Influence of Material and Process Parameters on Reduction-Swelling Characteristics of Sintered Iron Pellets
Abstract
1. Introduction
- (a)
- The degree of reduction (DOR).
- (b)
- The degree of densification (DOD).
- (c)
- The reduction swelling Index (RSI).
2. Material Details
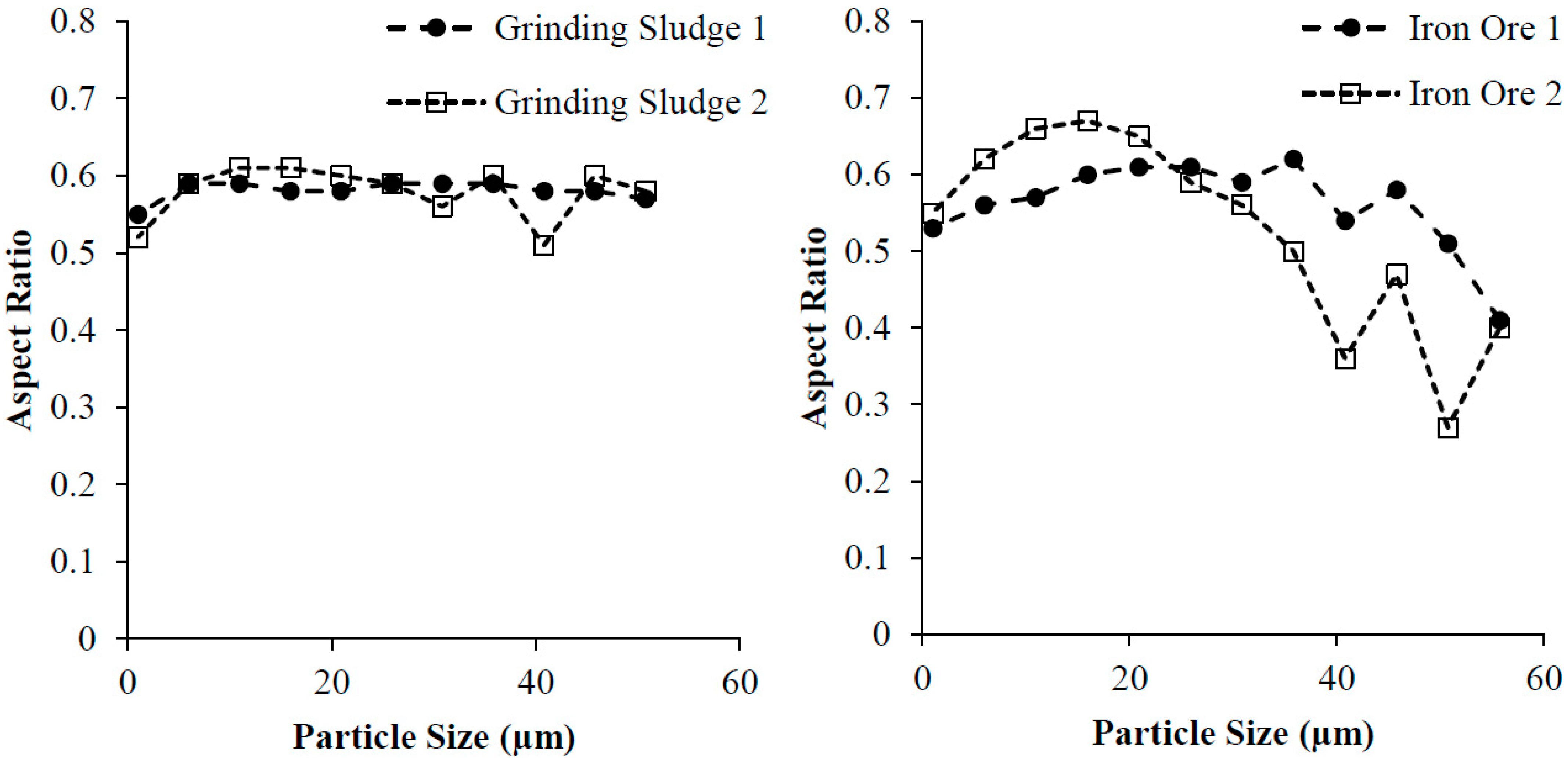
2.1. Grinding Sludge (GS) Powder
2.2. Iron Ore (IO) Powder
2.3. Reducing Agents
- (A)
- The grinding-sludge powder [92.78% Fe3O4, 1.92% FeO, 1.44% Fe Metallic, and 3.84% C]
- (B)
- Iron-ore powder [84.21% Fe2O3, 0.7% FeO, other oxides, and impurities including Al2O3 and SiO2]
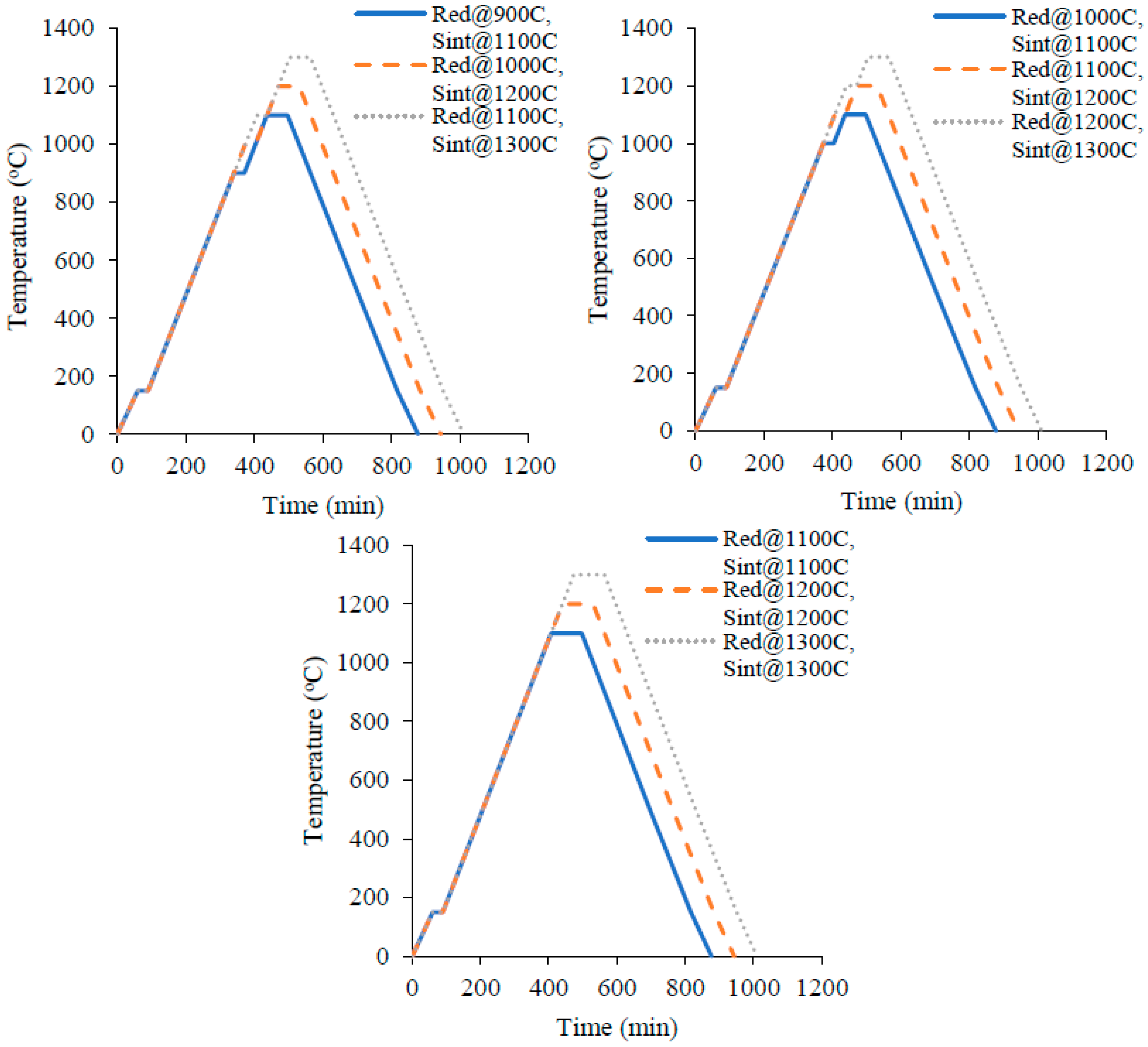
3. Design of Experiments Using the Taguchi Technique
- (a)
- The degree of reduction (DOR).
- (b)
- The degree of densification (DOD).
- (c)
- Mechanical properties.
| Sr. No. | Control Parameters | Levels | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 1 | A: Mixing Technique | Ball Milling | Mixer Grinding | Turbula Mixing |
| 2 | B: Reducing Agent Addition (%) | Stoichiometric | 25% excess | 50% excess |
| 3 | C: Reduction Temperature (°C) Below Sintering Temperature | 200 | 100 | 0 |
| 4 | D: Reducing Agent | Graphite | Carbon Black | Charcoal |
| 5 | E: Compaction Pressure (MPa) | 900 | 1050 | 1200 |
| 6 | F: Sintering Condition | Vacuum | Ar | N2 |
| 7 | G: Sintering Temperature (°C) | 1100 | 1200 | 1300 |
- (a)
- Column numbers 1, 2, 5, 9, 10, 12, and 13 were used for the following: (i) mixing technique, (ii) addition of reducing agent (%),(iii) reduction in temperature, (iv) type of reducing agent, (v) compaction pressure, (vi) sintering condition (atmosphere), and (vii) sintering temperature, respectively.
- (b)
- Column 3 and Column 4 were used to calculate the interaction between the mixing technique and the reducing agent (%).
- (c)
- Column 6 and Column 7 were used for an interaction between the mixing technique and reduction temperature, and
- (d)
- To study the mechanisms of reduction; direct–indirect reduction (C=>CO=>CO2) and direct reduction (C=>CO).
- To eliminate the effect of noise arising from the batch of powder (namely, different particle sizes).
- To eliminate effect of noise arising from positioning of the samples in the furnace.
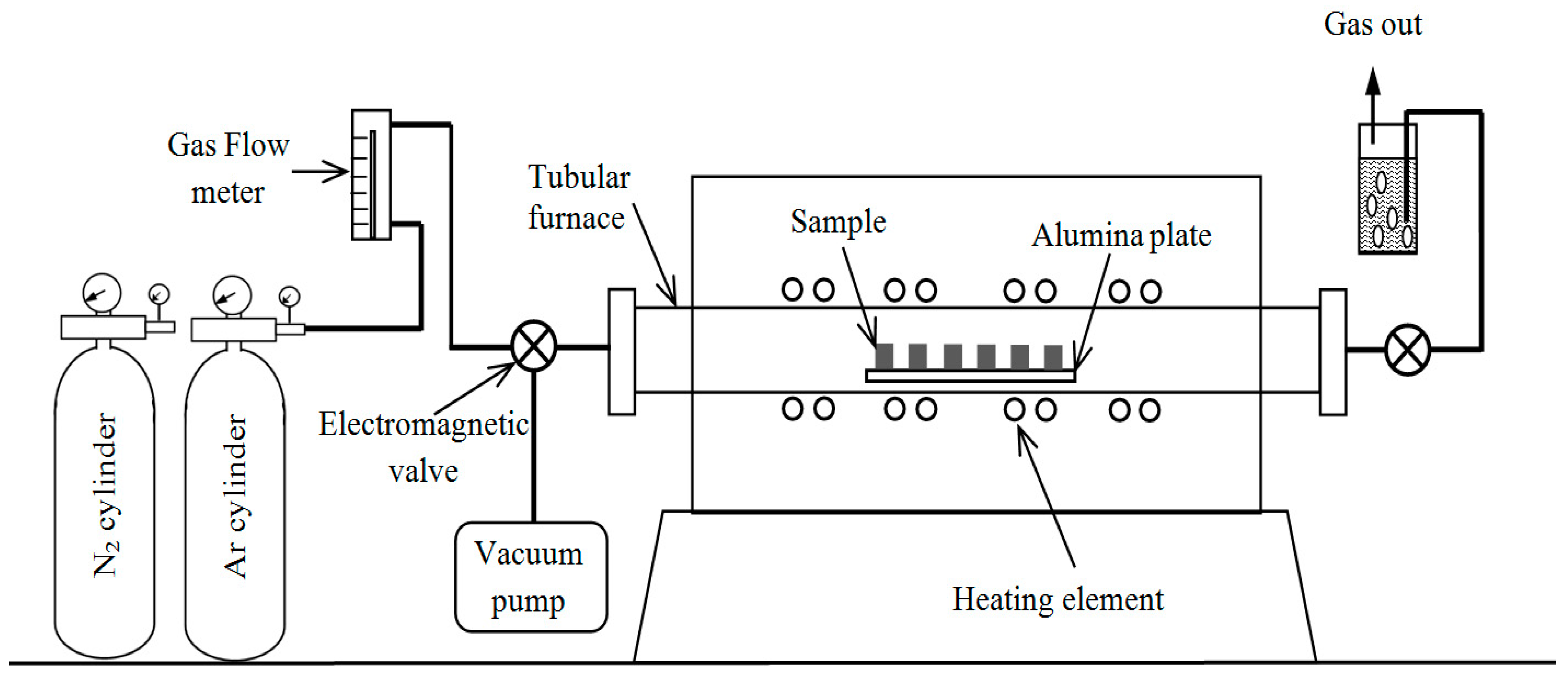
4. Experimental Details
5. Results and Discussion
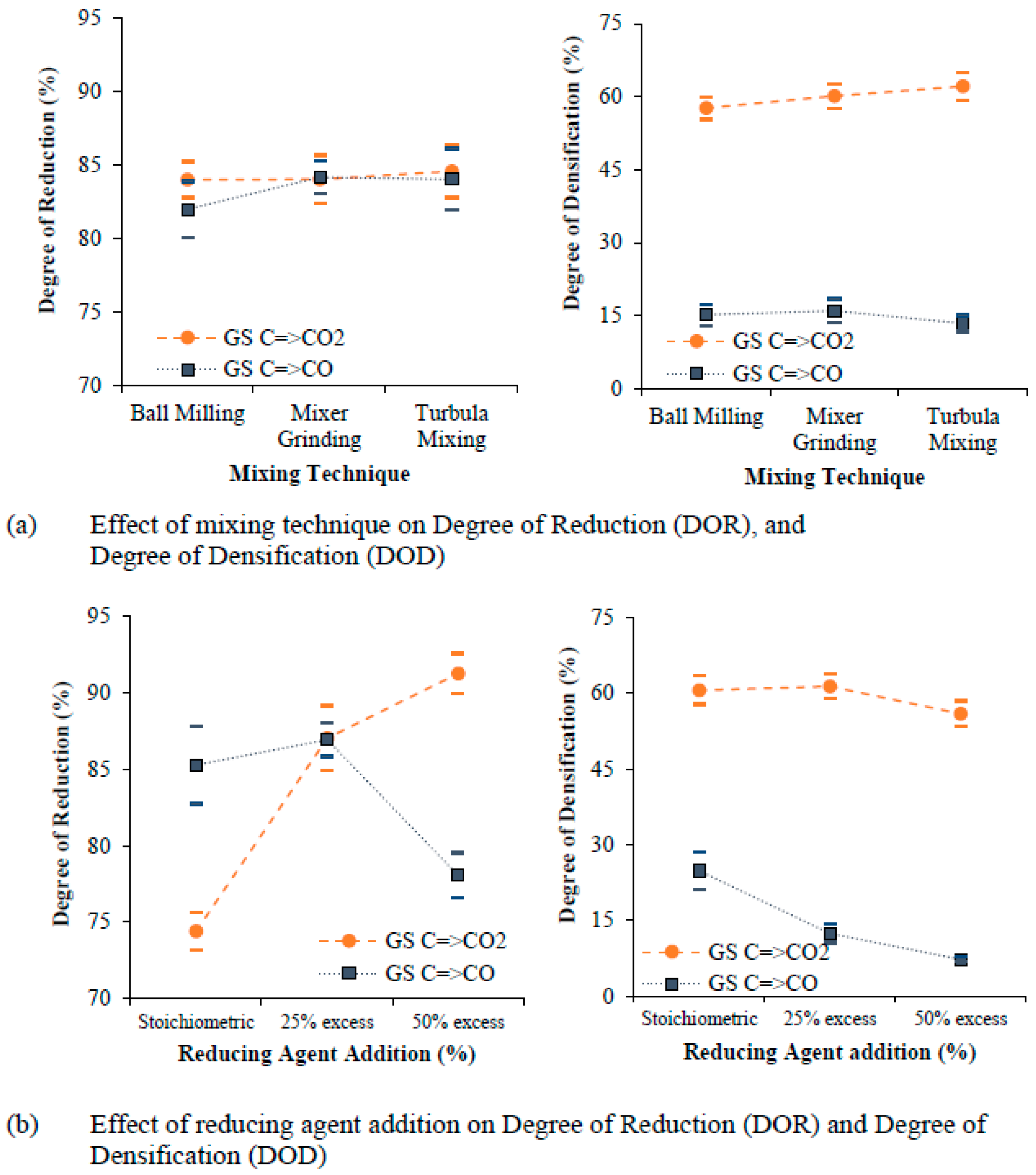
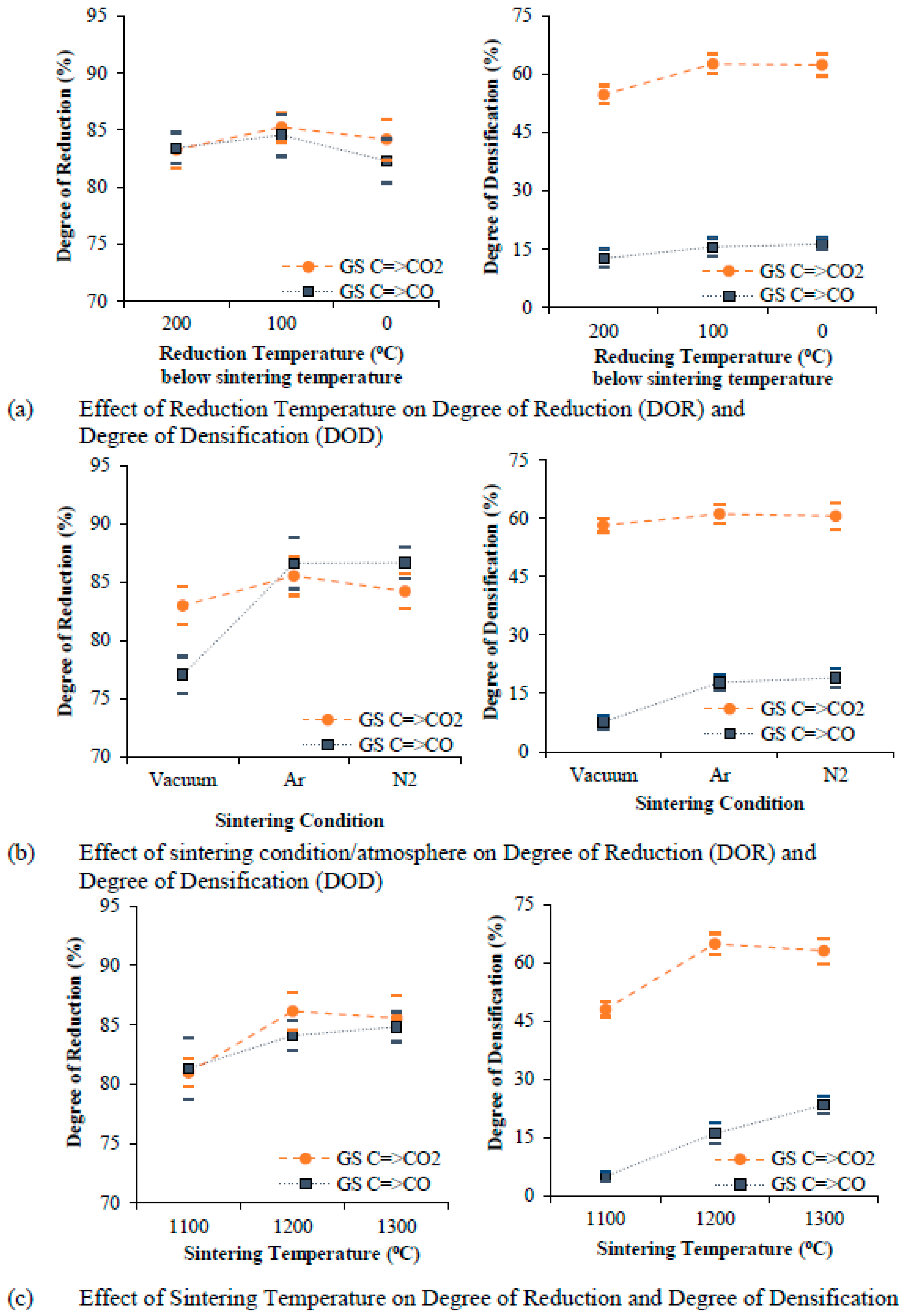
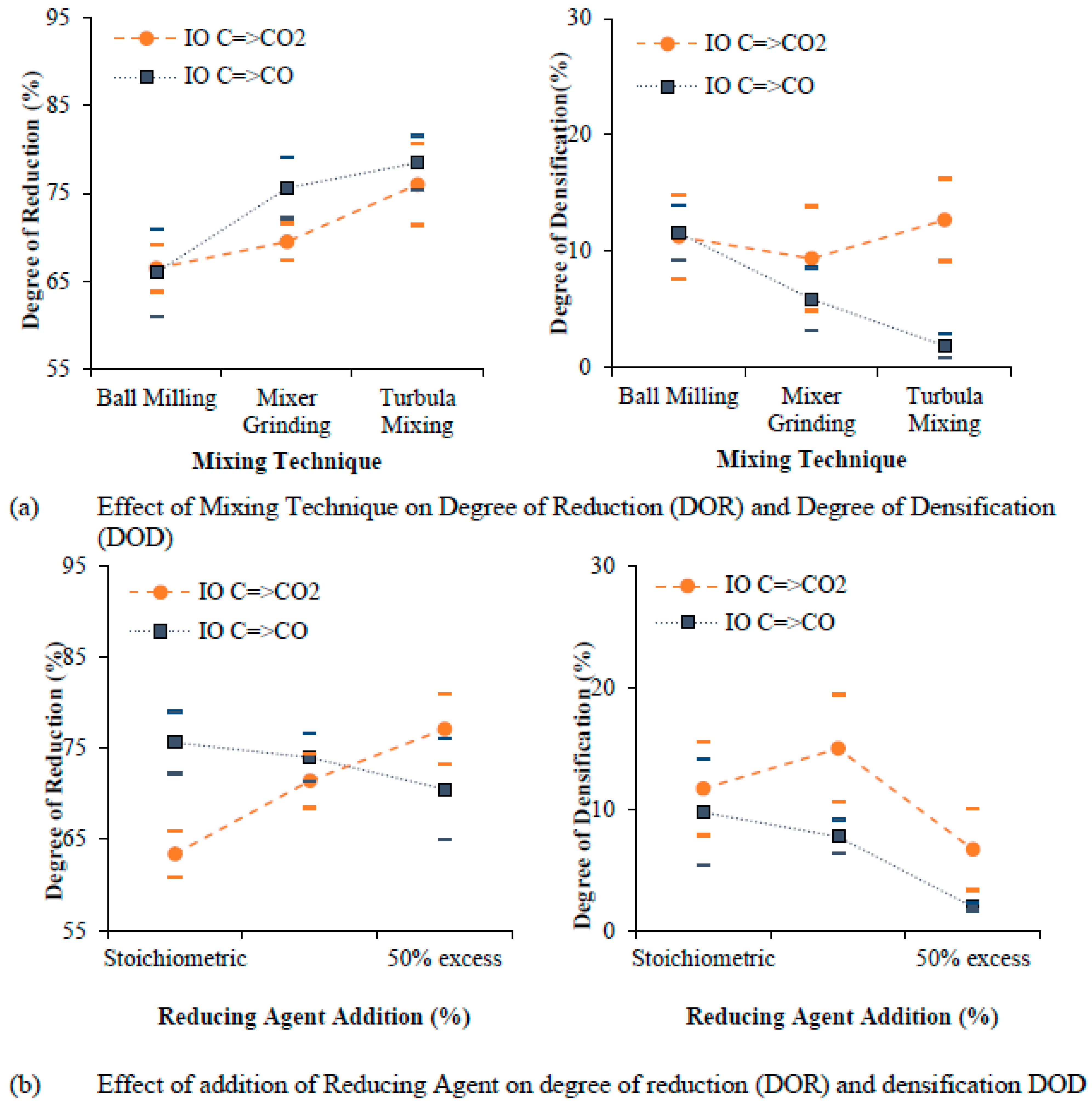
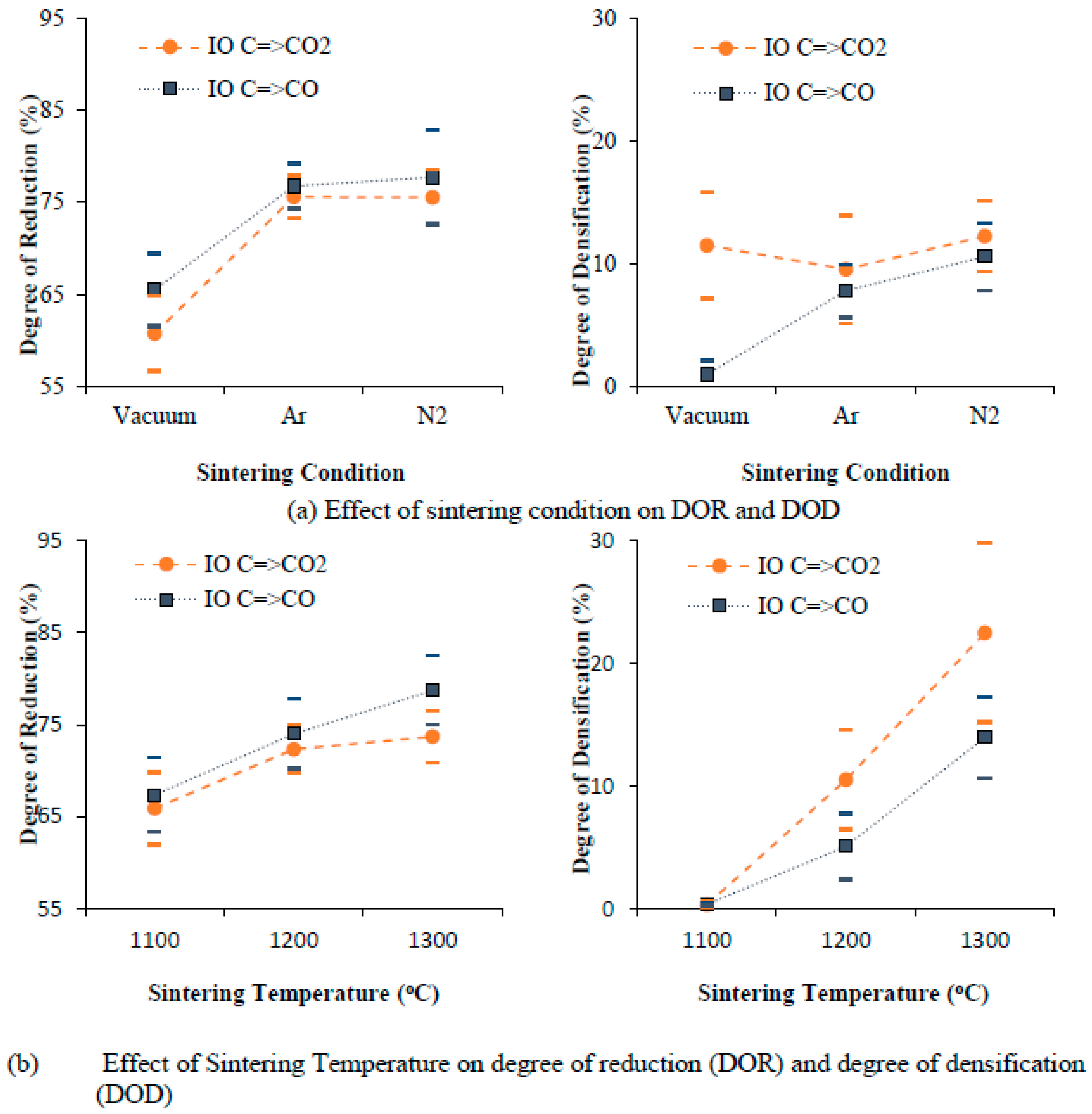
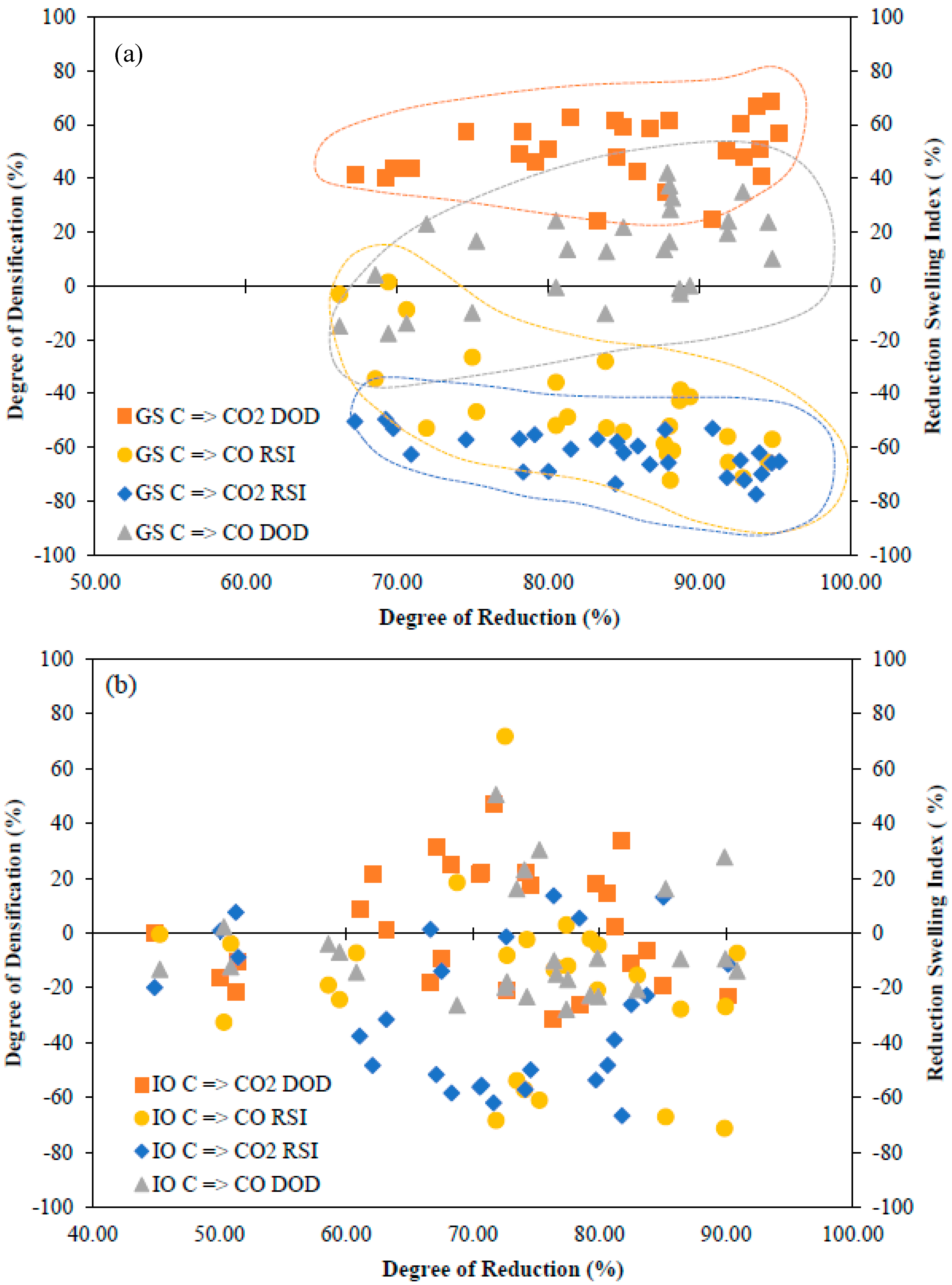
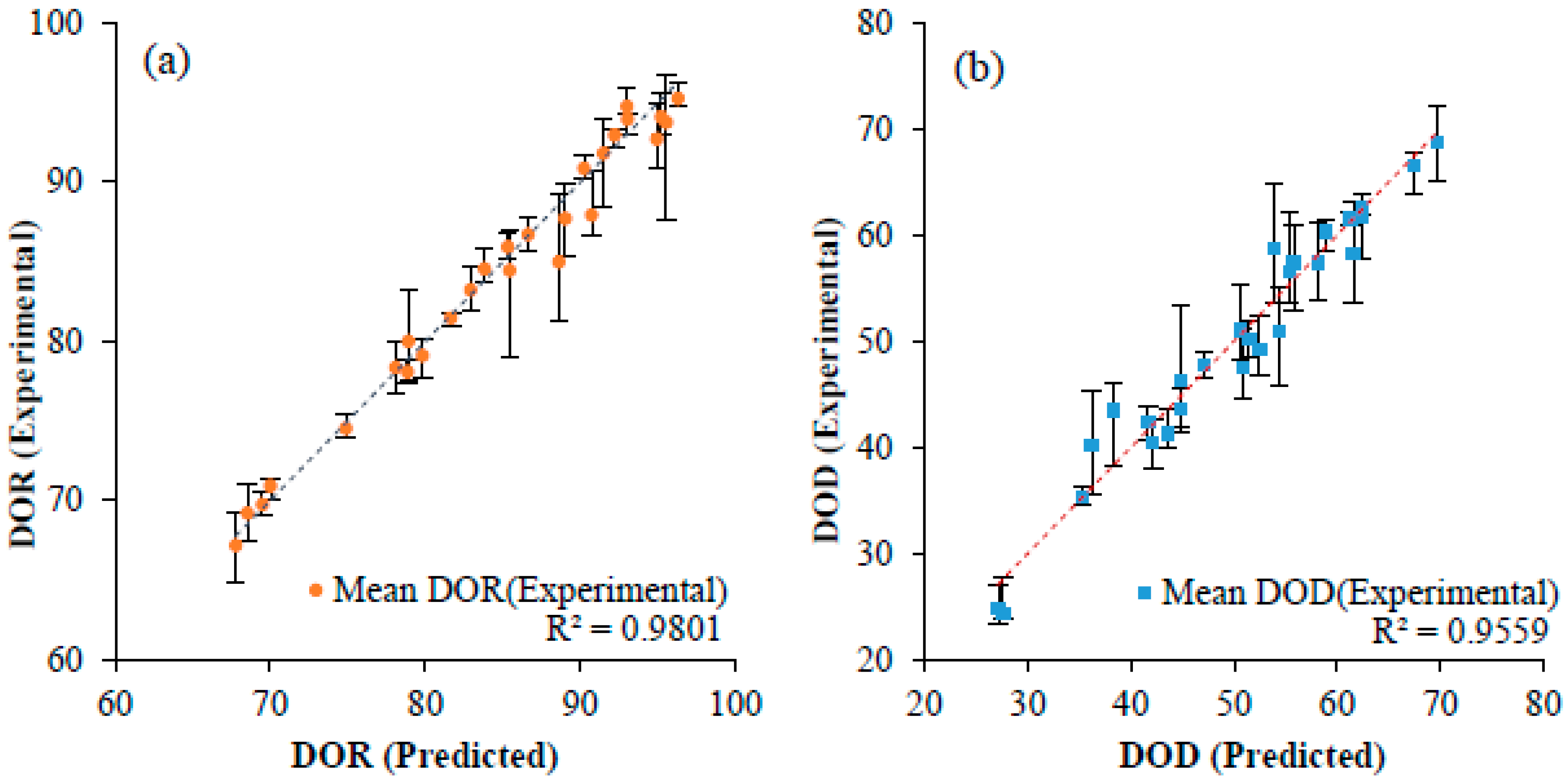
5.1. Degree of Reduction and Degree of Densification
5.2. Reduction Swelling Characteristics
5.3. Discussion on Utility of Taguchi Method for Reduction and Densification
6. Conclusions
- The source of iron oxide shows distinct characteristics of sintered pellets. Grinding sludge as a source of oxide powder shows a higher degree of reduction (DOR) when compared one-on-one to a conventional oxide source (the iron ore used for the production of iron).
- Graphite content in stoichiometric proportion gives the highest degree of densification, whereas an excess addition of graphite (between 25% in excess and 50% in excess) improves the degree of reduction (DOR) but creates a porous structure due to the formation and presence of excessive gaseous reaction products. Furthermore, an optimum graphite addition (25% in excess) helps to achieve the highest degree of reduction (DOR) and degree of densification (DOD).
- An intermediate compaction pressure of 1050 MPa and a sintering temperature of 1200 °C were found to be the optimum levels for reducing and sintering (achieving highest degree of reduction and degree of densification) of cylindrically shaped components from scrap powder.
- The degree of densification (DOD) is not significantly influenced by the degree of reduction (DOR) but is strongly influenced by the sintering parameters. The degree of reduction and the degree of densification (DOD) are higher for grinding sludge (GS) than for iron ore (IO) due to the presence of a higher quantity of unstable phases (Fe3O4) in the grinding sludge, which eases reduction.
- The mixing technique, compaction pressure, and a variation in the reduction temperature with respect to sintering temperature was not found to be influential in improving the degree of densification (DOD) and degree of reduction (DOR) for any of the two mechanisms of reduction studied.
- Carbon (particularly graphite) added to iron oxide and the C=>CO=>CO2 mechanism is utilized fully through both direct reduction and indirect reduction. The degree of densification (DOD) for the C=>CO mechanism of reduction was noticeably lower in all the experimental runs due to the presence of excess residual carbon subsequent to sintering.
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Exp. No. | A: Mixing Technique | B: Reducing Agent Addition (%) | C: Reduction Temperature (°C) below Sintering Temperature | D: Reducing Agent | E: Compaction Pressure (MPa) | F: Sintering Condition | G: Sintering Temp (°C) |
|---|---|---|---|---|---|---|---|
| 1 | Ball Milling | Stoic. | 200 | Graphite | 900 | Vacuum | 1100 |
| 2 | Ball Milling | Stoic. | 100 | Carbon Black | 1050 | Ar | 1200 |
| 3 | Ball Milling | Stoic. | 0 | Charcoal | 1200 | N2 | 1300 |
| 4 | Ball Milling | 25% excess | 200 | Carbon Black | 1050 | N2 | 1300 |
| 5 | Ball Milling | 25% excess | 100 | Charcoal | 1200 | Vacuum | 1100 |
| 6 | Ball Milling | 25% excess | 0 | Graphite | 900 | Ar | 1200 |
| 7 | Ball Milling | 50% excess | 200 | Charcoal | 1200 | Ar | 1200 |
| 8 | Ball Milling | 50% excess | 100 | Graphite | 900 | N2 | 1300 |
| 9 | Ball Milling | 50% excess | 0 | Carbon Black | 1050 | Vacuum | 1100 |
| 10 | Mixer Grinding | Stoic. | 200 | Carbon Black | 1200 | Ar | 1300 |
| 11 | Mixer Grinding | Stoic. | 100 | Charcoal | 900 | N2 | 1100 |
| 12 | Mixer Grinding | Stoic. | 0 | Graphite | 1050 | Vacuum | 1200 |
| 13 | Mixer Grinding | 25% excess | 200 | Charcoal | 900 | Vacuum | 1200 |
| 14 | Mixer Grinding | 25% excess | 100 | Graphite | 1050 | Ar | 1300 |
| 15 | Mixer Grinding | 25% excess | 0 | Carbon Black | 1200 | N2 | 1100 |
| 16 | Mixer Grinding | 50% excess | 200 | Graphite | 1050 | N2 | 1100 |
| 17 | Mixer Grinding | 50% excess | 100 | Carbon Black | 1200 | Vacuum | 1200 |
| 18 | Mixer Grinding | 50% excess | 0 | Charcoal | 900 | Ar | 1300 |
| 19 | Turbula Mixing | Stoic. | 200 | Charcoal | 1050 | N2 | 1200 |
| 20 | Turbula Mixing | Stoic. | 100 | Graphite | 1200 | Vacuum | 1300 |
| 21 | Turbula Mixing | Stoic. | 0 | Carbon Black | 900 | Ar | 1100 |
| 22 | Turbula Mixing | 25% excess | 200 | Graphite | 1200 | Ar | 1100 |
| 23 | Turbula Mixing | 25% excess | 100 | Carbon Black | 900 | N2 | 1200 |
| 24 | Turbula Mixing | 25% excess | 0 | Charcoal | 1050 | Vacuum | 1300 |
| 25 | Turbula Mixing | 50% excess | 200 | Carbon Black | 900 | Vacuum | 1300 |
| 26 | Turbula Mixing | 50% excess | 100 | Charcoal | 1050 | Ar | 1100 |
| 27 | Turbula Mixing | 50% excess | 0 | Graphite | 1200 | N2 | 1200 |
References
- Rane, K.; Date, P. Reduction and densification characteristics of iron oxide metallic waste during solid state recycling. Adv. Powder Technol. 2015, 26, 126–138. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, S.K. Assessment of Reduction Behavior of Hematite Iron Ore Pellets in Coal Fines for Application In Sponge Ironmaking. Miner. Process. Extr. Met. Rev. 2009, 30, 240–259. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanath, R.P.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrog. Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Cheng, G.-J.; Liu, J.-X.; Liu, Z.-G.; Chu, M.-S.; Xue, X.-X. Non-isothermal reduction mechanism and kinetics of high chromium vanadium–titanium magnetite pellets. Ironmak. Steelmak. 2015, 42, 17–26. [Google Scholar] [CrossRef]
- Donskoi, E.; McElwain, D.L.S.; Wibberley, L.J. Estimation and modeling of parameters for direct reduction in iron ore/coal composites: Part II. Kinetic parameters. Met. Mater. Trans. A 2003, 34, 255–266. [Google Scholar] [CrossRef]
- Bahgat, M.; Halim, K.S.A.; El-Kelesh, H.A.; Nasr, M.I. Enhancement of wüstite reducibility in blast furnace: Reaction kinetics and morphological changes. Ironmak. Steelmak. 2012, 39, 327–335. [Google Scholar] [CrossRef]
- Bahgat, M.; Halim, K.S.A.; El-Kelesh, H.A.; Nasr, M.I. Behaviour of wüstite prepared from Baharia iron ore sinter during reduction with CO–CO2–N2gas mixture. Miner. Process. Extr. Met. 2011, 120, 102–110. [Google Scholar] [CrossRef]
- Abdollahi, H.; Mahdavinejad, R.; Leavoli, R.P.; Ghambari, M.; Moradi, M. Investigation and optimization of properties of sintered iron/recycled grey cast iron powder metallurgy parts. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2014, 229, 1010–1020. [Google Scholar] [CrossRef]
- Abdollahi, H.; Mahdavinejad, R.; Ghambari, M.; Moradi, M. Investigation of green properties of iron/jet-milled grey cast iron compacts by response surface method. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2014, 228, 493–503. [Google Scholar] [CrossRef]
- Articles, T. Nakamura, Introduction of Grinding Swarf Recycling. Ntn Tech. Rev. 2004, 71, 80–83. [Google Scholar]
- Fu, P.; Dai, X.; Zhang, Q.; Beijing, T. Recycling of Steel-making Dust and Preparation of Iron Oxide Red. 2014, 86, pp. 1–7. Available online: https://silo.tips/download/recycling-of-steel-making-dust-and-preparation-of-iron-oxide-red (accessed on 1 December 2022).
- Materials, O. Iron Unit Recycling. 2001; 2000, pp. 1999–2000. Available online: https://www.energy.gov/sites/prod/files/2013/11/f4/roadmap_chap3.pdf (accessed on 1 December 2022).
- Delavari, M.; Salarvand, A.; Rahi, A.; Shahri, F. The effect of powder metallurgy process parameters on mechanical properties of micro and nano-iron powder. Int. J. Eng. Sci. Technol. 2011, 3, 86–94. [Google Scholar] [CrossRef]
- Komatina, M. The Sticking Problem During Direct Reduction of Fine Iron Ore In The Fluidized Bed Problem. ‘Stikinga’ Tokom Redukcije Rude Gvož Đ A, Malih Dimenzija Č Estica U Fluidizovanom Sloju. Metall. Mater. Eng. 2004. [Google Scholar]
- Emamian, A. A Study on Wear Resistance, Hardness and Impact Behaviour of Carburized Fe-Based Powder Metallurgy Parts for Automotive Applications. Mater. Sci. Appl. 2012, 3, 519–522. [Google Scholar] [CrossRef]
- Nerjavnega, L.; Nega, O.; Mim-tehnologijo, I.Z.; Butkovi, S.; Oru, M.; Mehmedovi, M. Effect Of Sintering Parameters On The Density, Microstructure And Mechanical Properties Of The Niobium-Modified Heat-Resistant Stainless Steel Gx40crnisi25-20 Produced By Mim Technology. Mater. Tehnol. 2012, 46, 185–190. [Google Scholar]
- Mashhadi, H.A.; Rastgoo, A.; Khaki, J.V. An Investigation on the Reduction of Iron Ore Pellets in Fixed Bed of Domestic Non-Coking Coals. Int. J. ISSI 2008, 5, 8–14. [Google Scholar]
- Manchili, S.K.; Wendel, J.; Hryha, E.; Nyborg, L. Analysis of iron oxide reduction kinetics in the nanometric scale using hydrogen. Nanomaterials 2020, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Sintered Iron-Based Materials Chapter 9. Available online: https://mail.pk.edu.pl/~mnykiel/iim/26/DOWNLOAD/pdf/CHAPT09.PDF (accessed on 1 December 2022).
- Dewidar, M. Influence of processing parameters and sintering atmosphere on the mechanical properties and microstructure of porous 316L stainless steel f or possible hard-tissue applications. Int. J. Mech. Mech. Eng. 2012, 12, 10–24. [Google Scholar]
- El-Geassy, A.A.; Halim, K.S.A.; Bahgat, M.; Mousa, E.A.; El-Shereafy, E.E.; El-Tawil, A.A. Carbothermic reduction of Fe2O3/C compacts: Comparative approach to kinetics and mechanism. Ironmak. Steelmak. 2013, 40, 534–544. [Google Scholar] [CrossRef]
- Donskoi, E.; McElwain, D.L.S.; Wibberley, L.J. Sensitivity analysis of a model for direct reduction in swelling coal char-hematite composite pellets. ANZIAM J. 2003, 44, 140–159. [Google Scholar] [CrossRef]
- Bahgat, M.; Halim, K.S.A.; Nasr, M.I. Effect of Nature Gas Injection on Reducibility of Wustite Prepared from Baharia Iron Ore Sinter. J. Metall. Eng. 2012, 1, 14–22. [Google Scholar]
- Xu, B.; Hou, T.; Chen, X.-L.; Li, Q.; Jiang, T.; Li, P. Effect of dolomite on reduction swelling property of iron ore pellets. J. Central South Univ. 2013, 20, 2806–2810. [Google Scholar] [CrossRef]
- Rane, K.K.; Date, P.P. Sustainable Recycling of Ferrous Metallic Scrap Using Powder Metallurgy Process. J. Sustain. Met. 2017, 3, 251–264. [Google Scholar] [CrossRef]
- Rane, K.; Date, P.P. Recycling Potential for Finely Divided Ferrous Metallic Scrap Using Powder Technology. Recycling 2018, 3, 59. [Google Scholar] [CrossRef]
- Nascimento, R.; Mourão, M.; Capocchi, J. Kinetics and catastrophic swelling during reduction of iron ore in carbon bearing pellets. Ironmak. Steelmak. 1999, 26, 182–186. [Google Scholar] [CrossRef]
- Heikkilä, A.; Iljana, M.; Bartusch, H.; Fabritius, T. Reduction of iron ore pellets, sinter, and lump ore under simulated blast furnace conditions. Steel Res. Int. 2020, 91, 2000047. [Google Scholar] [CrossRef]
- Al-tounsi, A.; Ireland, H. Effect of sintering parameters on the mechanical and physical properties of sinter formed materials. 1992. Available online: https://doras.dcu.ie/18312/.
- Çavdar, U.; Sad, B.; At, E. Effect of The Copper Amount In Iron-Based Powder-Metal Compacts. Vpliv Vsebnosti Bakra V Kovinskih Stisnjencih Iz. Mater. Tehnol. 2015, 49, 57–62. [Google Scholar]
- Philips, T.; Dwyer, J.J.; Zurecki, Z. Controlling Properties of Sintered Steel Powder Metal Components Using Atmosphere Composition as a Variable; Air Products and Chemicals Inc.: Allentown, PA, USA, 2006. [Google Scholar]
- Yi, L.; Huang, Z.; Jiang, T.; Wang, L.; Qi, T. Swelling behavior of iron ore pellet reduced by H2–CO mixtures. Powder Technol. 2015, 269, 290–295. [Google Scholar] [CrossRef]
- Iljana, M.; Mattila, O.; Alatarvas, T.; Visuri, V.V.; Kurikkala, J.; Paananen, T.; Fabritius, T. Dynamic and isothermal reduction swelling behaviour of olivine and acid iron ore pellets under simulated blast furnace shaft conditions. ISIJ Int. 2020, 52, 1257–1265. [Google Scholar] [CrossRef]




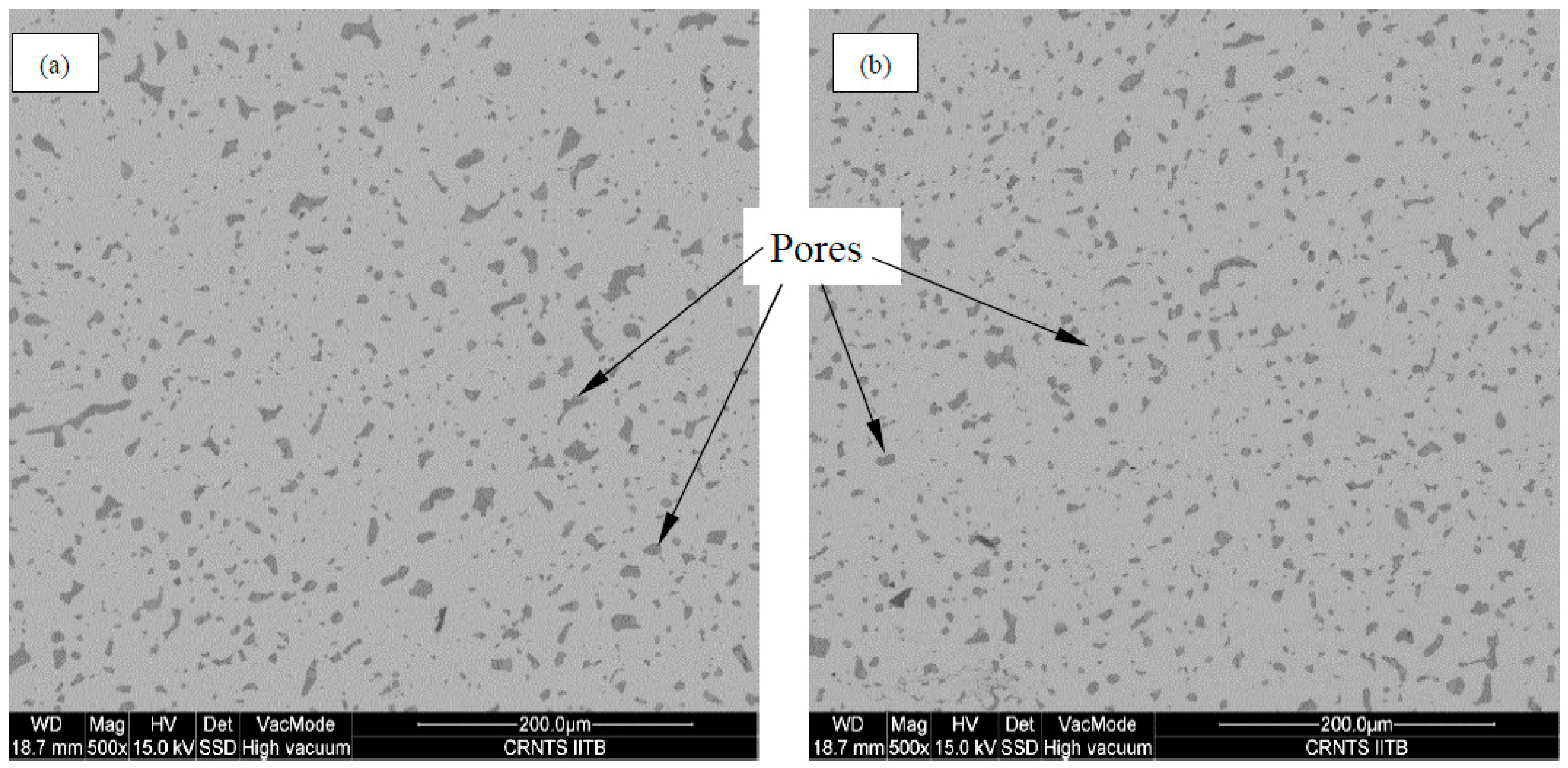

| Material | Constituents % | |||||
|---|---|---|---|---|---|---|
| FeTotal | Fe2O3 | Fe3O4 | FeO | FeMetallic | Impurity/ Non-Metallic | |
| Grinding Sludge (GS) | 75.72 | Nil | 92.78 | 1.92 | 1.44 | Nil |
| Iron Ore (IO) | 59.77 | 84.21 | Nil | 0.70 | Nil | 1.73 CaO, 0.24 MgO, 6.56 SiO2, 5.28 Al2O3, 0.16 MnO, 0.31 TiO2, 0.051NiO2, 0.08 P2O5 |
| GS Powder | IO Powder | |||
|---|---|---|---|---|
| Grinding Sludge 1 | Grinding Sludge 2 | Iron Ore 1 | Iron Ore 2 | |
| Average particle size (µm) | 21.77 | 29.19 | 22.62 | 18.59 |
| Surface Area (m2/g) | 7.87 | 7.95 | 8.52 | 8.76 |
| Reducing Agents | Moisture (%) | Volatile Matter (%) | Ash (%) | Fixed Carbon (%) |
|---|---|---|---|---|
| Graphite | 0.67 | 00.18 | 01.34 | 97.81 |
| Charcoal | 1.21 | 21.66 | 09.86 | 67.27 |
| Carbon Black | 3.20 | 07.66 | 09.06 | 80.08 |
| Elements (%) | Graphite | Charcoal | Carbon Black |
|---|---|---|---|
| C | 33.102 | 66.383 | 39.714 |
| H | 5.589 | 2.006 | 9.792 |
| N | 2.441 | 0.328 | 2.513 |
| O | 0.637 | 6.563 | 2.838 |
| Proportion of C Addition | Mechanism 1 Direct-Indirect; C=>CO=>CO2 | Mechanism 2 Direct; C=>CO | ||||
|---|---|---|---|---|---|---|
| Stoic. | 25% Excess | 50% Excess | Stoic. | 25% Excess | 50% Excess | |
| for Grinding Sludge | 0.0985 | 0.0123 | 0.1477 | 0.1955 | 0.2443 | 0.2932 |
| for Iron Ore | 0.0949 | 0.1186 | 0.1423 | 0.1911 | 0.2388 | 0.2866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rane, K.; Date, P.; Srivatsan, T.S. Influence of Material and Process Parameters on Reduction-Swelling Characteristics of Sintered Iron Pellets. Metals 2023, 13, 141. https://doi.org/10.3390/met13010141
Rane K, Date P, Srivatsan TS. Influence of Material and Process Parameters on Reduction-Swelling Characteristics of Sintered Iron Pellets. Metals. 2023; 13(1):141. https://doi.org/10.3390/met13010141
Chicago/Turabian StyleRane, Kedarnath, Prashant Date, and T. S. Srivatsan. 2023. "Influence of Material and Process Parameters on Reduction-Swelling Characteristics of Sintered Iron Pellets" Metals 13, no. 1: 141. https://doi.org/10.3390/met13010141
APA StyleRane, K., Date, P., & Srivatsan, T. S. (2023). Influence of Material and Process Parameters on Reduction-Swelling Characteristics of Sintered Iron Pellets. Metals, 13(1), 141. https://doi.org/10.3390/met13010141





