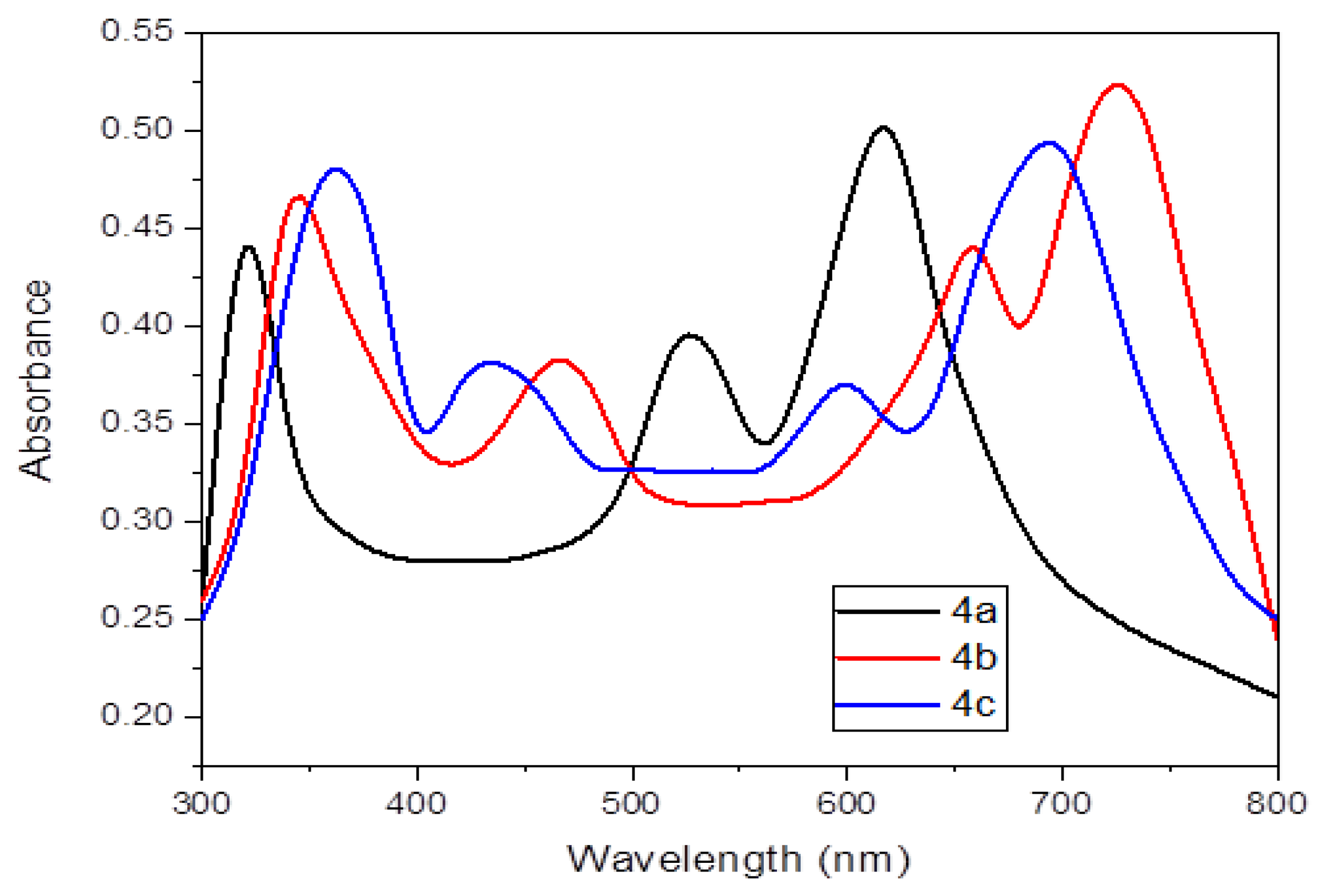
A prominent band at 310 nm may be seen in the absorption spectra of 2H-Pz 4a in methylene chloride solvent (Figure 7). At 544 and 632 nm, the π (bonding orbital)-π* (anti-bonding orbital) transformations in 2H-Pz 4a are induced by the bandgap in between the electronic states (HOMO) and the empty conduction orbit (LUMO). The absorption spectra published in the literature [28] accord well with these bands. In dichloromethane solution, the optoelectronic absorbances of the metal complexes 4b and 4c are shown in Figure 7.
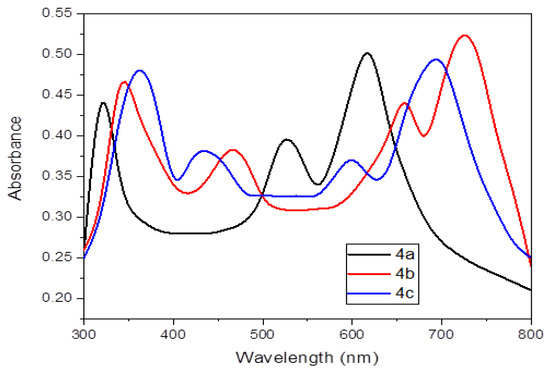
Figure 7.
Ultraviolet-visible spectra of the porphyrazines 4a–4c.
At greater energy B-bands (absorbance bands near 355 nm and shoulder bands near 454 nm), two transitions dominate that are attributed to the π-π* and/or d-π* transformation in the fused pyrrolobenzopyrazine ring structure [29,30]. The 1s → 4d transition is generally regarded for the shoulder band [31].
At lower wavelengths, combination of the central metal atom with ligands causes spin-allowed shifts, according to early conceptual investigations [25,32]. It will be demonstrated that all M-Pz polymers have states in the 432–478 nm region below the Q-band excitation. Those modes are created through pulse interchange bonding and d-d excitation. Around 612 nm and 714 nm, there are two strong reduced Q-peaks, which are widespread. Metal–ligand linkage charge-transfer bands are thought to influence the Q and B frequency bands [33]. These porphyrazines could be useful in a range of optoelectronic applications since metal combinations’ absorption spectrum extended past 800 nm (Figure 7).
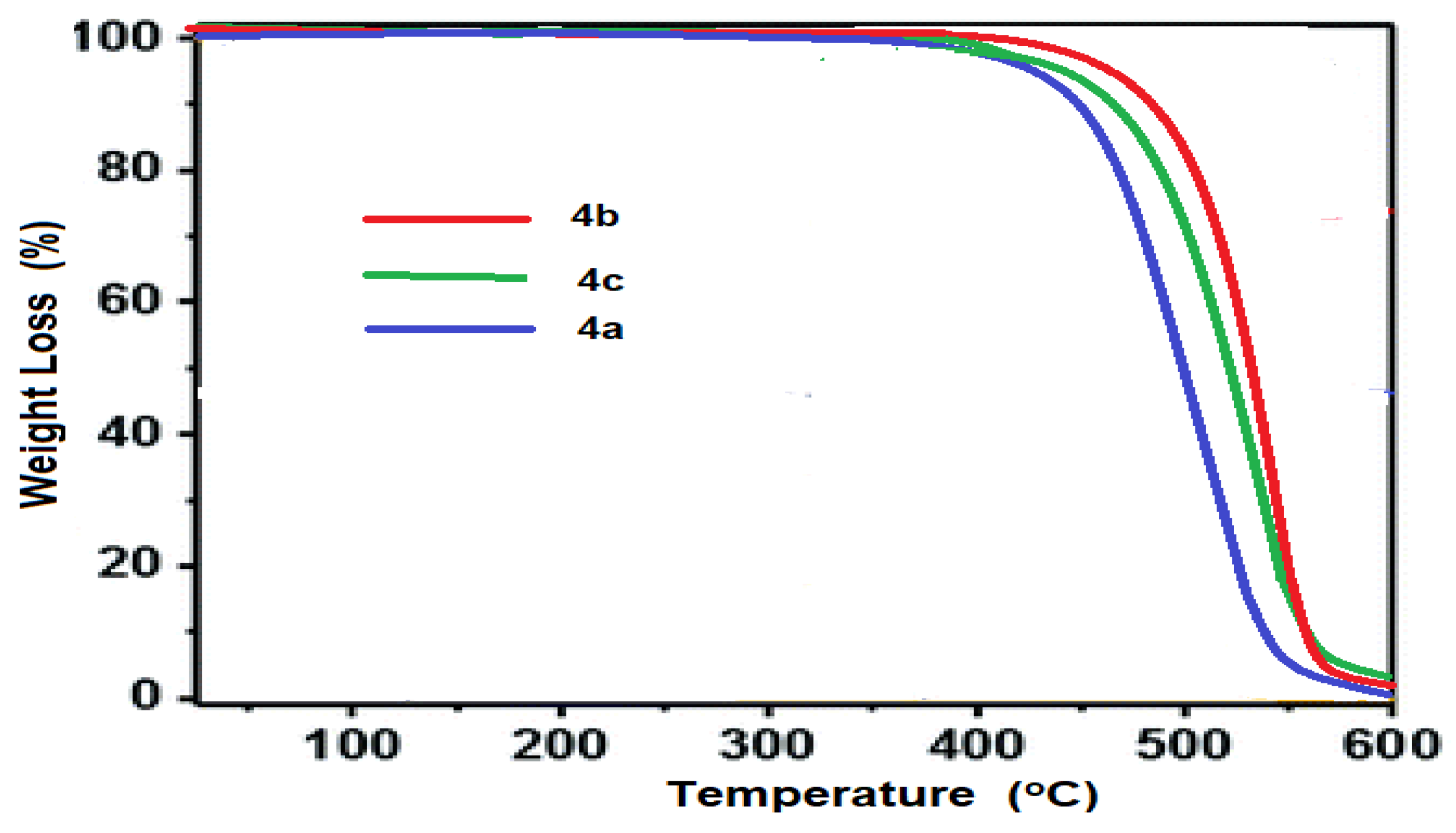
3.5. Thermal Analysis
The thermal stability of porphyrazine polymers was investigated as well. Figure 8 shows the TGA thermograms of the produced porphyrazines. The thermograms demonstrate that metal-free porphyrazine network 4a has a one-step decomposition temperature (Td) at 428 °C that really is significantly comparable. On heating at a rate of 10 K/min, metal-porphyrazine networks 4b and 4c show enhanced thermal stability, with a one-step decomposition occurring at 437 °C for Zn-Pz and 434 °C for Ni-Pz.
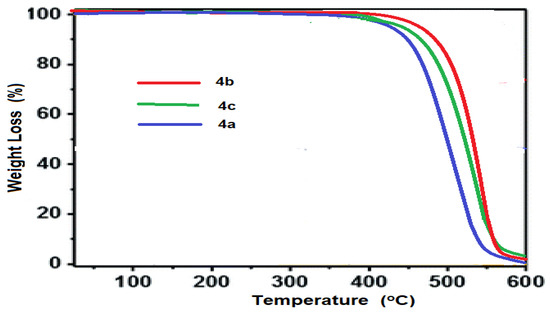
Figure 8.
Thermogravimetric analysis (TGA) of porphyrazine networks 4a–4c.
3.6. Effectiveness of the Manufactured Polymeric Materials as Catalysts
Oxygen can perform at elevated heat and in the oxidation reaction, a variety of oxidation processes occur, such as substituting oxygen inside a phenyl ring to produce a quinol or quinone. Rollmann [34] developed a bi-functional elastomeric catalyst that included all Co (II) tetraphenyl porphyrin and a proton uptake base. This approach successfully accelerated the oxidizing of thiol groups by oxygen molecules. The performance of Cu and Ni accelerators for the oxidizing of phenol dispersions was evaluated [35].
In the air atmosphere, the synthesized porphyrazine networks 4a–4c were utilized as catalytic materials for the oxidizing of thiol derivative of aroma molecules to respective disulfide derivatives. Table 1 illustrates the BET surface areas of the synthesized catalysts.
According to nitrogen adsorption estimates, the porphyrazines possess large specific surface area in the ranges of 430–466 m2g−1, with significant sorption at reduced pressure (P/P0 0.02), confirming porous structure [36]. There were minor differences in metal-porphyrazine BET surface sizes.
The maximal production of the related disulfides was 97 percent and 96 percent after 15 min of oxidation of thiophenol and benzyl thiol, respectively (see Experimental). As a result, metal-porphyrazines are considerably more efficient thiol oxidation catalysts, highlighting the importance of the heterocycle rings in the catalyzed reaction [25,37]. Quinone rings distributed along the polymer structure were reported to be beneficial in the catalytic pathway, as expected.
Following the completion of the process, spectrum examination of the employed catalysts revealed that the catalyst containing the quinone ring had been converted into quinol rings, following the completion of the thiol oxidation process. The reduction in quinone rings in the produced porphyrazine networks results in the production of disulfides, which is a key component in this process. It was reported that disulfide bonds are generated by quinone reduction [38].
The oxidation of phenols is a complicated process that has yet to be clearly realized. The oxidizing of phenolic molecules by oxygen molecules is usually assumed to be an electrophilic process, with level phase step being the interaction between both the aromatic-oxy radicals and O2. In the aerobic conditions, the phenolic moieties can self-oxidize [39].
As a result, we observed that the catalysts 4a–4c that were generated may be utilized several times. According to Table 2, which compares the yields of disulfides produced by the oxidative catalytic method with those reported for the catalytic oxidation of thiols [40,41], metallic porphyrazine catalysts exhibit greater catalytic activity than porphyrazine catalysts free of metal. Complexes’ activities are affected by the shape of complexing metal as well as the catalysts’ BET surface ratings.

Table 2.
Percent yields of disulfides produced by an oxidative catalyzed reaction.
Thus, the number of reactant molecules transformed per catalytic site in a minute for specific reaction conditions is known as the turnover number (TON), which is a useful way to express catalytic activity. The range of the turnover number (TON), which reflects catalytic activity, is 820.7 to 964.0 (Table 2). Since electron-state fluctuations are more likely to occur at bulk-like sites and have a catalytic role in the breaking and creation of chemical bonds as well as producing a high turnover, it is thought that the catalytic process predominates at high metal coordination sites.
The catalysts were studied for reuse over a period of seven cycles, with the same value in conversion seen in each cycle. To screen out the catalyst, the free polymer was redissolved in dichloromethane (DCM). After the reaction had been quenched, IR spectra of all recovered catalysts were acquired and correlated to the original catalyst. This indicated that not all disulfides had been released from active centers, highlighting the significance of the dynamic exchange process.
4. Conclusions
The condensation reaction of 2,3,5,6-tetraamino-1,4-benzoquinone with 4,5-dichloro-3,6-dihydroxy-phthalonitrile yielded the tetra nitrile derivative p-benzoquinone [2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazine)-6,7-dinitrile]. Tetra-nitrile monomer was cyclo-tetramerized, yielding the matching network polymer, tetra p-benzoquinone [2,3-b:2,3-b]. bis[(5,8-dihydroxybenzopyrazino) porphyrazine (2H-Pz). The analogous tetra p-benzoquinone [2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazino) porphyrazinate metal II-based networks (M-Pz), M = Zn, or Ni, were obtained by cyclo-tetramerizing the tetra-nitril monomer. The synthesized substituted porphyrazines have a high UV–Vis absorption, suggesting that they could be useful as basic components for novel optical and electrical substances. Since the absorption peaks of the produced porphyrazine polymers reach exceed 800 nm in the ultraviolet-visible spectra, these porphyrazines could be beneficial in a variety of optoelectronics. In the presence of air, the synthesized porphyrazines were found to be effective accelerators for the conversion of derivatives of thiol molecules to respective disulfides.
Author Contributions
Conceptualization, H.H.A.-R. and E.F.; methodology, H.H.A.-R. and E.F.; software, T.A.-W. and O.A.A.A. validation, H.H.A.-R. and E.F.; formal analysis, H.H.A.-R. and E.F.; investigation, H.H.A.-R. and E.F.; resources, T.A.-W. and O.A.A.A.; data curation, T.A.-W. and O.A.A.A.; writing—original draft preparation, M.A. and S.M.A.; writing—review and editing, M.A. and S.M.A.; visualization, M.A. and S.M.A.; supervision, M.A. and S.M.A.; project administration, T.A.-W. and O.A.A.A.; funding acquisition, T.A.-W. and O.A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R25), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia & Deanship of Scientific Research at Taif University for funding this work through Taif University Researchers Supporting Project number (TURSP-2020/220), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R25), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia & Deanship of Scientific Research at Taif University for funding this work through Taif University Researchers Supporting Project number (TURSP-2020/220), Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cook, A.H.; Linstead, R.P. Phthalocyanines. XI. The preparation of octaphenylporphyrazines from diphenylmaleinitrile. J. Chem. Soc. 1937, 929–933. [Google Scholar] [CrossRef]
- Ghosh, A.; Fitzgerald, J.; Gassman, P.G.; Almof, J. Electronic Distinction between Porphyrins and Tetraazaporphyrins. Insights from X-ray Photoelectron Spectra of Free Base Porphyrin, Porphyrazine, and Phthalocyanine Ligands. Inorg. Chem. 1994, 33, 6057–6060. [Google Scholar] [CrossRef]
- Koczorowski, T.; Ber, J.; Sokolnicki, T.; Teubert, A.; Szczolko, W.; Goslinski, T. Electrochemical and catalytic assessment of peripheral bromoaryl-substituted manganese and iron porphyrazines. Dye. Pigm. 2020, 178, 108370. [Google Scholar] [CrossRef]
- Izquierdo, M.A.; Aurimas Vyšniauskas, A.; Svetlana, A.; Lermontova, S.A.; Grigoryev, I.S.; Natalia, Y.; Shilyagina, N.Y.; Irina, V.; Balalaeva, I.V.; Larisa, G.; et al. Dual use of porphyrazines as sensitizers and viscosity markers in photodynamic therapy. J. Mater. Chem. B. 2014, 3, 1089–1096. [Google Scholar] [CrossRef]
- Korzhenevskii, A.B.; Efimova, S.V.; Zelenov, A.A.; Zelenov, O.I. Metal complexes of polymeric tetrapyrazinoporphyrazine of the network structure: III. Catalytic properties. Russ. J. Gen. Chem. 2006, 76, 822–825. [Google Scholar] [CrossRef]
- Zou, L.; Feng, D.; Tian-Fu Liu, T.F.; Ying-Pin Chen, Y.P.; Fordham, S.; Shuai Yuan, S.; Tiana, J.; Zhou, H.C. Facile one-pot synthesis of porphyrin based porous polymer networks (PPNs) as biomimetic catalysts. Chem. Commun. 2015, 51, 4005–4008. [Google Scholar] [CrossRef] [PubMed]
- Faust, R.; Weber, C. Three-step synthesis and absorption and emission properties of peripherally peralkynylated tetrapyrazinoporphyrazines. J. Org. Chem. 1999, 64, 2571. [Google Scholar] [CrossRef]
- Wang, C.S.; Bryce, M.R.; Batsanov, A.S.; Howard, J.A.K. Synthesis of Pyrazinoporphyrazine Derivatives Functionalised with Tetrathiafulvalene (TTF) Units: X-ray Crystal Structures of Two Related ttf Cyclophanes and Two Bis(1,3-Dithiole-2-Thione) Intermediates. Chem. Eur. J. 1997, 3, 1679–1690. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Jiang, X.; Zhao, C. A New Method for the Synthesis of Cobalt Complex of Tetra-2,3-(5-tert-butyl-pyrazino) porphyrazine. IOP Conf. Ser. Earth Environ. Sci. 2021, 680, 012066. [Google Scholar] [CrossRef]
- Mani, N.S.; Beall, L.S.; White, A.J.P.; Williams, D.J.; Barrett, A.G.M.; Hoffman, B.M. Serendipitous desymmetrization during porphyrazine synthesis: An X-ray crystallographic study of 2,3,7,8,12,13,17,18-octakis(dimethylamino)-2-secoporphyrazine-2,3-dione. J. Chem. Soc. Chem. Commun. 1994, 17, 1943–1944. [Google Scholar] [CrossRef]
- Shindy, H.A.; El-Maghraby, M.A.; Eissa, F.M. Cyanine dyes of new heterocyclic ring systems: Synthesis and structure-spectra studies. Dyes Pigm. 2006, 68, 11–18. [Google Scholar] [CrossRef]
- Schutten, J.H.; Beelen, T.P.M. The role of hydrogen peroxide during the autoxidation of thiols promoted by bifunctional polymer-bonded cobalt phthalocyanine catalysts. Mol. Catal. 1980, 10, 85–97. [Google Scholar] [CrossRef]
- Brouwer, W.M.; Piet, P.; German, A.L. Autooxidation of thiols with cobalt (II) phthalocyanine tetrasodium sulfonate attached to poly(vinylamine): II. Kinetic measurments. Mol. Catal. 1985, 22, 297–308. [Google Scholar] [CrossRef]
- Brouwer, W.M.; Traa, P.A.M.; de Weerd, T.J.W.; Piet, F.; German, A.L. Autoxidation of thiols with cobalt (II) phthalocyanine-tetra-sodium sulfonate attached to poly(vinylamine), 6: Immobilized catalysts by crosslinking of poly(vinylamine). Die Angew. Makromol. Chem. 1984, 128, 133–147. [Google Scholar] [CrossRef]
- Arai, H.; Yashiro, M. Catalytic oxidation of ethylene using functional quinone-polymer-anchored palladium catalysts. J. Molec. Catal. 1978, 3, 427–434. [Google Scholar] [CrossRef]
- Abdel-Razik, H.H.; El-Sayed, S.; Hassen, A. Dielectric properties of new fully conjugated 2H- and metal-pyrazinoporphyrazine network polymers. J. Appl. Polym. Sci. 2011, 121, 3579–3589. [Google Scholar] [CrossRef]
- Abdel-Razik, H.H.; Asghar, B.H.; Kenawy, E. Synthesis, characterization and spectroscopic investigation of pyrazinoporphyrazine network polymer-supported metal (II)-based catalysts. Chin. J. Polym. Sci. 2013, 31, 242–250. [Google Scholar] [CrossRef]
- Bellucci, N.; Donzello, M.P.; Elisa Viola, E.; Ercolani, C. Homo/Heteropentanuclear Porphyrazine MgII, ZnII, and PdII Macrocycles with Externally Pending PdCl2 and Pd(CBT)2 Units: Synthesis, Physicochemical Characterization, and Photoactivity Studies. Inorg. Chem. 2021, 60, 12029–12038. [Google Scholar] [CrossRef]
- Zhabanov, Y.A.; Ryzhov, I.V.; Kuzmin, I.A.; Eroshin, A.V.; Stuzhin, P.A. DFT Study of Molecular and Electronic Structure of Y, La and Lu Complexes with Porphyrazine and Tetrakis(1,2,5-thiadiazole)porphyrazine. Molecules 2021, 26, 113. [Google Scholar] [CrossRef]
- Fahim, A.M.; Magar, H.S.; Nasar, E.; Abdelrazek, F.M.; Aboelnaga, A. Synthesis of Cu-Porphyrazines by Annulated Diazepine Rings with Electrochemical, Conductance Activities and Computational Studies. J. Inorg. Organomet. Polym. 2022, 32, 240–266. [Google Scholar] [CrossRef]
- Donzello, M.P.; Capobianco, G.; Pettiti, I.; Ercolani, C.; Stuzhin, P.A. Tetra-2,3-Pyrazinoporphyrazines with Externally Appended Pyridine Rings 22 Synthesis, Physicochemical and Photoactivity Studies on In(III) Mono- and Heteropentanuclear Complexes. Molecules 2022, 27, 849. [Google Scholar] [CrossRef] [PubMed]
- Paulina, S.M.; Koczorowski, T.; Szczolko, W.; Dlugaszewska, J.; Anna Teubert, A.; Hanna, P.K.; Goslinski, T.; Sobotta, L. Cationic porphyrazines with morpholinoethyl substituents—Syntheses, optical properties, and photocytotoxicities. Dye. Pigm. 2022, 197, 109937. [Google Scholar] [CrossRef]
- Abu Ali, O.A.; Abdel-Razik, H.H.; Abualnaja, M.; Fayad, E. Investigation of Structural and Optical Properties of Some [1,4]Dithiine-porphyrazine Dyes. Molecules 2022, 27, 1651. [Google Scholar] [CrossRef] [PubMed]
- Bruder, I.; Schöneboom, J.; Dinnebier, R.; Ojala, A.; Schäfer, S.; Sens, R.; Erk, P.; Weis, J. What determines the performance of metal phthalocyanines (MPc, M=Zn, Cu, Ni, Fe) in organic heterojunction solar cells? A combined experimental and theoretical investigation. Org. Electron. 2010, 11, 377–387. [Google Scholar] [CrossRef]
- Iranpoor, N.; Zeynizadeh, B. Air oxidative coupling of thiols to disulfides catalyzed by Fe (III)/NaI. Synthesis 1999, 1999, 49–50. [Google Scholar] [CrossRef]
- Tarakanova, E.N.; Hamdoush, M.; Eroshin, A.V.; Ryzhov, I.V.; Zhabanov, Y.A.; Stuzhin, P.A. Tetra(1,2,5-thiadiazolo)porphyrazines. 10. Synthesis, spectral characterization and DFT study of complexes with yttrium(III) and lutetium(III). Polyhedron 2021, 193, 114877. [Google Scholar] [CrossRef]
- Yaman, H.; Kayan, A. Synthesis of novel single site tin porphyrin complexes and the catalytic activity of tin tetrakis(4-fluorophenyl)porphyrin over ε-caprolactone. J. Porphyr. Phthalocyanines 2017, 21, 231–237. [Google Scholar] [CrossRef]
- Kim, J.; Jaung, J.Y.; Ahn, H. Tetrapyrazinoindoloporphyrazine Langmuir-Blodgett films. Macromol. Res. 2008, 16, 367–372. [Google Scholar] [CrossRef]
- Han, J.; Jaung, J.Y.; Ahn, H. Study of the thermal decomposition of tetrapyrazino [2,3-b]indoloporphyrazine films. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 545–548. [Google Scholar] [CrossRef]
- Puigdollers, J.; Voz, C.; Fonrodona, M.; Cheylan, S.; Stella, M.; Andreu, J.; Vetter, M.; Alcubilla, R. Copper phthalocyanine thin-film transistors with polymeric gate dielectric. J. Non-Cryst. Solids 2006, 352, 1778–1782. [Google Scholar] [CrossRef]
- Wizel, S.; Margel, S.; Gedanken, A.; Rojas, T.C.; Fernandez, A.; Prozorov, R. The preparation of metal–polymer composite materials using ultrasound radiation: Part II. Differences in physical properties of cobalt–polymer and iron–polymer composites. J. Mater. Res. 1999, 14, 3913–3920. [Google Scholar] [CrossRef]
- Cory, M.G.; Zerner, M.C. Metal-ligand exchange coupling in transition-metal complexes. Chem. Rev. 1991, 91, 813–822. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, D.; Gan, F. Ellipsometric spectra of cobalt phthalocyanine films. Phys. B Condens. Matter. 1995, 212, 189–194. [Google Scholar] [CrossRef]
- Rollmann, L.D. Porous, polymer-bonded metalloporphyrins. J. Am. Chem. Soc. 1975, 97, 2132–2136. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Salagre, P.; Fabregat, A.; Sueiras, J.E. Characterization and activity of copper and nickel catalysts for the oxidation of phenol aqueous solutions. Appl. Catal. B. 1998, 18, 307–315. [Google Scholar] [CrossRef]
- McKeown, N.B.; Makhseed, S.; Budd, P.M. Phthalocyanine-based nanoporous network polymers. Chem. Commun. 2002, 23, 2780–2781. [Google Scholar] [CrossRef]
- Sonavane, S.U.; Chidambaram, M.; Almog, J.; Sasson, Y. Rapid and efficient synthesis of symmetrical alkyl disulfides under phase transfer conditions. Tetrahedron Lett. 2007, 48, 6048–6050. [Google Scholar] [CrossRef]
- Bader, M.W.; Xie, T.; Yu, C.A.; Bardwell, J.C. Disulfide Bonds are generated by quinone reduction. J. Biol. Chem. 2000, 275, 26082–26088. [Google Scholar] [CrossRef]
- Hay, A.S. Polymerization by oxidative coupling. II. Oxidation of 2,6-disubstituted phenols. J. Polym. Sci. 1962, 58, 581–591. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N.S.S.; Shafiei, N. Recent progresses in polymer supported cobalt complexes/nanoparticles for sustainable and selective oxidation reactions. Mol. Catal. 2020, 484, 110775. [Google Scholar] [CrossRef]
- Mert, H.; Tunca, U.; Hiza, G. Thiophenol Derivatives as a Reducing Agent for In Situ Generation of Cu(I) Species via Electron Transfer Reaction in Copper-Catalyzed Living/Controlled Radical Polymerization of Styrene. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5923–5932. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).