Corrosion Induced Morphology Evolution in Stressed Solids
Abstract
1. Introduction
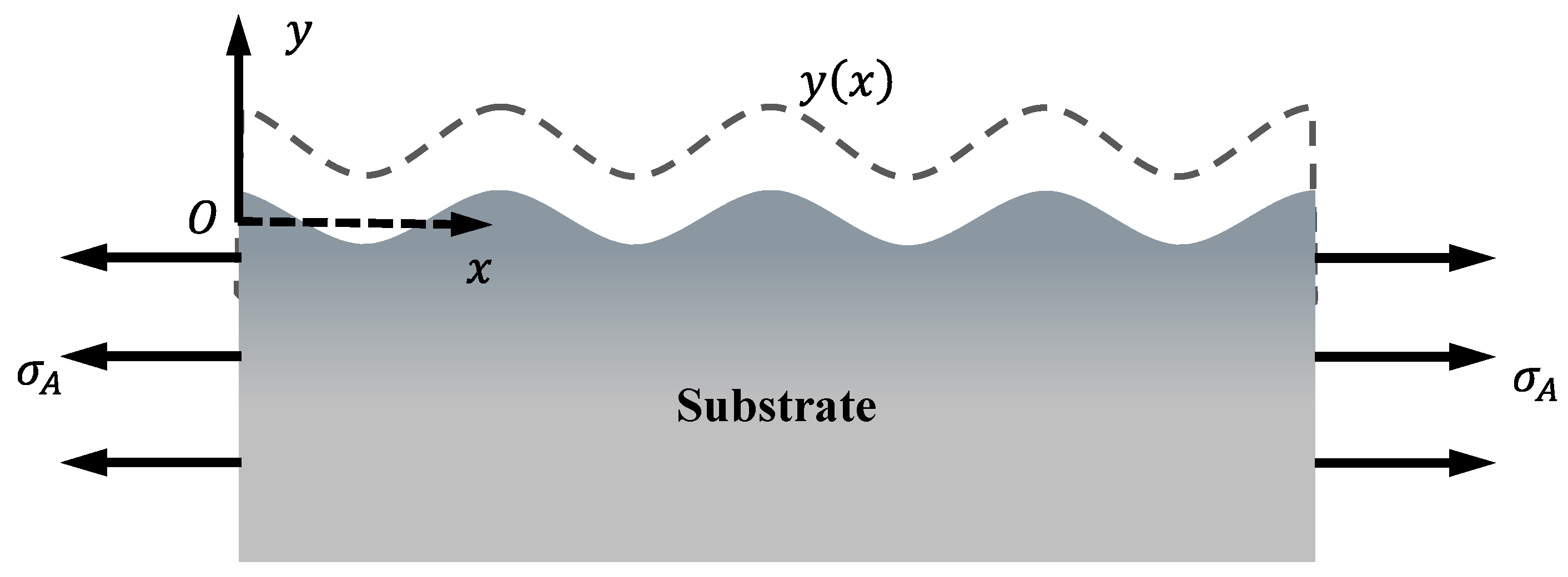
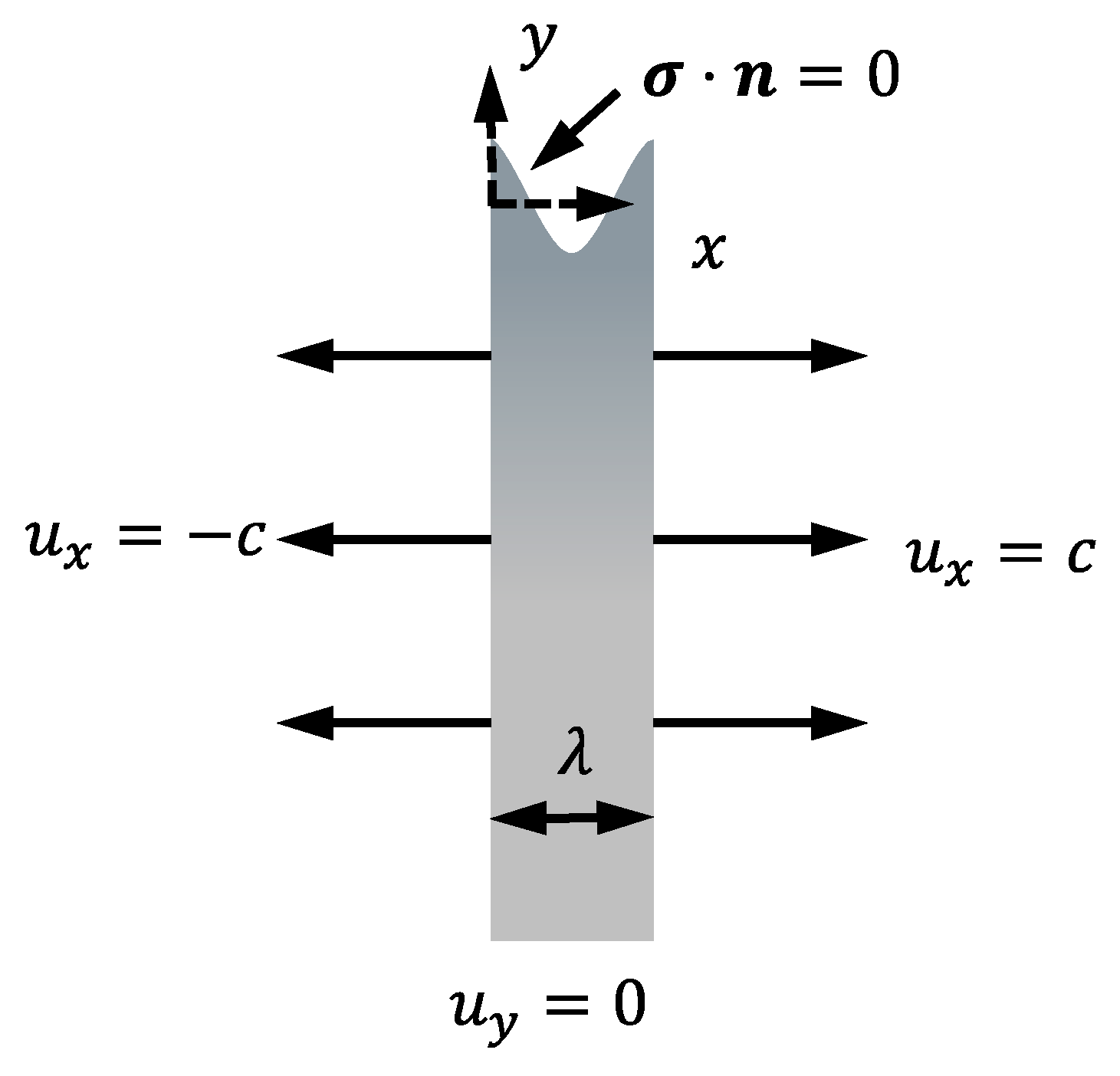
2. Stress Corrosion Model
2.1. Morphology Evolution
2.2. Corrosion Kinetics
2.3. Mechanical Governing Equations
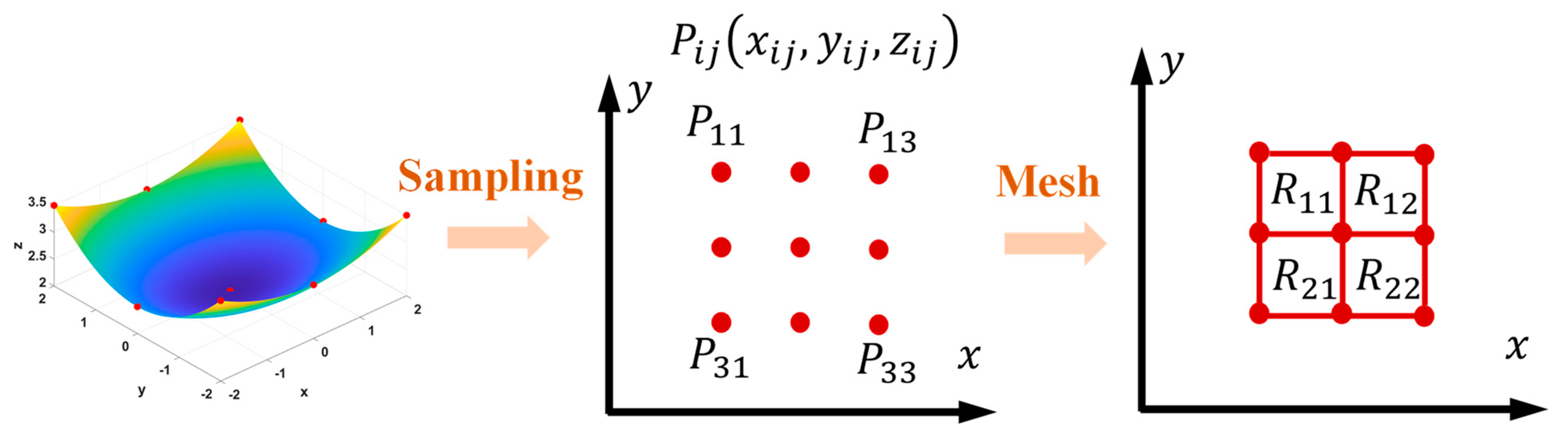
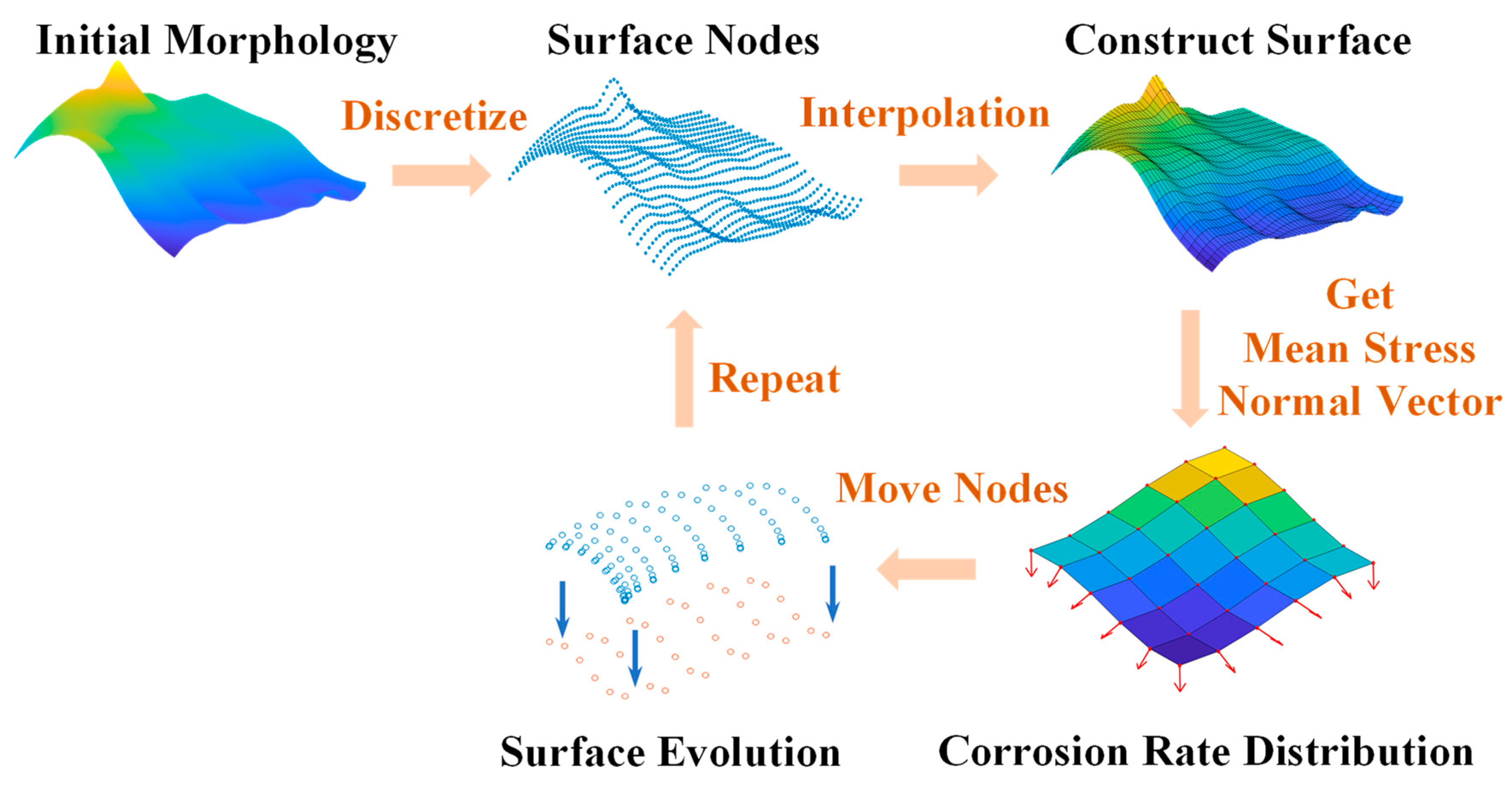
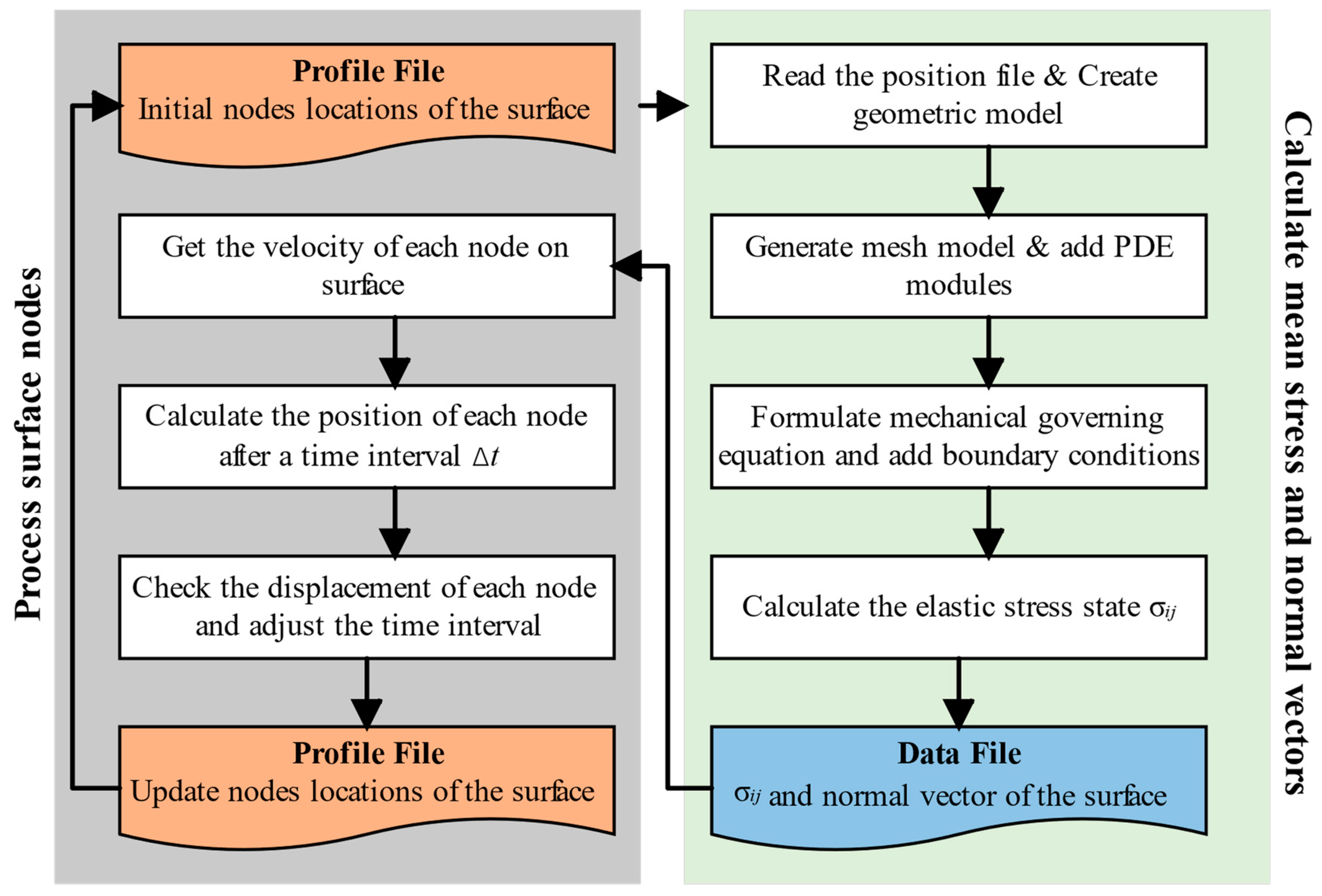
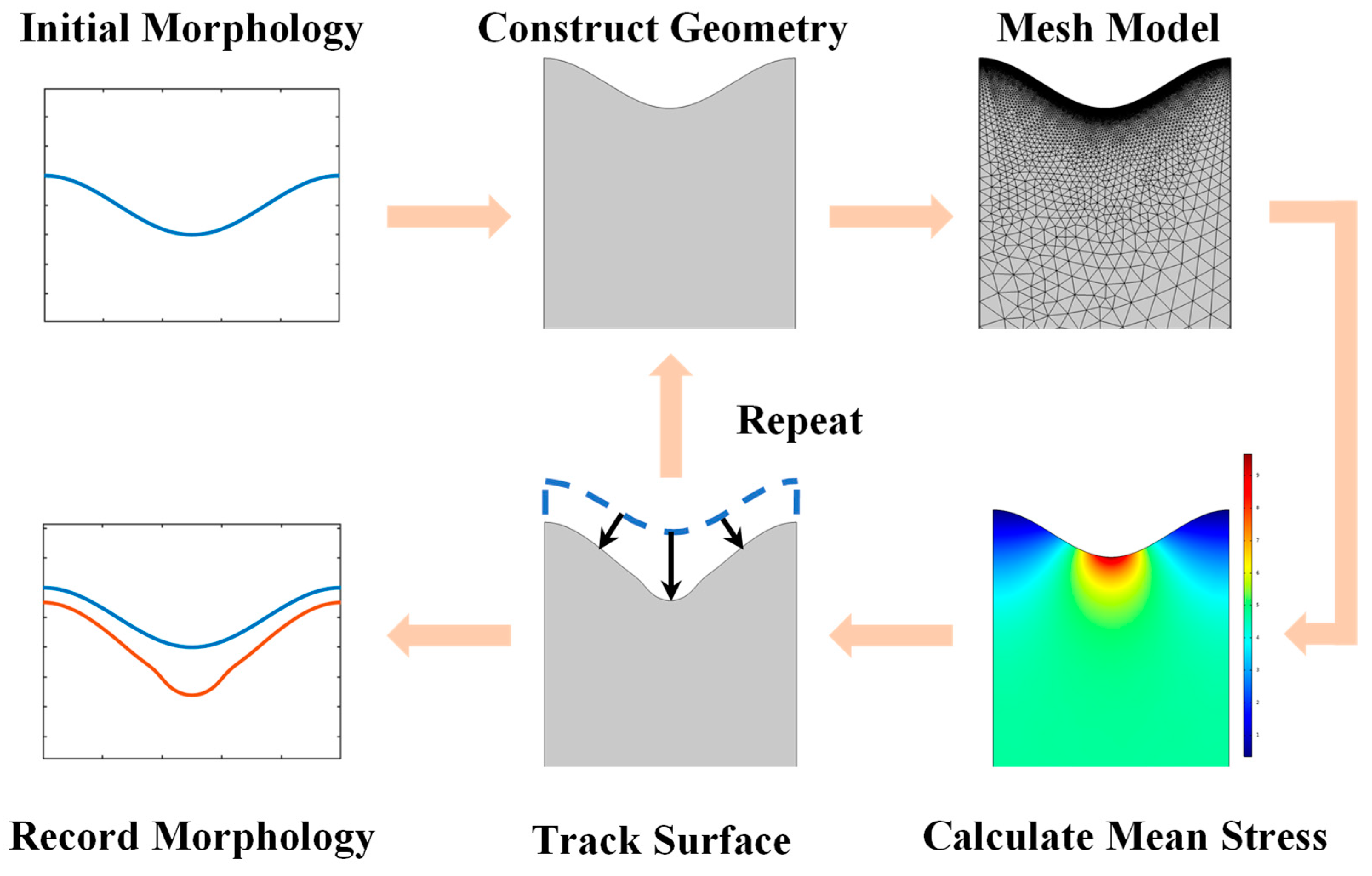
3. Numerical Method
- (1)
- Generate the initial positions of the surface nodes at the beginning of the corrosion;
- (2)
- Construct the surface by interpolation, then generate a mesh model and calculate the stress state via the FEM;
- (3)
- Outputs the elastic stress state and normal vector of each surface element in a data file;
- (4)
- Calculates the velocity of each surface nodal point by Equations (13)–(15). Then, the new position information of each node after Δt is obtained by Equations (23)–(25);
- (5)
- Checks the corrosion depth of each node and adjusts the time interval Δt when the maximum of the depths exceeds the critical length. Then, it stores the new surface nodes and updates the positions of the surface nodes;
- (6)
- Go back to step (2) and repeat.
4. Simulation Results and Discussions
- Crevice pattern:
- Pit pattern: and
- Flattening pattern: and
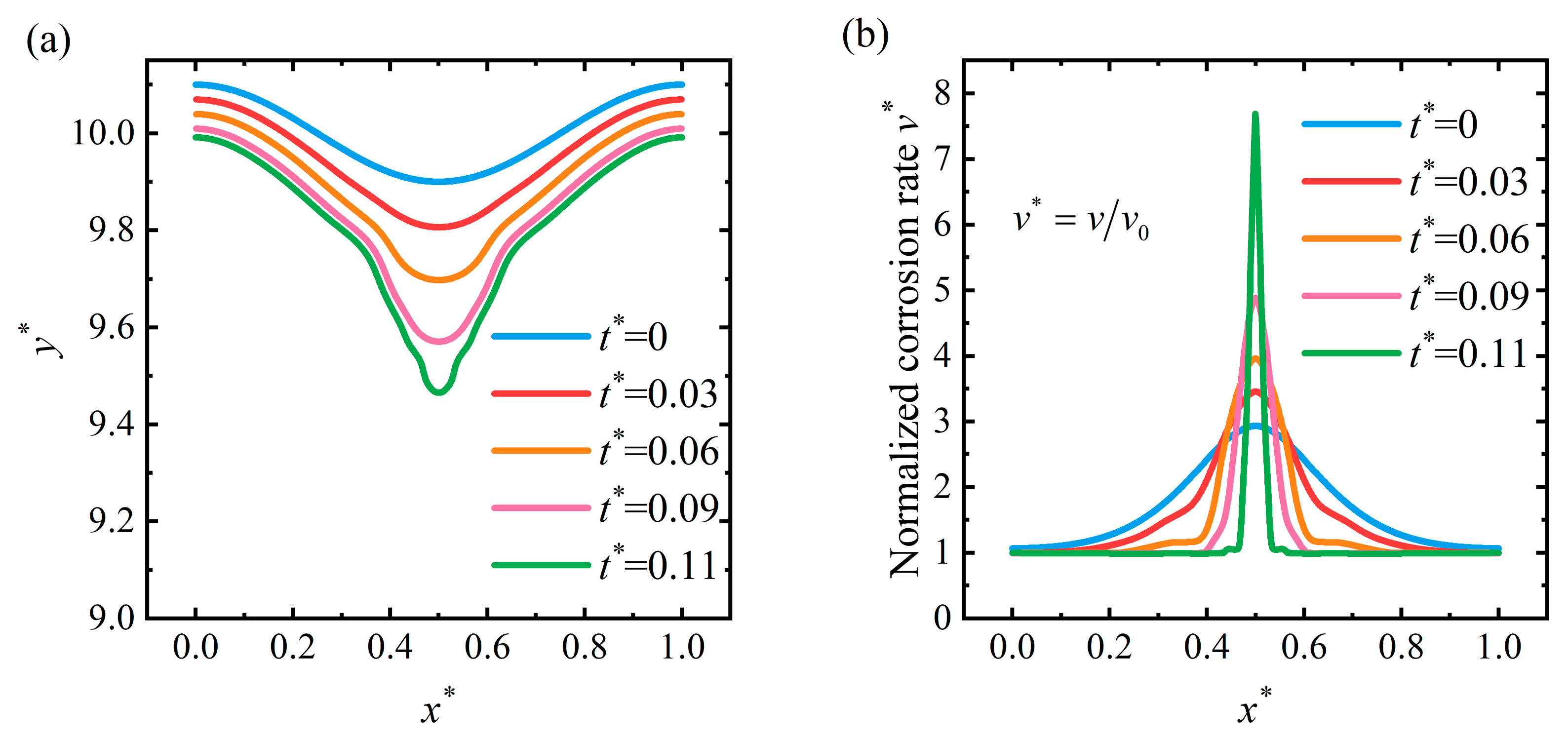
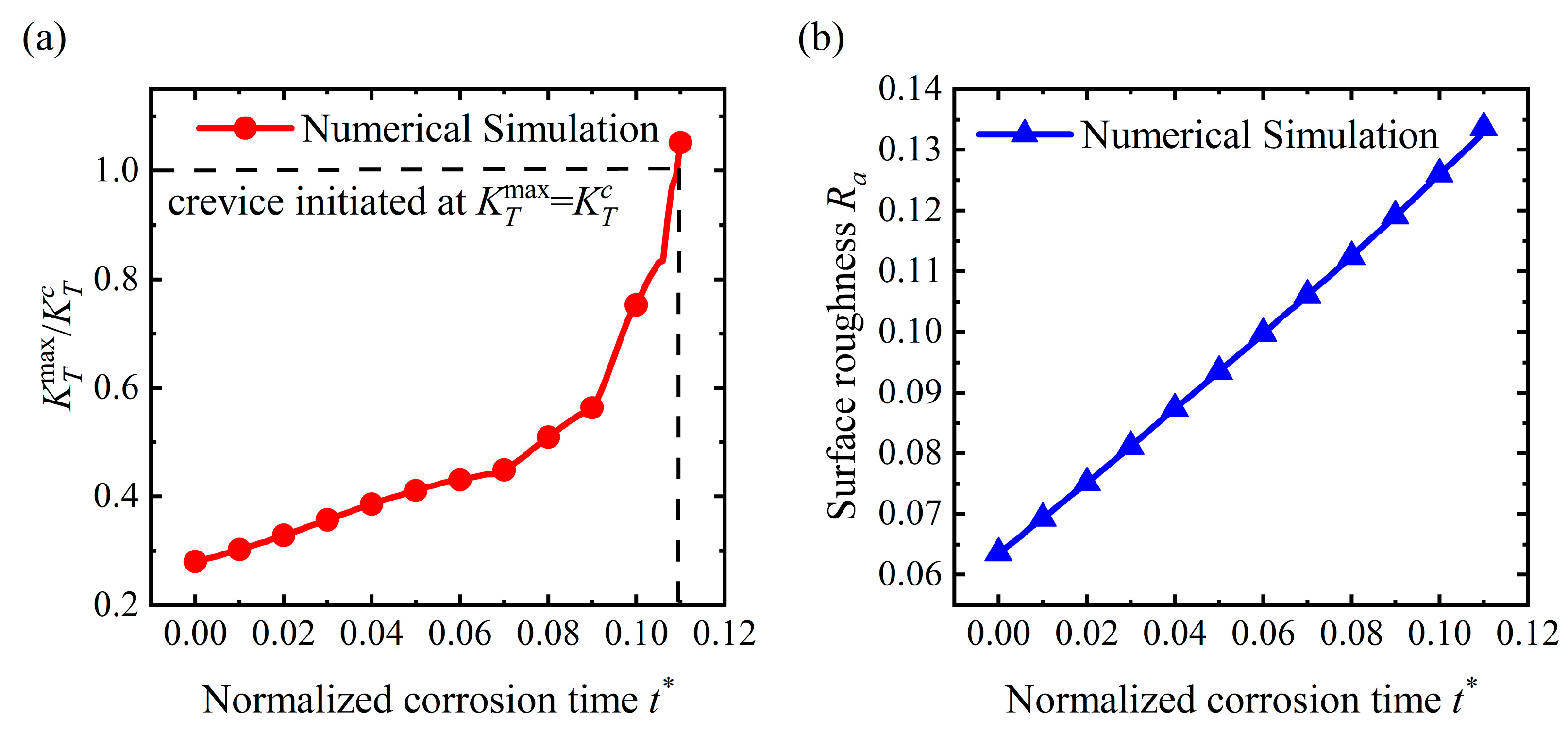
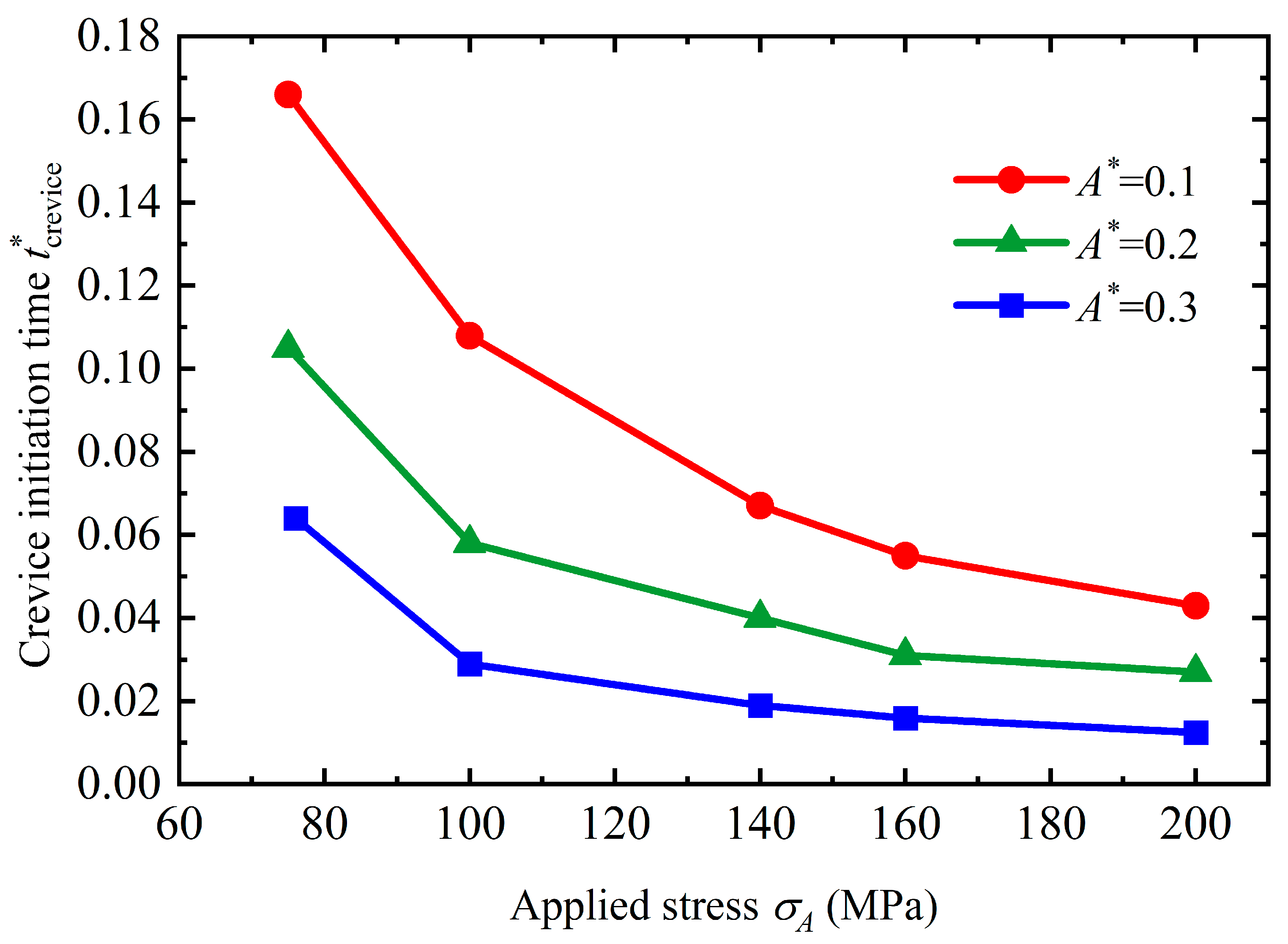
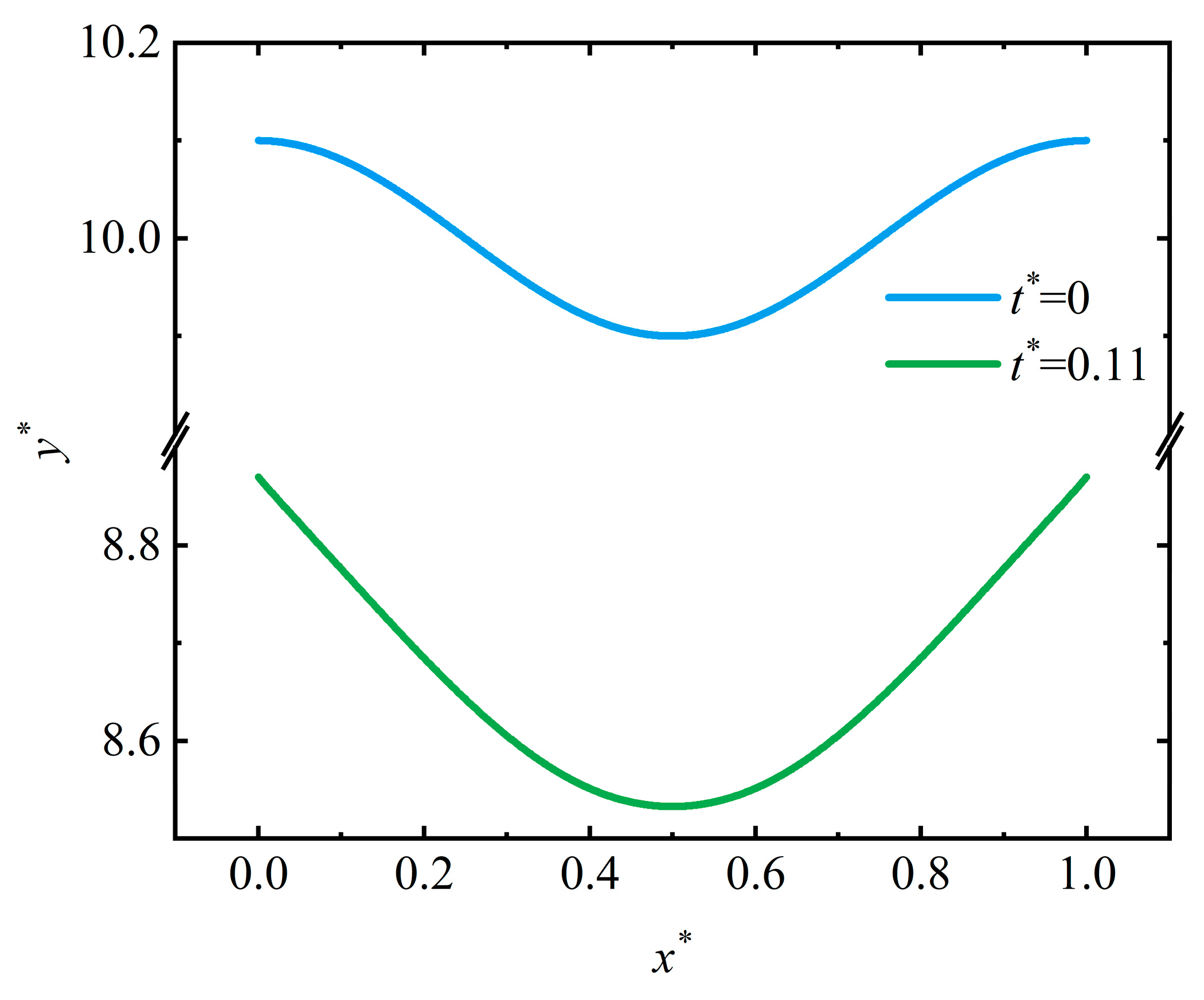
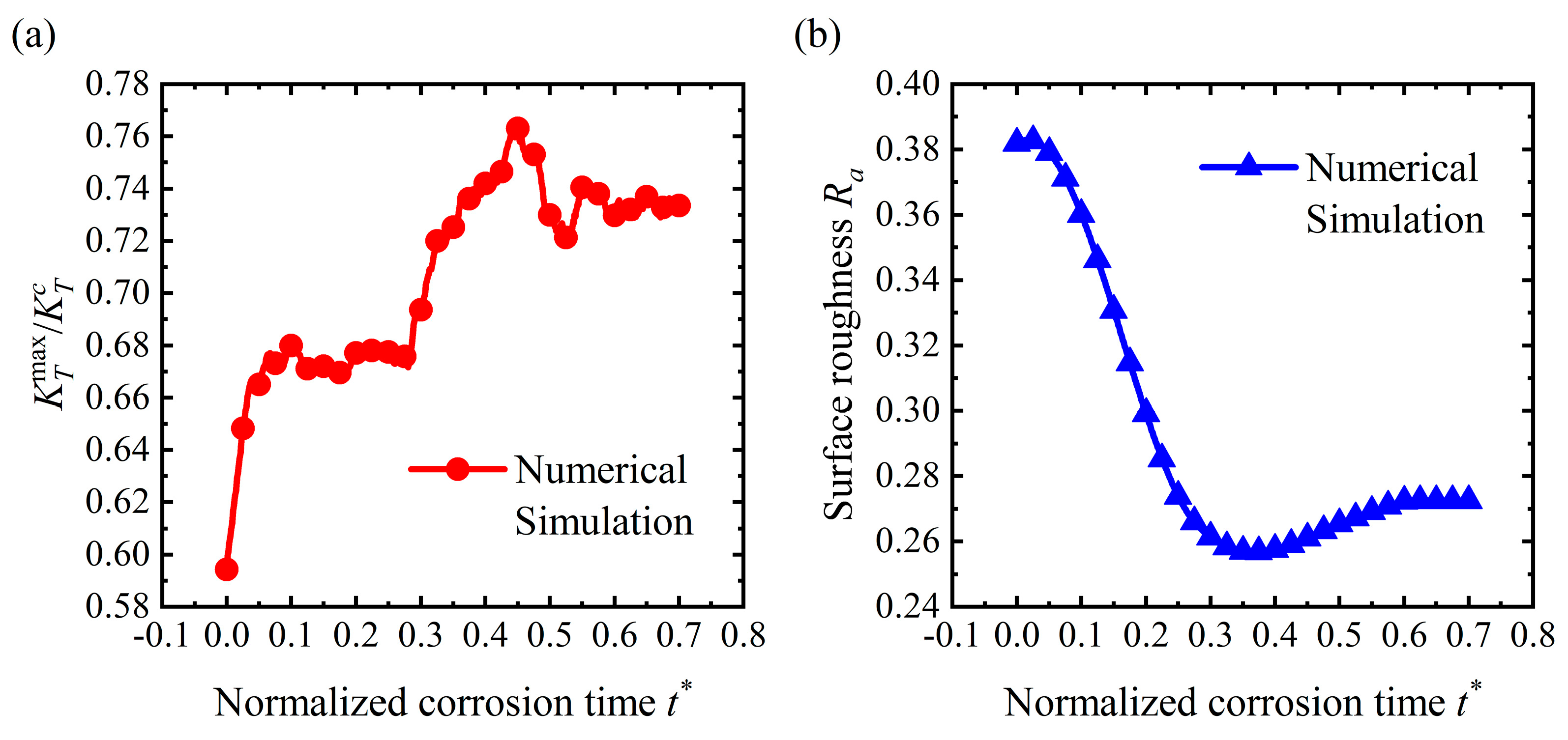
4.1. Crevice Pattern
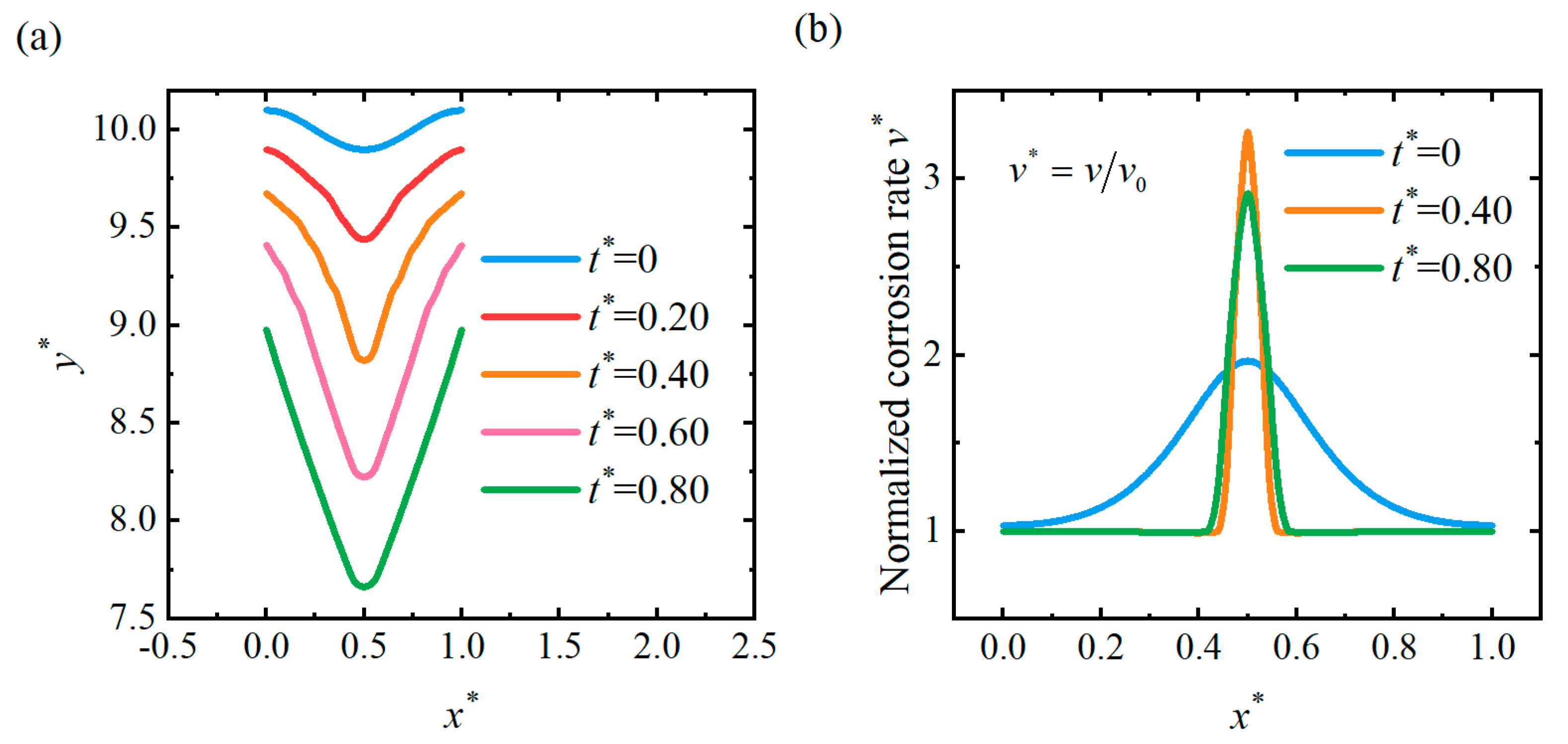
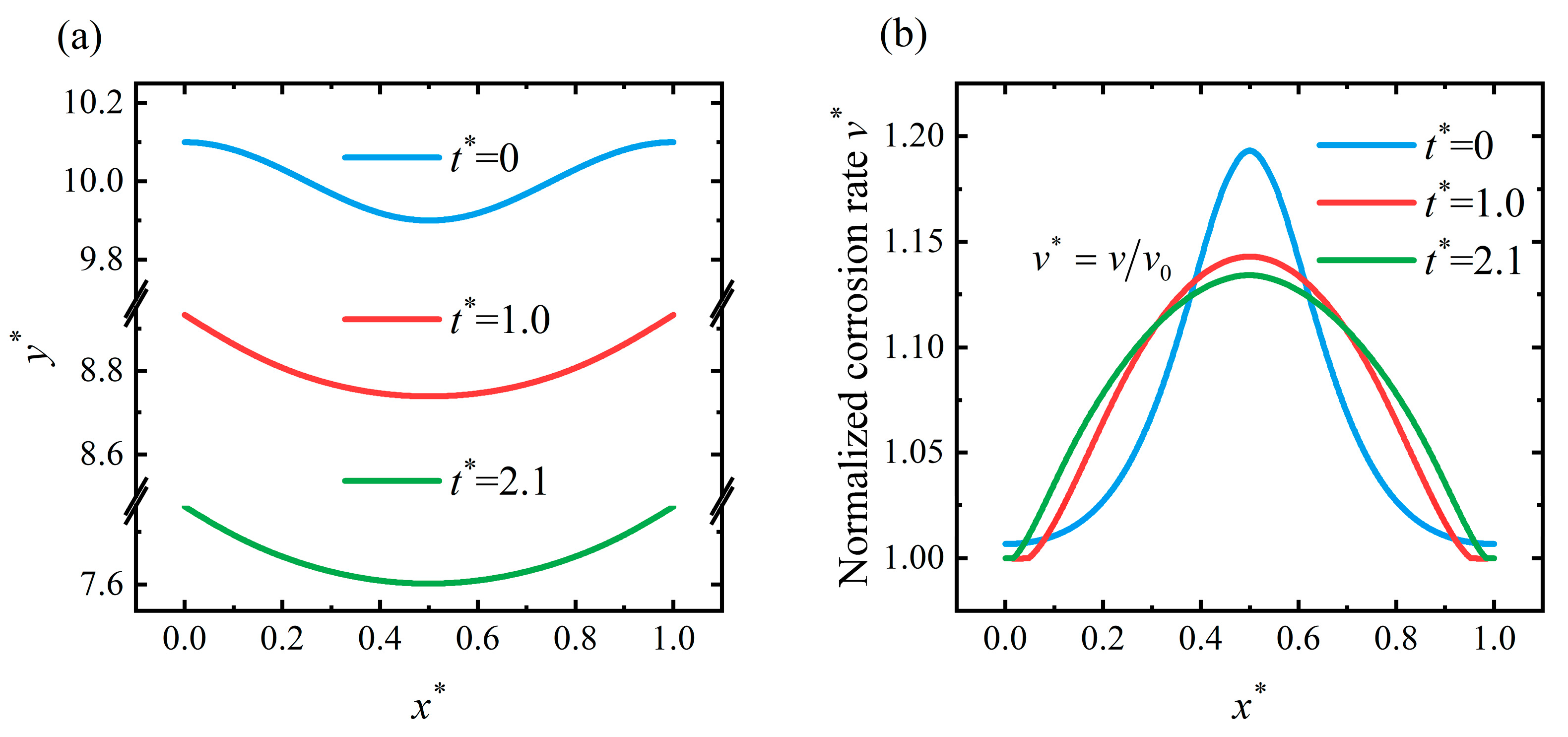
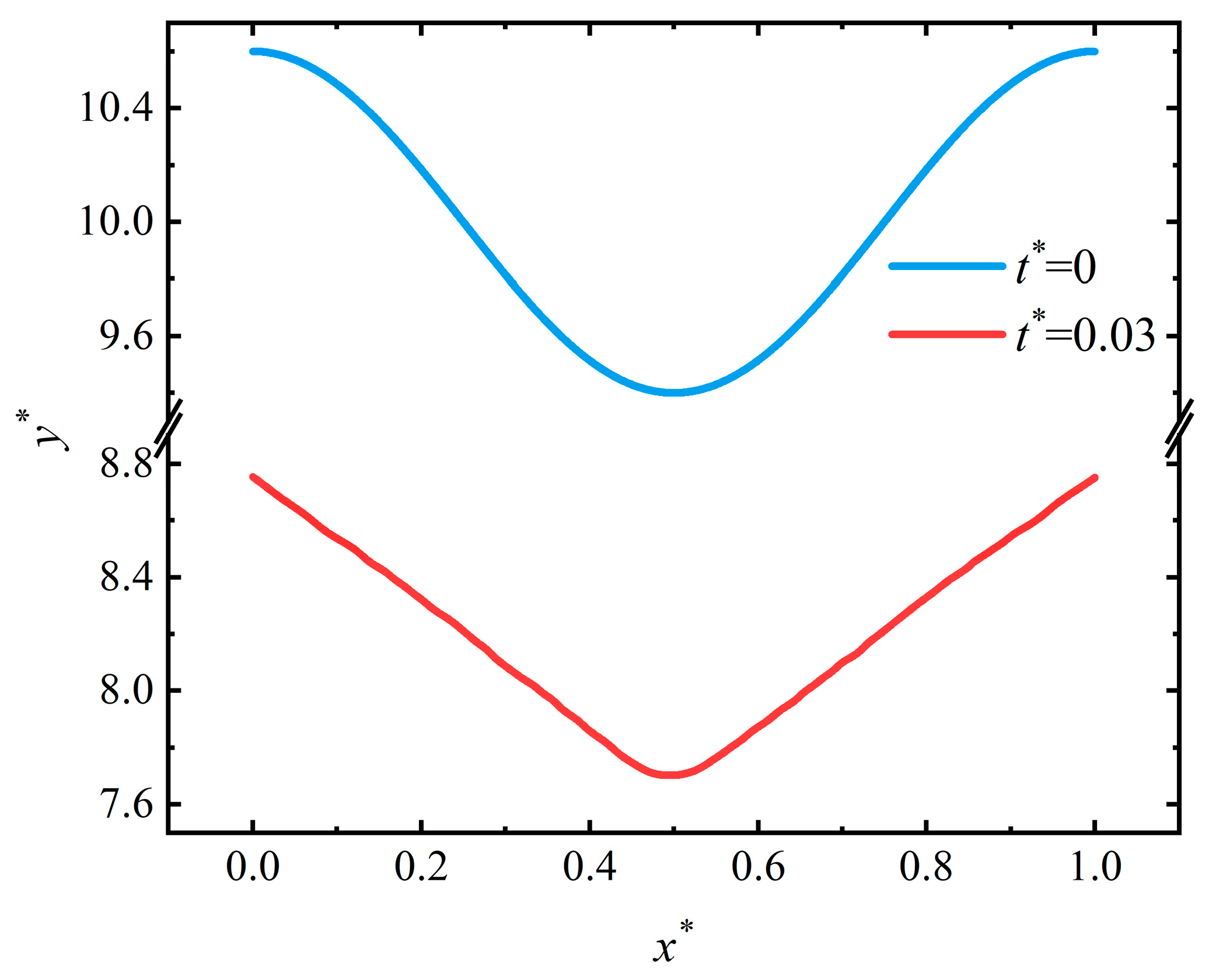
4.2. Pit Pattern
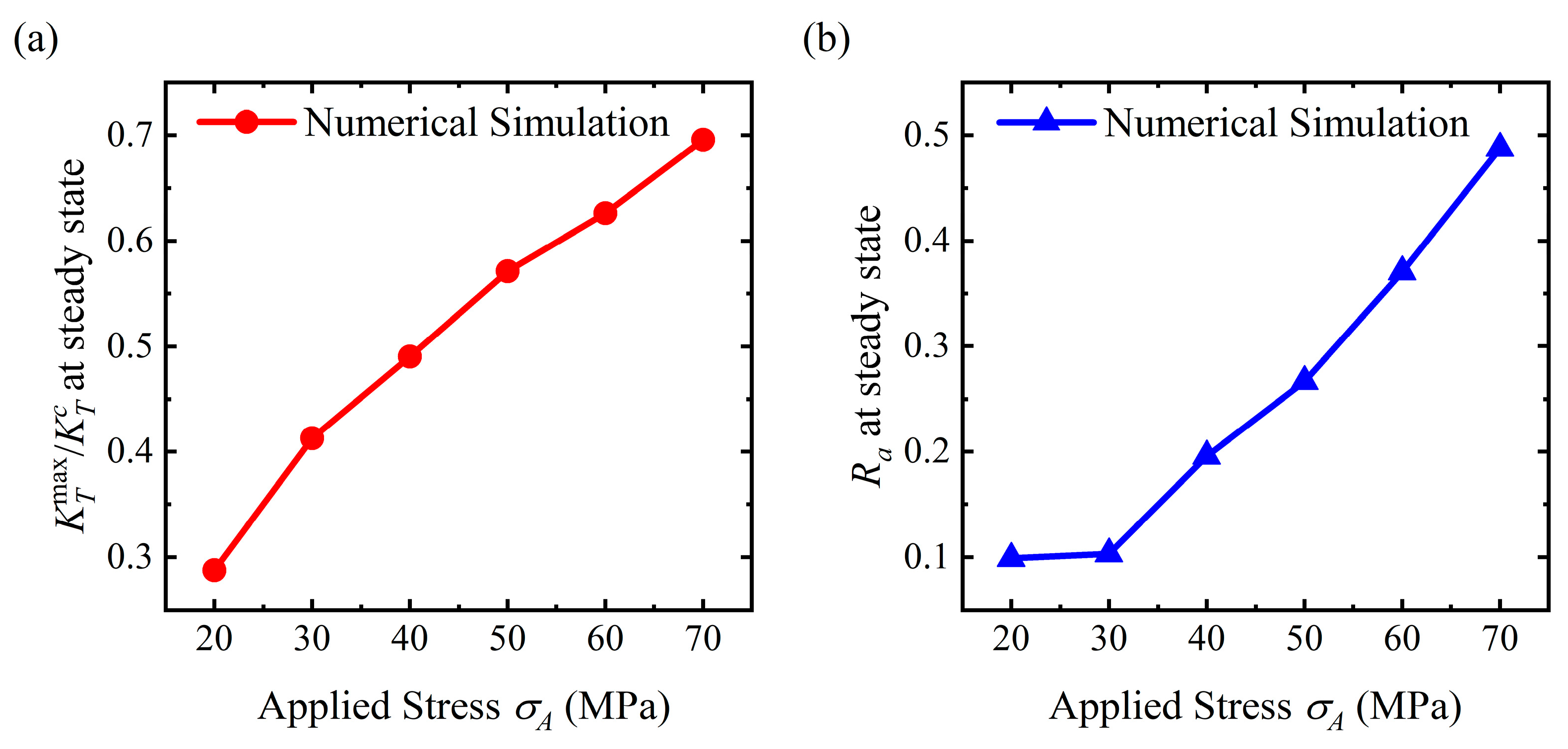
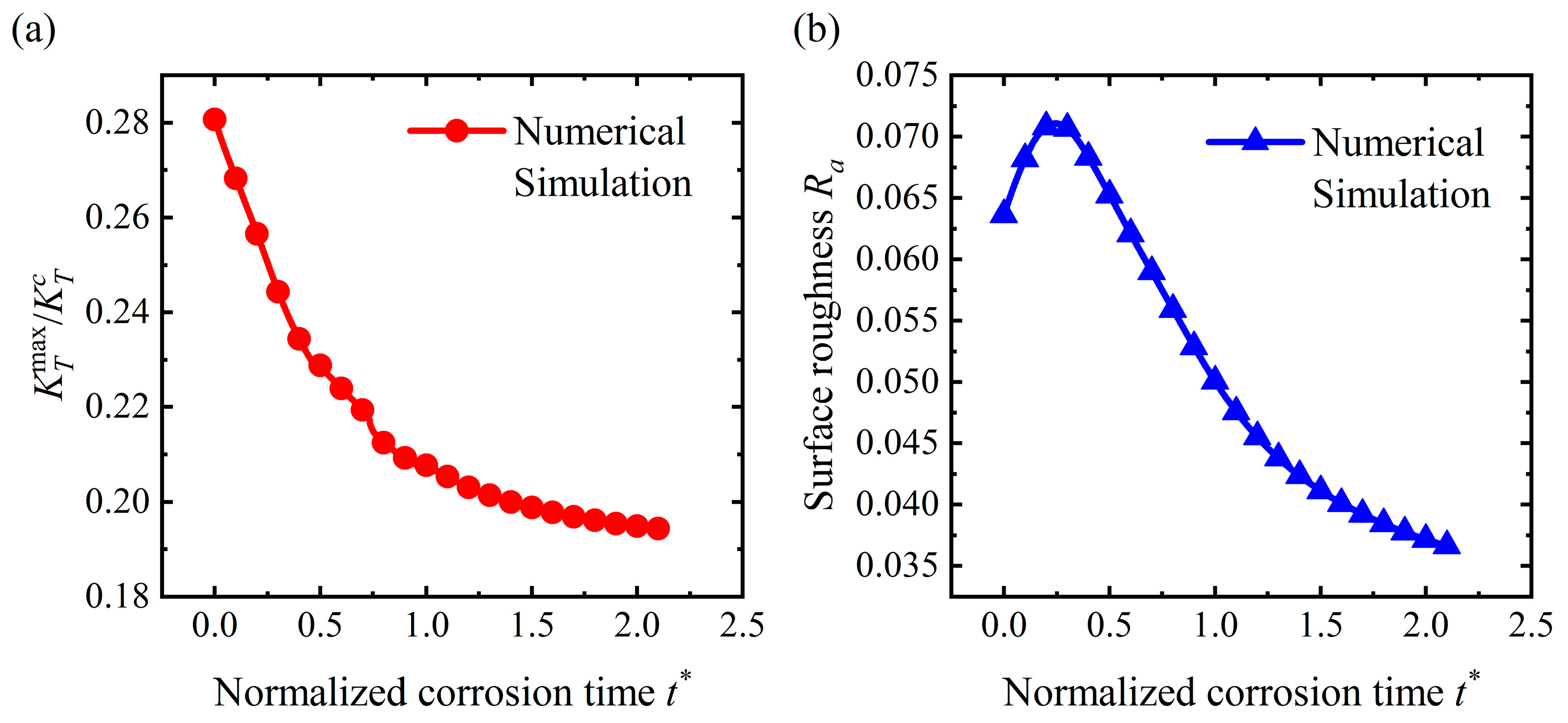
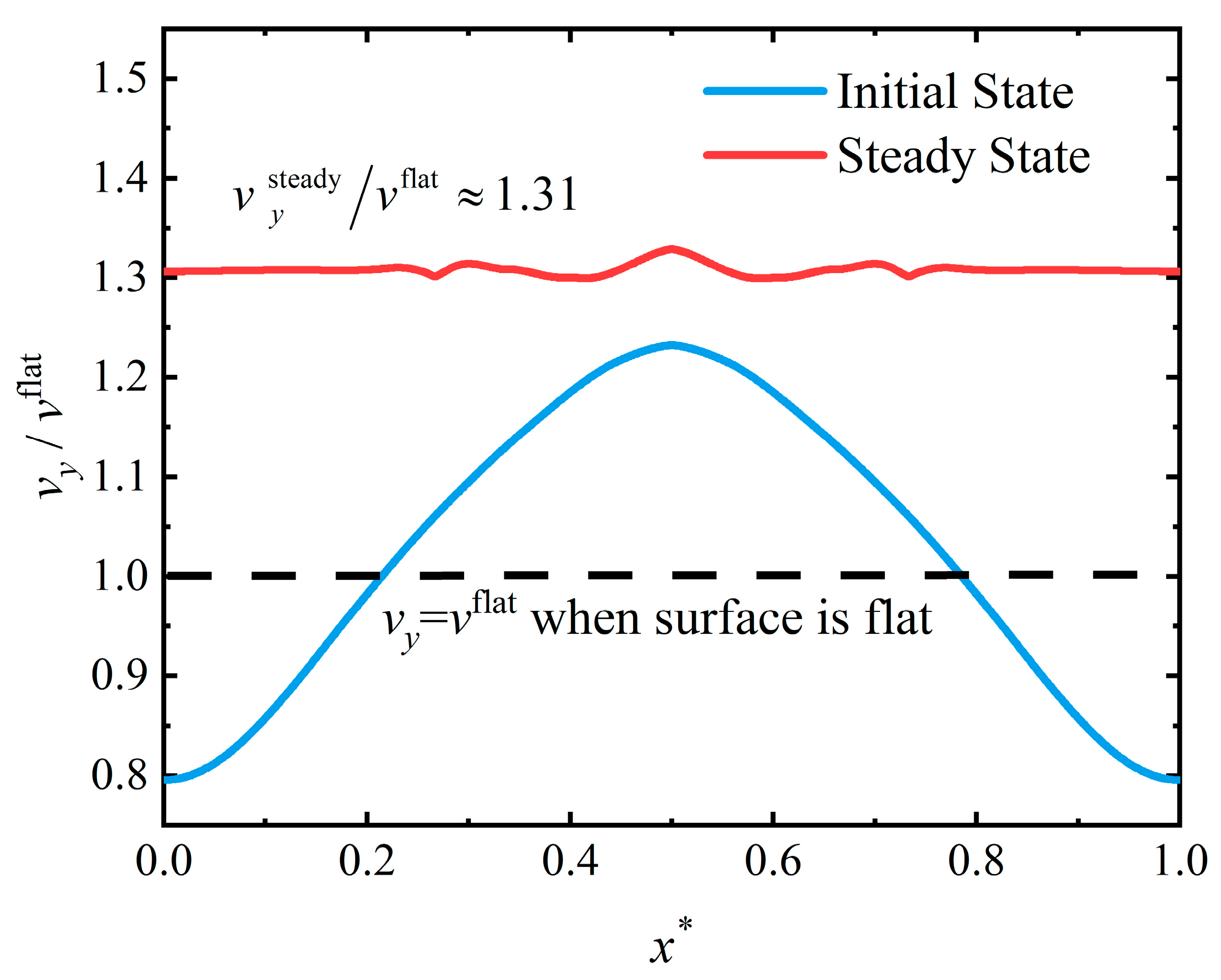
4.3. Flattening Pattern
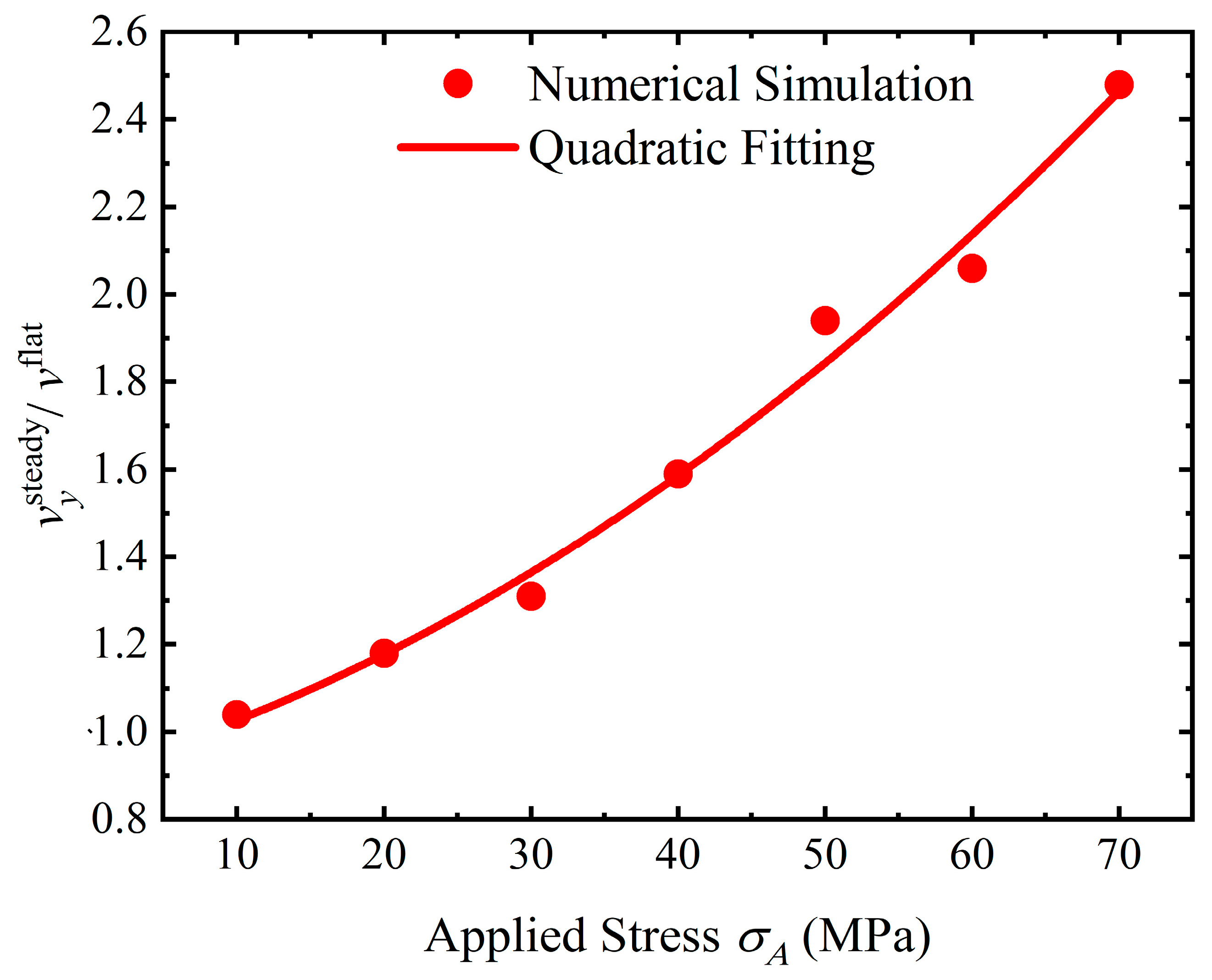
4.4. Discussion
5. Conclusions
- Stress could dominate the surface morphology evolution during corrosion. As stress increases, the corrosion morphology will transfer from a flat surface to a pit and then a crevice. The flatter surface and the corrosion pit are stable morphologies that will maintain a fixed shape. Once the stress exceeds the critical value for a crevice pattern, unstable crevices are generated on the surface;
- For the stable corrosion morphology, surface roughness and maximum stress concentration under a steady state increase with an increase in the applied stress. When the applied stress exceeds the critical value for the pit pattern, the surface will become rougher than the initial state, i.e., a pit pattern. When the applied stress is below the critical value for pit patterning, the surface will become flatter during the corrosion process, i.e., the flattening pattern;
- Stress can magnify the influence of morphologies on the service life of the structure. Once a crevice is generated on the surface, the service life will reduce dramatically. The initiation time for crevice generation is reduced as the applied stress rises. As a pit or flatter surface is formed, the service life of the structure is reduced when compared with the flat surface. Corrosion acceleration due to surface morphology presents a quadratically increasing relationship with a rise in the applied stress. In general, stress can reduce the service life of the structure with a rough surface by forming corrosion morphologies and increasing the dissolution rate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| amplitude of sinusoidal wave | corrosion depth | ||
| normalized amplitude | normalized corrosion depth | ||
| standard activity of the reactant | molar volume | ||
| mechano-electrochemical activity | corrosion rate | ||
| elastic modulus | corrosion rate | ||
| Faraday constant | initial corrosion rate of the un-stressed material | ||
| corrosion current density | vertical corrosion rate | ||
| anodic current density | vertical corrosion rate at steady state | ||
| cathodic current density | corrosion rate of the flat surface | ||
| mass flux | valence | ||
| stress concentration factor | transfer coefficients | ||
| maximal stress concentration factor | electrochemical potential | ||
| initial stress concentration factor | standard chemical potential | ||
| critical stress concentration factor for crevice initiation | electrical potential | ||
| constant of forward reaction rate | density | ||
| constant of reverse reaction rate | stress components | ||
| molar mass | mean stress | ||
| normal vector | applied stress | ||
| surface roughness | critical stress for crevice initiation | ||
| initial surface roughness | critical stress for pit generation | ||
| gas constant | strain components | ||
| temperature | amplitude of sinusoidal wave | ||
| time | |||
| normalized time | |||
| crevice generation time |
References
- Pronina, Y.; Sedova, O. Analytical Solution for the Lifetime of a Spherical Shell of Arbitrary Thickness Under the Pressure of Corrosive Environments: The Effect of Thermal and Elastic Stresses. J. Appl. Mech. 2021, 88, 061004. [Google Scholar] [CrossRef]
- Ståhle, P.; Hansen, E. Phase field modelling of stress corrosion. Eng. Fail. Anal. 2015, 47, 241–251. [Google Scholar] [CrossRef]
- Cui, C.; Ma, R.; Martínez-Pañeda, E. A phase field formulation for dissolution-driven stress corrosion cracking. J. Mech. Phys. Solids 2021, 147, 104254. [Google Scholar] [CrossRef]
- Zhu, L.; Cui, Z.; Cui, H.; Wang, X.; Li, Y. The effect of applied stress on the crevice corrosion of 304 stainless steel in 3.5 wt% NaCl solution. Corros. Sci. 2022, 196, 110039. [Google Scholar] [CrossRef]
- Cherry, B.; Price, S. Pitting, crevice and stress corrosion cracking studies of cold drawn eutectoid steels. Corros. Sci. 1980, 20, 1163–1183. [Google Scholar] [CrossRef]
- Sharland, S. A review of the theoretical modelling of crevice and pitting corrosion. Corros. Sci. 1987, 27, 289–323. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Zhang, G. Synergistic effect of stress and crevice on the corrosion of N80 carbon steel in the CO 2 -saturated NaCl solution containing acetic acid. Corros. Sci. 2017, 123, 228–242. [Google Scholar] [CrossRef]
- Burstein, G.T.; Pistorius, P. Surface Roughness and the Metastable Pitting of Stainless Steel in Chloride Solutions. Corrosion 1995, 51, 380–385. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. Influence of surface roughness on the corrosion behaviour of magnesium alloy. Mater. Des. 2011, 32, 2350–2354. [Google Scholar] [CrossRef]
- Li, W.; Li, D. Influence of surface morphology on corrosion and electronic behavior. Acta Mater. 2006, 54, 445–452. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Wu, T.; Yan, M.; Yu, C.; Sun, C. Effect of elastic stress and alternating current on corrosion of X80 pipeline steel in simulated soil solution. Eng. Fail. Anal. 2019, 100, 192–205. [Google Scholar] [CrossRef]
- Bocchi, S.; Cabrini, M.; D’Urso, G.; Giardini, C.; Lorenzi, S.; Pastore, T. Stress enhanced intergranular corrosion of friction stir welded AA2024-T3. Eng. Fail. Anal. 2020, 111, 104483. [Google Scholar] [CrossRef]
- Kentish, P. Stress corrosion cracking of gas pipelines–Effect of surface roughness, orientations and flattening. Corros. Sci. 2007, 49, 2521–2533. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chen, W. Near-neutral pH corrosion and stress corrosion crack initiation of a mill-scaled pipeline steel under the combined effect of oxygen and paint primer. Corros. Sci. 2021, 187, 109511. [Google Scholar] [CrossRef]
- Zadow, L.; Gamboa, E.; Lavigne, O. Inclined stress corrosion cracks in gas pipeline steels: Morphology and implications. Mater. Corros. 2015, 66, 1092–1100. [Google Scholar] [CrossRef]
- Toribio, J.; Kharin, V. Factors Affecting the Intrinsic Character of the Crack Growth Kinetics Curve in Stress Corrosion Cracking: A Review. Corros. Rev. 2001, 19, 207–252. [Google Scholar] [CrossRef]
- Dai, H.; Shi, S.; Guo, C.; Chen, X. Pits formation and stress corrosion cracking behavior of Q345R in hydrofluoric acid. Corros. Sci. 2020, 166, 108443. [Google Scholar] [CrossRef]
- Chen, Z.; Jafarzadeh, S.; Zhao, J.; Bobaru, F. A coupled mechano-chemical peridynamic model for pit-to-crack transition in stress-corrosion cracking. J. Mech. Phys. Solids 2021, 146, 104203. [Google Scholar] [CrossRef]
- Ståhle, P.; Bjerkén, C.; Jivkov, A. On dissolution driven crack growth. Int. J. Solids Struct. 2007, 44, 1880–1890. [Google Scholar] [CrossRef]
- Sun, S.; Chen, X.; Badwe, N.; Sieradzki, K. Potential-dependent dynamic fracture of nanoporous gold. Nat. Mater. 2015, 14, 894–898. [Google Scholar] [CrossRef]
- Nisbet, W.; Lorimer, G.; Newman, R. A transmission electron microscopy study of stress corrosion cracking in stainless steels. Corros. Sci. 1993, 35, 457–469. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Solid Surfaces; World Scientific Publishing Company: Singapore, 1994. [Google Scholar]
- Gutman, E.; Solovioff, G.; Eliezer, D. The mechanochemical behavior of type 316L stainless steel. Corros. Sci. 1996, 38, 1141–1145. [Google Scholar] [CrossRef]
- Wang, H.; Han, E.-H. Simulation of metastable corrosion pit development under mechanical stress. Electrochim. Acta 2013, 90, 128–134. [Google Scholar] [CrossRef]
- Choi, K.Y.; Kim, S.S. Morphological analysis and classification of types of surface corrosion damage by digital image processing. Corros. Sci. 2005, 47, 1–15. [Google Scholar] [CrossRef]
- Betts, A.J.; Boulton, L.H. Crevice corrosion: Review of mechanisms, modelling, and mitigation. Br. Corros. J. 1993, 28, 279–296. [Google Scholar] [CrossRef]
- Rosenfeld, I.L.; Marshakov, I.K. Mechanism of Crevice Corrosion. Corrosion 2013, 20, 115t–125t. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, G. Corrosion behaviour of 13Cr stainless steel under stress and crevice in 3.5 wt.% NaCl solution. Corros. Sci. 2020, 163, 108290. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Wang, J.; Wang, G.; Han, E.-H.; Ke, W. Anisotropic 3D growth of corrosion pits initiated at MnS inclusions for A537 steel during corrosion fatigue. Corros. Sci. 2010, 52, 2867–2877. [Google Scholar] [CrossRef]
- Fan, S.; Tian, C.; Liu, Y.; Chen, Z. Surface stability in stress-assisted corrosion: A peridynamic investigation. Electrochim. Acta 2022, 423, 140570. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Zhao, J.; Shakouri, M.; Bobaru, F. A peridynamic model for crevice corrosion damage. Electrochim. Acta 2022, 401, 139512. [Google Scholar] [CrossRef]
- Duddu, R.; Kota, N.; Qidwai, S.M. An Extended Finite Element Method Based Approach for Modeling Crevice and Pitting Corrosion. J. Appl. Mech. 2016, 83, 081003. [Google Scholar] [CrossRef]
- Tan, H. In vivo surface roughness evolution of a stressed metallic implant. J. Mech. Phys. Solids 2016, 95, 430–440. [Google Scholar] [CrossRef]
- Yang, H.-Q.; Zhang, Q.; Tu, S.-S.; Wang, Y.; Li, Y.-M.; Huang, Y. A study on time-variant corrosion model for immersed steel plate elements considering the effect of mechanical stress. Ocean Eng. 2016, 125, 134–146. [Google Scholar] [CrossRef]
- Qiao, L.; Mao, X. Thermodynamic analysis on the role of hydrogen in anodic stress corrosion cracking. Acta Met. Et Mater. 1995, 43, 4001–4006. [Google Scholar] [CrossRef]
- Pronina, Y. Analytical solution for the general mechanochemical corrosion of an ideal elastic–plastic thick-walled tube under pressure. Int. J. Solids Struct. 2013, 50, 3626–3633. [Google Scholar] [CrossRef]
- Vasudevan, A.K. Applied Stress Affecting the Environmentally Assisted Cracking. Met. Mater. Trans. A 2013, 44, 1254–1267. [Google Scholar] [CrossRef]
- Croll, S. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coatings 2020, 148, 105847. [Google Scholar] [CrossRef]
- Späth, H. Two Dimensional Spline Interpolation Algorithms; A. K. Peters, Ltd.: Natick, MA, USA, 1995. [Google Scholar]
- Zienkiewicz, O.C.; Taylor, R.L.; Zhu, J.Z. (Eds.) Chapter 7-Elasticity: Two- and Three-Dimensional Finite Elements. In The Finite Element Method: Its Basis and Fundamentals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 211–255. [Google Scholar]
- Cheng, Z.; Zhang, Q.; Wang, D.; Lu, W. Cyclic stress-assisted surface diffusion and stress concentration of machined surface topography. Eng. Fract. Mech. 2020, 234, 107087. [Google Scholar] [CrossRef]
- Yang, W.; Srolovitz, D. Surface morphology evolution in stressed solids: Surface diffusion controlled crack initiation. J. Mech. Phys. Solids 1994, 42, 1551–1574. [Google Scholar] [CrossRef]
- Katardjiev, I.; Carter, G.; Nobes, M.; Berg, S.; Blom, H. Three-dimensional simulation of surface evolution during growth and erosion. J. Vac. Sci. Technol. A Vac. Surf. Film. 1994, 12, 61–68. [Google Scholar] [CrossRef]
- Kamaya, M.; Haruna, T. Crack initiation model for sensitized 304 stainless steel in high temperature water. Corros. Sci. 2006, 48, 2442–2456. [Google Scholar] [CrossRef]
- Kamaya, M.; Haruna, T. Influence of local stress on initiation behavior of stress corrosion cracking for sensitized 304 stainless steel. Corros. Sci. 2007, 49, 3303–3324. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Zhang, W.; Li, X.; Han, M.; Long, B.; Jiang, P. Corrosion Induced Morphology Evolution in Stressed Solids. Metals 2023, 13, 108. https://doi.org/10.3390/met13010108
Dong Z, Zhang W, Li X, Han M, Long B, Jiang P. Corrosion Induced Morphology Evolution in Stressed Solids. Metals. 2023; 13(1):108. https://doi.org/10.3390/met13010108
Chicago/Turabian StyleDong, Zhelin, Weixu Zhang, Xiaozhen Li, Mingda Han, Bin Long, and Peng Jiang. 2023. "Corrosion Induced Morphology Evolution in Stressed Solids" Metals 13, no. 1: 108. https://doi.org/10.3390/met13010108
APA StyleDong, Z., Zhang, W., Li, X., Han, M., Long, B., & Jiang, P. (2023). Corrosion Induced Morphology Evolution in Stressed Solids. Metals, 13(1), 108. https://doi.org/10.3390/met13010108






