Abstract
This laboratory study aimed to reveal the inner connects between the microstructure and corrosion properties of a RE microalloyed ultra-thin cast strip (UCS) steel. The microstructure mainly consisted of homogeneous polygonal ferrite (PF) with a small amount of pearlite (P), while adding multiple alloying elements led to the appearance of granular bainite (GB) and bainitic ferrite (BF). RE elements strongly promoted the homogenization and refinement of microstructure by segregating towards the solid–liquid interface. Potentiodynamic polarization, EIS, and weight loss curves under wet–dry immersion test confirmed that the corrosion behavior was significantly improved by RE, while the addition of RE had no obvious change on tensile strength. The corrosion resistance of the homogeneous single-phase microstructure was proved to be better than that of multiphase microstructure. Hence, RE had a remarkable influence on improving corrosion resistance when the experimental steels processed single-phase microstructures.
1. Introduction
In recent years, twin-roll strip casting technology has been rapidly developed. The relatively mature technologies, including Nucor’s CASTRIP®, POSCO’s POSTRIP®, and EUROSTRIP® in Europe and BAOSTRIP® and E2STRIP in China, have also entered the stage of industrial implementation [1,2,3,4]. Shagang introduced CASTRIP® technology from Nucor steel company and built the first ultra-thin cast strip (UCS) production line with independent innovation in 2018. Compared to traditional hot continuous rolling and thin slab continuous casting lines, CASTRIP® technology is a novel forming technology by means of which thin strips can be directly produced from molten melt with less energy consumption, low investment, and small occupation [2,5]. The development of CASTRIP® technology aims to simplify the steel production flow by minimizing the size of the casting machine and simplifying the requirements for the hot reduction of steel strip [6].
CASTRIP® technology has been successfully applied in manufacturing low-carbon steels and Nb-microalloyed steels [7]. As an important structure steel, low-carbon steel is particularly vulnerable to corrosion in industrial environments. However, a number of studies have been devoted to the complex strengthening mechanisms, including microstructure hardening and solid solution hardening operating in Nb-microalloyed steels during deformation, while few people pay attention to the corrosion problem. The addition of rare earth (RE) elements into steels have been reported to improve corrosion resistance by changing the properties, morphology, and distribution of inclusions [8,9,10,11]. The beneficial effect of RE in optimizing inclusions is to form smaller spherical RE-oxy-sulfides and, thus, to reduce the occurrence of pitting corrosion. The addition of RE in steel is beneficial to purify liquid steel, refine dendrite, and inhibit columnar crystal growth. RE inclusions and RE atoms enriched at the interfaces also play a role in influencing the diffusion of other elements and restraining the growth of new phases [12,13,14]. Therefore, the aim of the present study was to further investigate the potential effect of rare earth on microstructure transformation. Systematic research has been carried out by the authors to understand the effect of rare earth elements on the corrosion properties of CASTRIP® steels.
2. Experimental
2.1. Sample Preparation
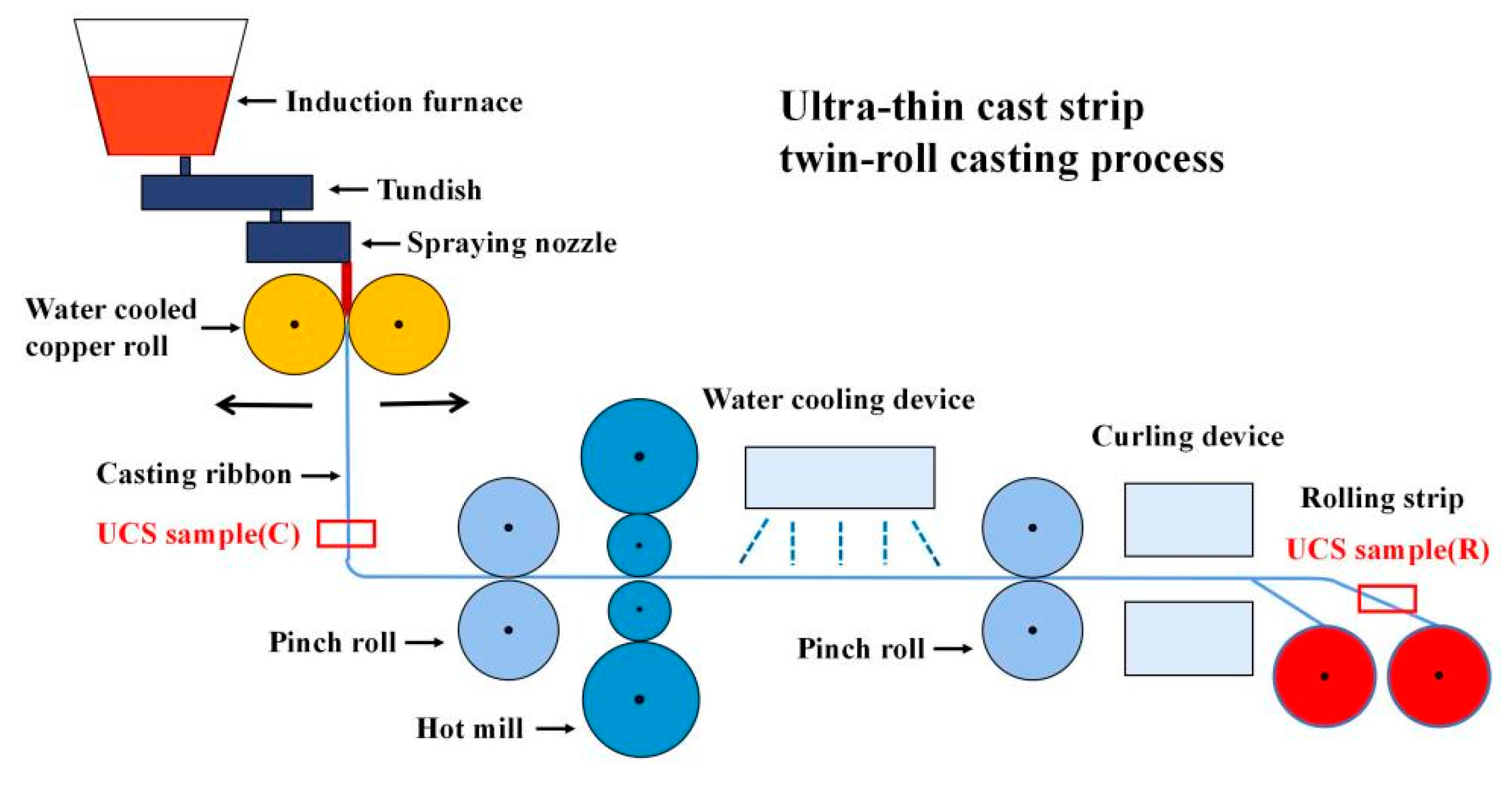
A schematic diagram of the processing route is given in Figure 1. Molten steel flowed into a pair of copper rolls through metal delivery system. After solidification, the molten steel was extruded from the gap between copper rolls to produce casting ribbon with a thickness of 1.4–2.1 mm, and then a rolling strip with a thickness of 0.7–1.9 mm was produced by a single rolling.
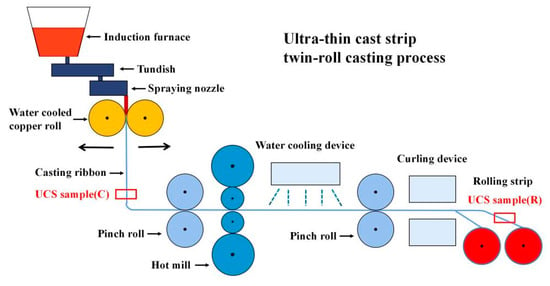
Figure 1.
Schematic diagram of UCS processing route.
In this work, a certain amount of RE elements (Ce and La) were added into UCS-Q235B steels. Samples were taken from casting ribbon with a thickness of 1.7 mm and rolling strip with a thickness of 1.0 mm, labeled as UCS-Q235BRE(C) and UCS-Q235BRE(R), respectively, in Figure 1. In order to illustrate the role of RE elements in steel, samples obtained from UCS-Q235B(R) steel and UCS-SPA-H(R) steel rolling strips were selected as contrast samples. The average chemical composition of final experimental steels are listed in Table 1.

Table 1.
Chemical composition of experimental steels (wt.%).
2.2. Examination of Microstructure
Specimens were prepared by polishing and etching in a weak solution of nitric acid and alcohol. Optical metallography was carried out on an optical microscope (OM) (Nikon MA100, Tokyo, Japan). The morphology of inclusions was observed using a thermal field scanning electron microscopy (SEM) and combined with energy dispersive X-ray spectroscopy (EDS) (Hitachi SU-1500, Tokyo, Japan). The microstructure and dislocation morphology were observed by transmission electron microscopy (TEM) (JEOL JEM-2100F, Tokyo, Japan).
2.3. Characterization of Properties
Two tensile parallel samples with a gauge length of 50 mm were prepared to fracture at room temperature. Three parallel samples (55 mm × 50 mm × 1.5 mm) were prepared for calculating the rate of corrosion weight loss under wet–dry cycle immersion test. Immersion tests were carried out at 45 °C in a solution of 0.01 mol/L NaHSO3. One immersion cycle consisted of 15 min of infiltration and 45 min of exposure. Time points of analyses were set to 24 h, 48 h, 72 h, and 120 h. Before immersion tests were conducted, the specimens were cleaned ultrasonically and weighed in a dry environment. The rust on the surface was chemically moved in a specific solution consisted of 500 mL HCl, 500 mL distilled water, and 3.5 g hexamethylenetetramine. After corrosion products were removed, the specimens were reweighed.
The electrochemical measurements consisting of potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and linear polarization resistance were conducted in an electrochemical workstation (Gamry Reference 600, Philadelphia, PA, USA) in a conventional glass cell. The test temperature was 20 °C. A saturated calomel electrode (SCE) and a Pt counter electrode were used as the reference and the counter electrodes, respectively. The test solution was deaerated by nitrogen treatment before the experiment began. In the case of EIS measurements, a sinusoidal AC voltage signal of amplitude 10 mV was applied, and the frequency ranged from 100 kHz to 10 mHz. The polarization tests were conducted in the potential range of −1.0 to 0 V (vs. Eref) and a scanning rate of 1 mV/s.
3. Results
3.1. Microstructure
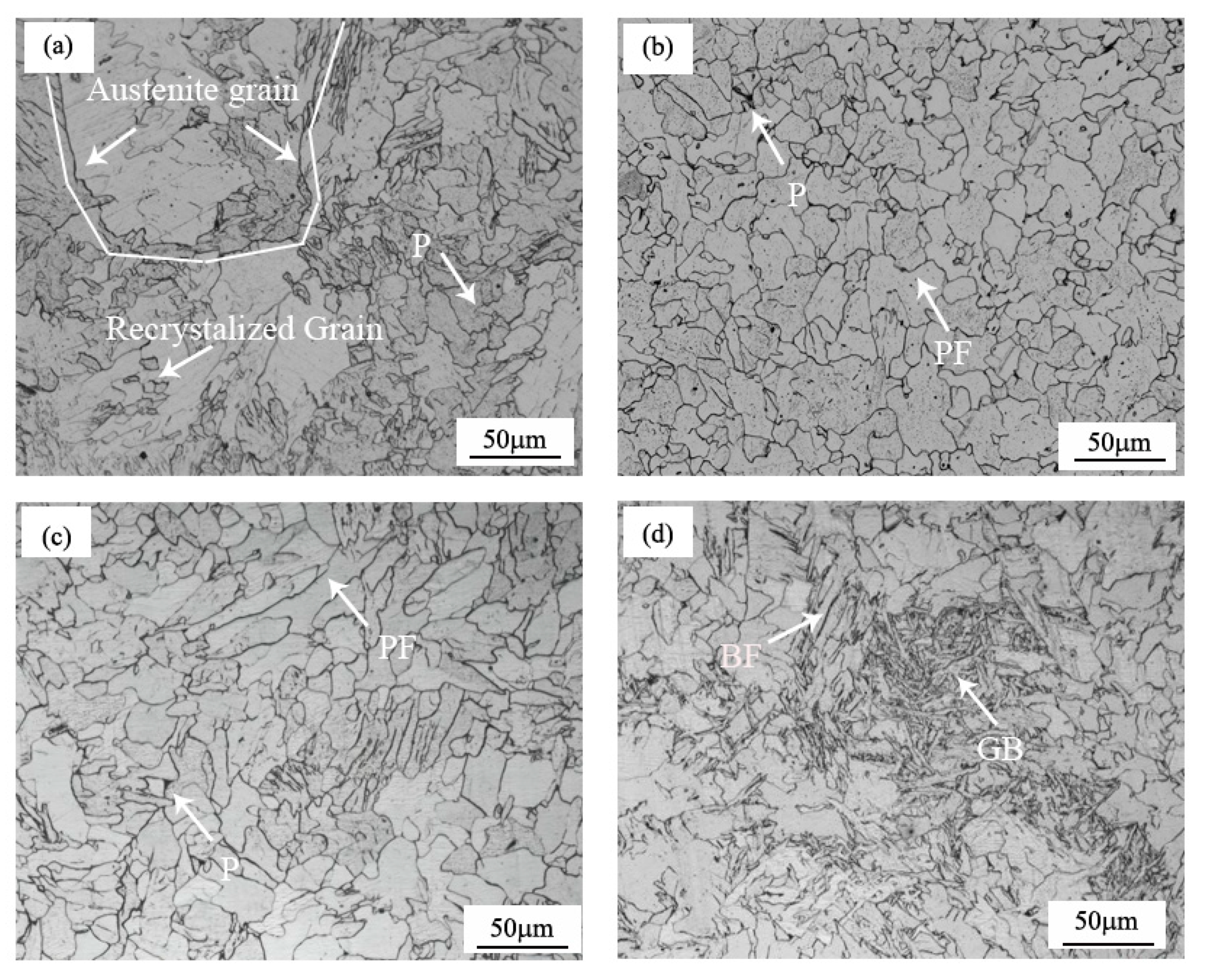
The microstructure of the samples varied with the different procedures, as shown in Figure 2. The microstructure of the UCS Q235BRE(C) sample was shown in Figure 2a. It could be seen that fine recrystallized grains were formed, and the prior austenite grain size was more than 150 µm, which was relatively coarse, compared with the fine austenite grain size produced during conventional hot rolled strip rolling. The coarse austenite grain size retarded the austenite transformation process, compared with conventional fine grained hot rolled strip steels [15]. Regardless of the addition of RE, the samples of UCS Q235BRE(R) and UCS Q235B(R) had a similar microstructure, both consisting of polygonal ferrite (PF) and a small amount of pearlite (P), as shown in Figure 2b,c. The volume fractions of the polygonal ferrite in these two samples were both 98%. The difference was that the grain size after RE alloying was significantly refined from 41μm in the sample of Q235B(R) to 24 μm in the sample of Q235BRE(R). Figure 2d shows the microstructure of the UCS SPA-H(R) samples, which consisted of 57% polygonal ferrite, 32% granular bainite (GB), and 11% bainitic ferrite (BF). The volume fraction of each constituent of the samples was estimated by using the analysis software Image-Pro and named according to the ISIJ transformation products nomenclature. The volume fractions of the different steels are shown in Table 2.
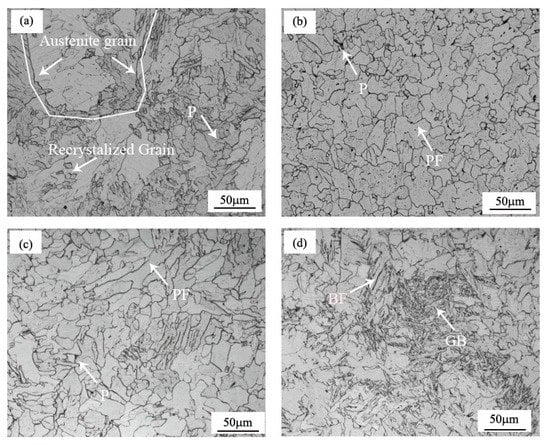
Figure 2.
Optical microstructure of (a) UCS Q235BRE(C); (b) UCS Q235BRE(R); (c) UCS Q235B(R); (d) UCS SPA-H.

Table 2.
Microstructure summary of the different samples.
3.2. Inclusion
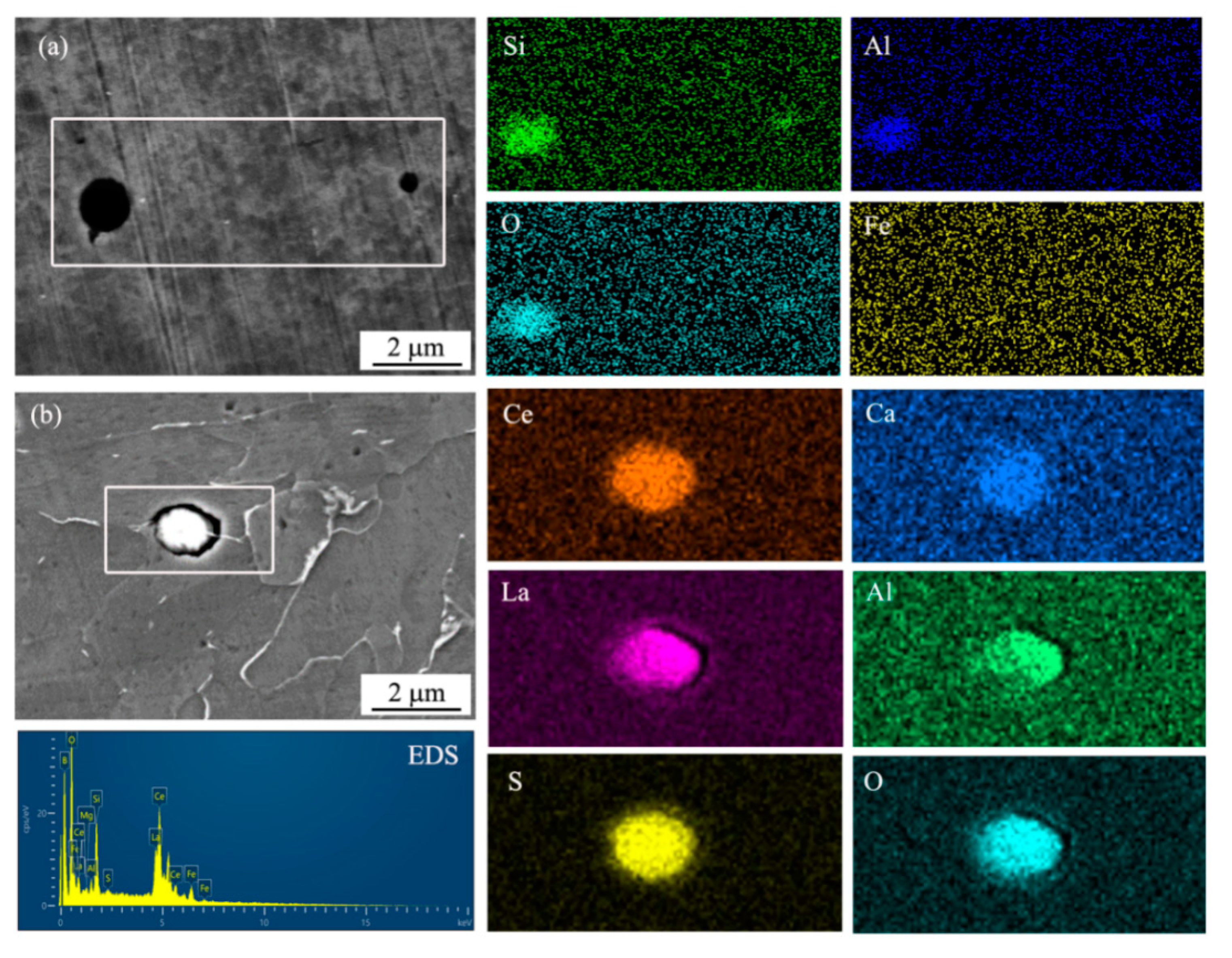
In order to determine the inclusion types, SEM observation and EDS mapping analysis of inclusions were conducted, as shown in Figure 3. The designation of chemical elements demonstrated the enrichment of the elements in the inclusions. It was found that the inclusion in UCS Q235B (R) steel was enriched with Si, Al, and O (Figure 3a). Ce, La, S, O, Ca, and Al are concentrated in the inclusion in UCS Q235BRE (R) steel in Figure 3b.
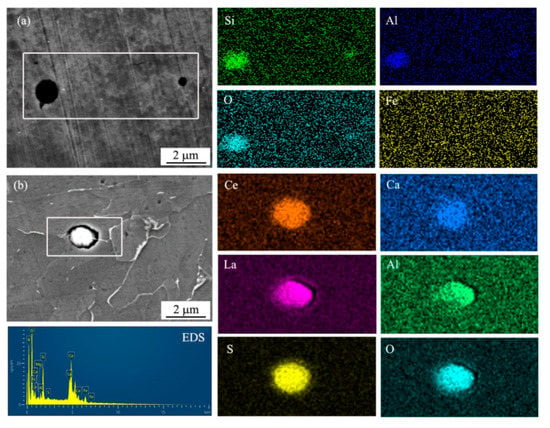
Figure 3.
Morphology of inclusions characterized by EDS mapping (a) in UCS Q235B(R) steel and (b) in UCS Q235BRE(R) steel.
The current steel produced by CASTRIP® procedure was designed to be a silicon-manganese steel with low carbon (<0.05%). The purpose of this alloy composition system aimed to ensure that the deoxidized products were composed of liquid MnO ans SiO2 to prevent the nozzle from clogging and improve the conductivity of the casting roll. It has been reported that rapid solidification can promote uniform distribution of spherical inclusions without deformation [16,17]. Therefore, the inclusions were composed of MnO, SiO2, and Al2O3 with an average size of less than 2 μm, as shown in Figure 3a. After RE alloying, the inclusion was modified into RE inclusions, although the size and shape had no obvious change, as indicated in Figure 3b.
3.3. Tensile Properties
The tensile properties of the steels conducted at room temperature were listed in Table 3. The addition of RE had no significant effect on the yield strength, ultimate strength, and elongation of steels.

Table 3.
Tensile strength of experimental steels.
3.4. Corrosion Properties
Weight loss measurements were performed in wet–dry immersion tests. In the weight loss method, the average corrosion rate of the steel was determined by the following expression [18]:
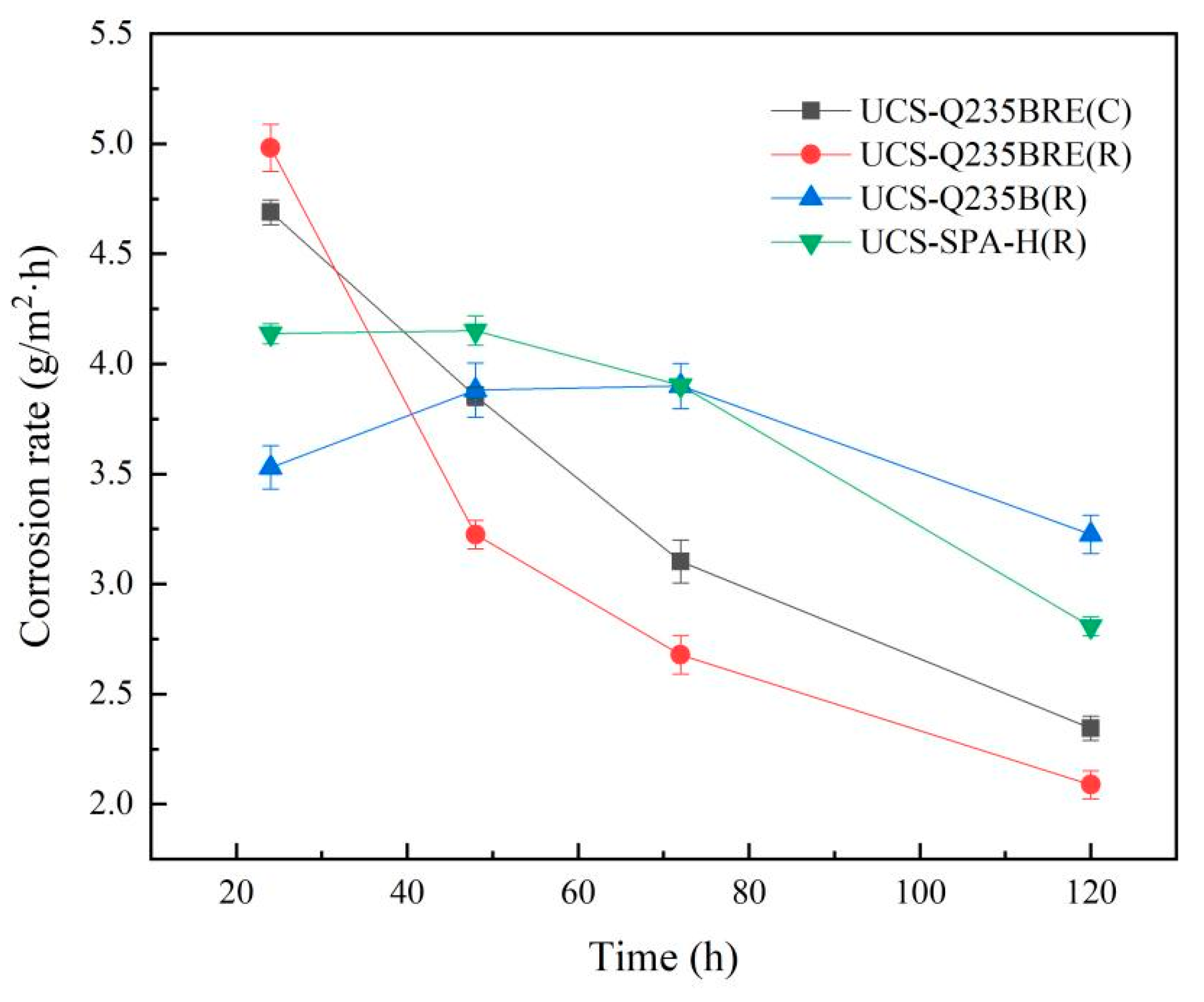
where G0 is initial sample weight (g), G1 is sample weight after immersion test (g), S the area of exposure (m2), and T the time of exposure (h). The results of weight loss measurements are given in Table 4. After immersion, the weight loss of the steels was ranked in the following order: UCS-Q235B(R) steel > UCS-SPA-H(R) steel > UCS-Q235BRE(C) steel > UCS-Q235BRE(RE) steel. Figure 4 shows the relationship between corrosion rate as a function of time obtained by wet–dry immersion tests.

Table 4.
Corrosion rates of experimental steels (g/m2·h).
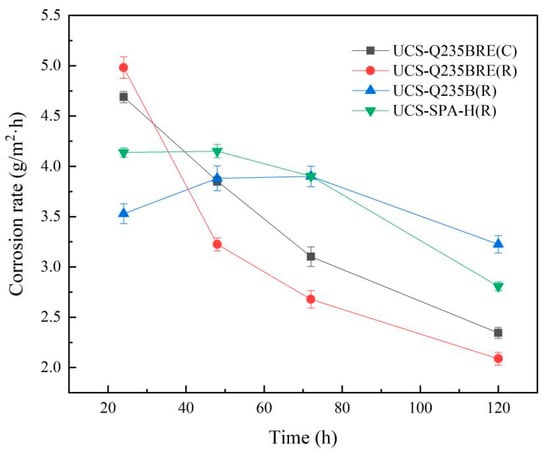
Figure 4.
Corrosion rate curves obtained in wet–dry immersion test.
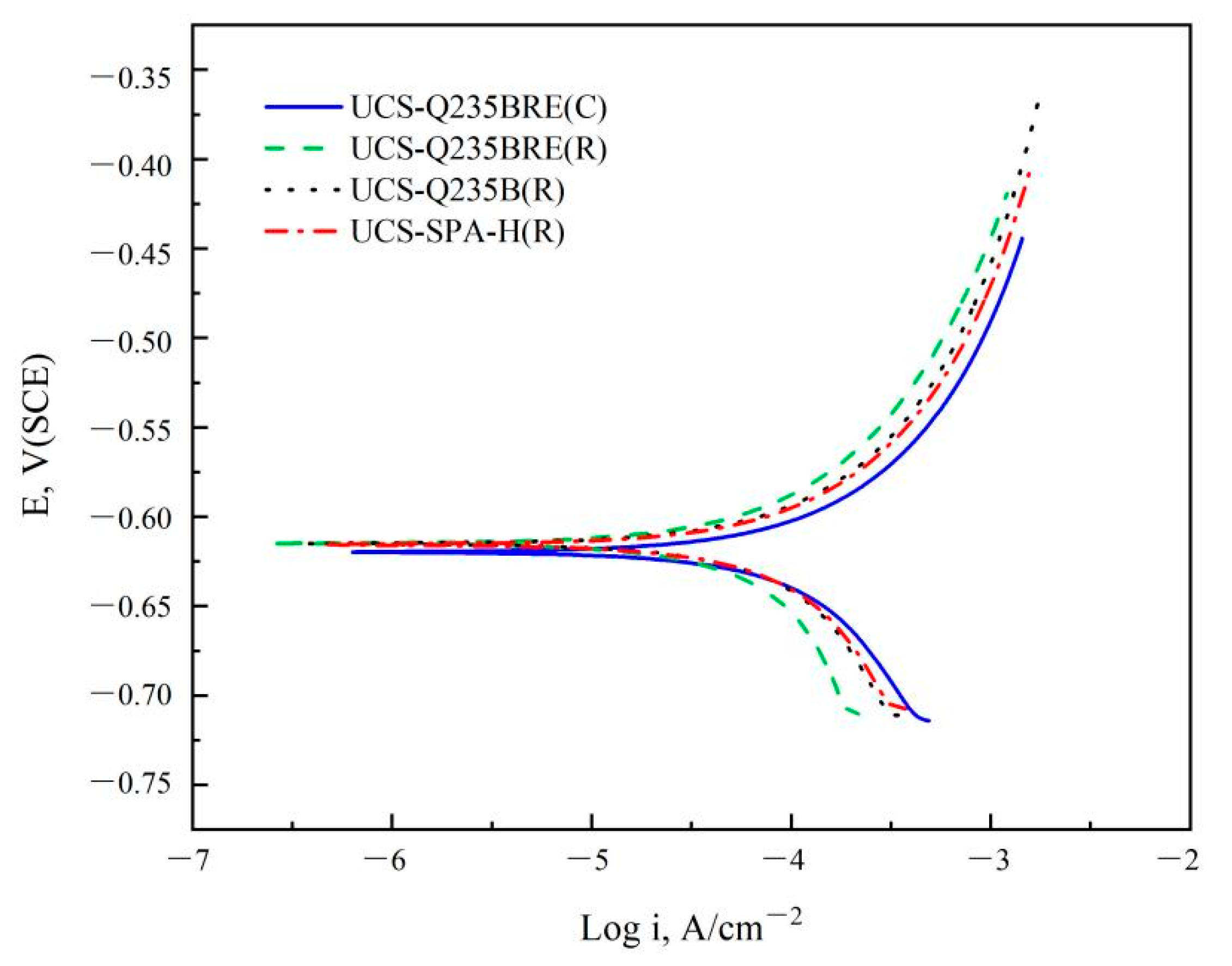
Figure 5 shows the potentiodynamic polarization curves of specimens in the solution of 3.5% NaCl at the temperature of 20 °C. The potentiodynamic polarization results were summarized in Table 5, which exhibited the beneficial effect of RE. All steels demonstrated active corrosion behavior and no passivation, indicating that the anodic current density continuously increased with increasing potential. The curves indicated that the addition of RE barely influenced the corrosion potential, while increasing RE content decreased the corrosion current density.
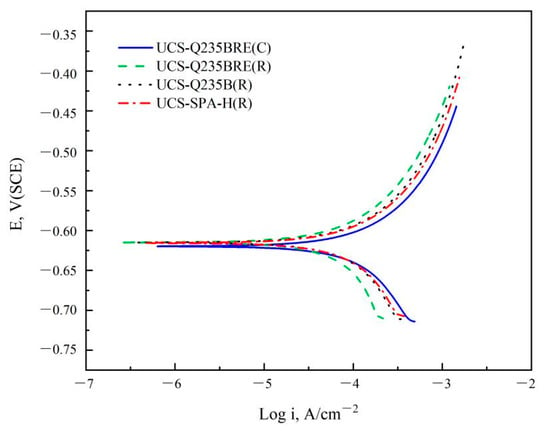
Figure 5.
Potentiodynamic polarization curves of specimens in the solution of 3.5% NaCl at a temperature of 20 °C.

Table 5.
Electrochemical parameters of potentiodynamic measurements in the solution of 3.5% NaCl at a temperature of 20 °C.
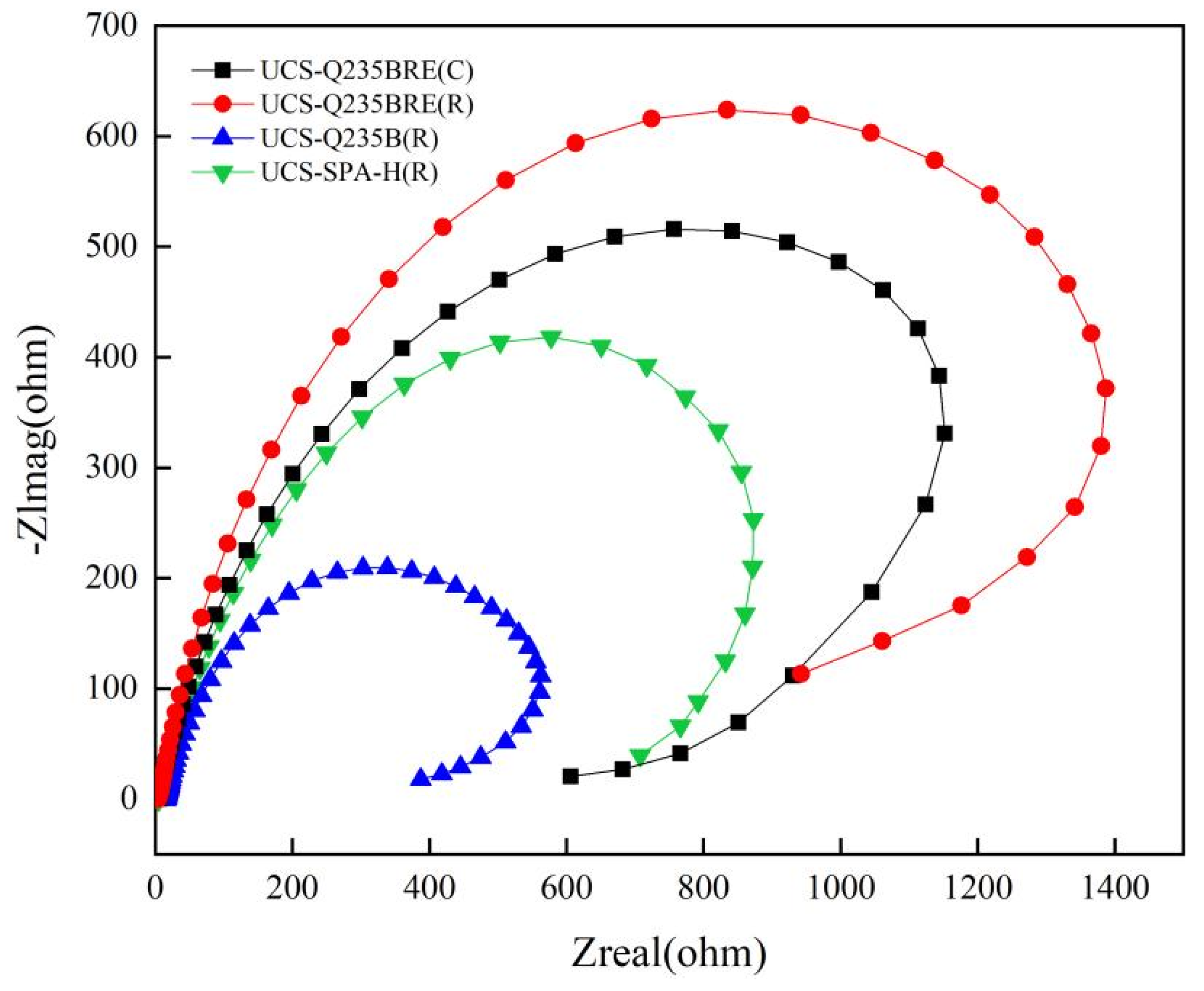
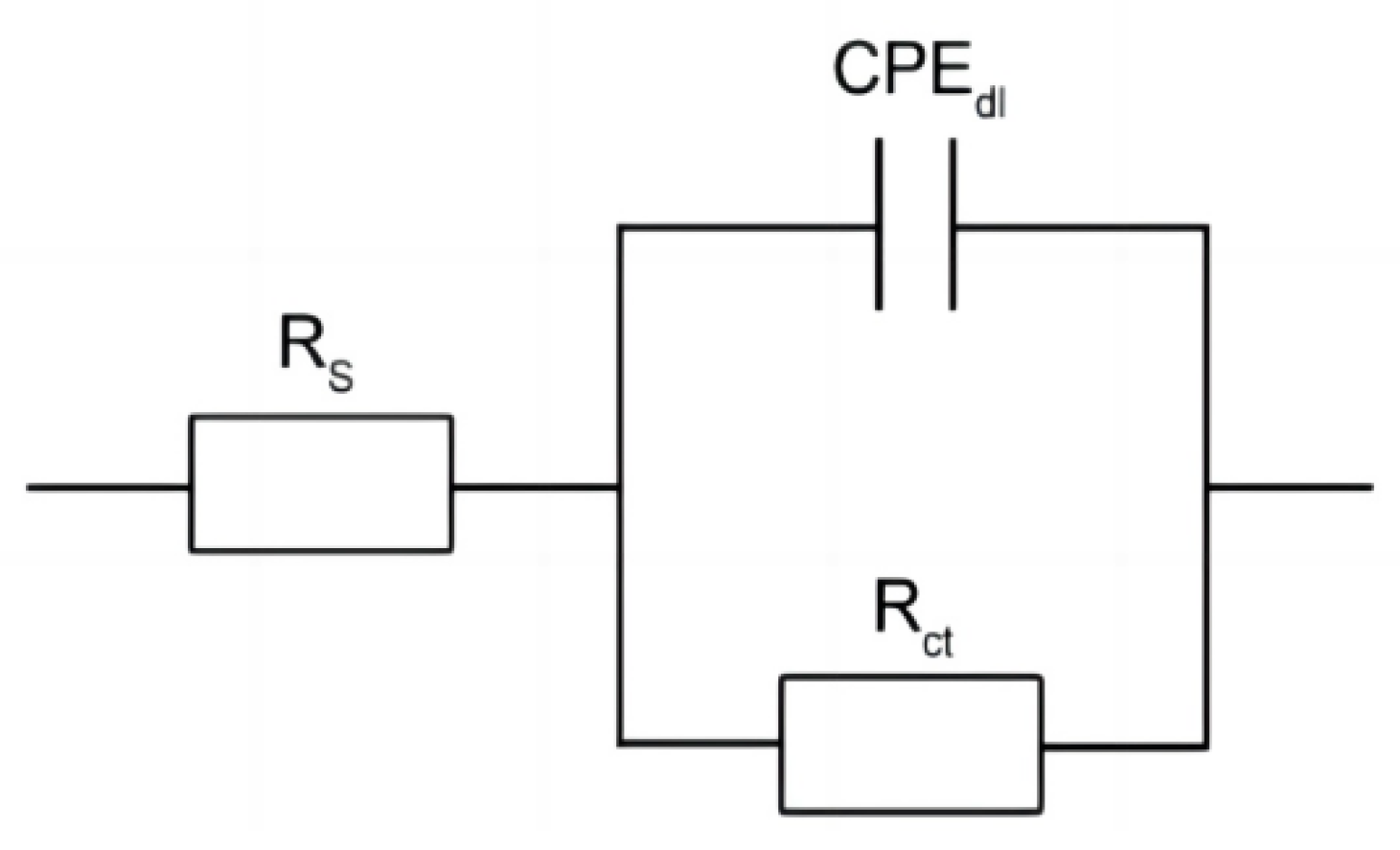
The Nyquist and Bode plots under open-circuit potential were presented in Figure 6. The impedance spectra of the steels showed a single semicircle [19,20]. Based on the above impedance spectrum features, the physical model and equivalent circuit for fitting the EIS data are offered in Figure 7. A solution resistance Rs of the test electrolyte between the working electrode, reference electrode, and charge transfer resistance Rct are presented. A constant phase element (CPE) represents the capacitance to allow for depressed semicircles.
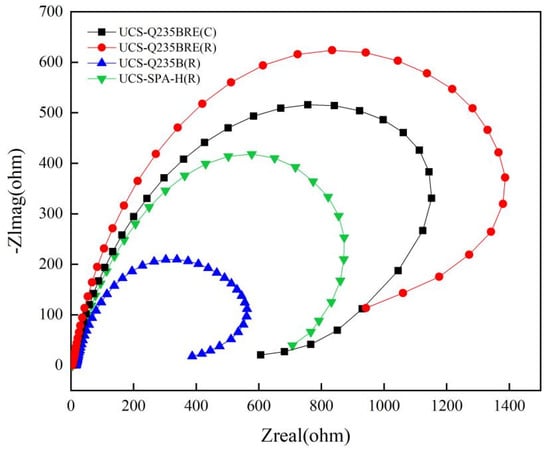
Figure 6.
Nyquist plots for specimens.
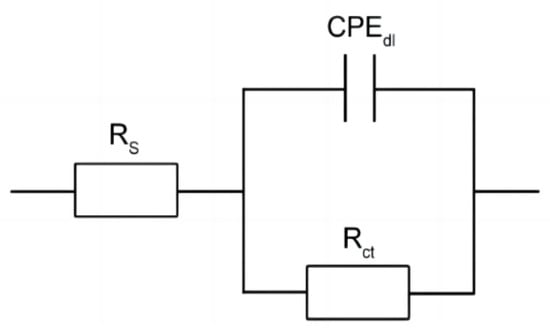
Figure 7.
Equivalent circuit for EIS data fitting in the solution of 3.5% NaCl.
Table 6 presents the fitted EIS data obtained by the ZSimpWin program. The corrosion procedure of steels was controlled by the charge transfer resistance (Rct). The increase in diameter of the arc in Nyquist plots indicated that the corrosion resistance was improved. Obtained from Figure 6, the value of Rct was completely increased with the addition of RE.

Table 6.
Impedance parameters of specimens.
4. Discussion
4.1. Effect of RE on Microstructure
With the decrease of temperature, an irregular film or ferrite grain boundary was formed at the austenite grain boundary during the process of CASTRIP®. Then, nucleation began at the interface between α and γ and extended into the undeformed region. In addition, the cooling caused acicular ferrite to nucleate preferentially at the places around inclusions and other interfaces [21,22,23]. The key factors affecting the nucleation of acicular ferrite include the type and size of inclusions, the size of the austenite grains, the hardenability of steel, and the cooling rate. Larger grains with sizes of 0.5–5 μm promoted the inner nucleation of the acicular ferrite, which was beneficial to the formation of the final microstructure, without damaging the formability of the strip.
Oxygen and sulfur were captured by rare earth elements to form RE-inclusions, which could become the non-spontaneous nucleus in the steels, making the equiaxed crystal grains refined [24,25]. In addition, the solubility of rare earths in the solid phase was very small. Once the molten steel solidifies, rare earth elements segregate at the front of solid–liquid interface in the liquid phase, preventing the growth of crystals and leading to grain refinement. Therefore, the microstructure was more uniform, with a smaller grain size in UCS-Q235BRE(R) steel than that in UCS-Q235B(R) steel.
4.2. Relationship between Corrosion Properties and Microstructure
The corrosion process of steel was essentially an electrochemical process. Improving the uniformity of microstructure was equivalent to reducing the potential difference between the anode and cathode of corrosion cell, which led to the reduction of corrosion rate, especially slowing down the trend of local corrosion [26,27]. It can be seen from the metallographic photograph in Figure 2 that the microstructure of UCS-Q235BRE(R) steel was a uniform single PF with a small amount of P. There were no obvious potential differences between phases. The grain refinement caused by RE resulted in the dilution of segregated alloying elements at GBs, thus alleviating the intergranular corrosion, which was responsible for the improvement of the corrosion behavior. UCS-SPA-H(R) steel was a composite structure of PF, GB, and BF. The potential difference between phases was relatively high, although several weathering-resistant elements, such as Cu, P, Cr, and Ni, were added into UCS-SPA-H(R) steel. The improvement of corrosion resistance by alloying elements was mainly reflected in the following three aspects, including reducing the conductive property of the rust layer and changing the type and structure of the corrosion products, accelerating the uniform dissolution of matrix and conversion from Fe2+ to Fe3+ and blocking the cracks in rust layer. Under the special process of CASTRIP®, the element alloying led to a multi-structure, which negated the effect of weathering-resistant elements on the corrosion performance [28]. In other words, the uniform structure contributed more to corrosion resistance, compared to certain alloying elements.
From the results of this study, it could be seen that the corrosion resistance of low alloy steel was mainly determined by the influence of the microstruture. In low alloy steel, the content of alloying elements was too low to have a direct effect on corrosion process, while the effect of the microstructure became prominent.
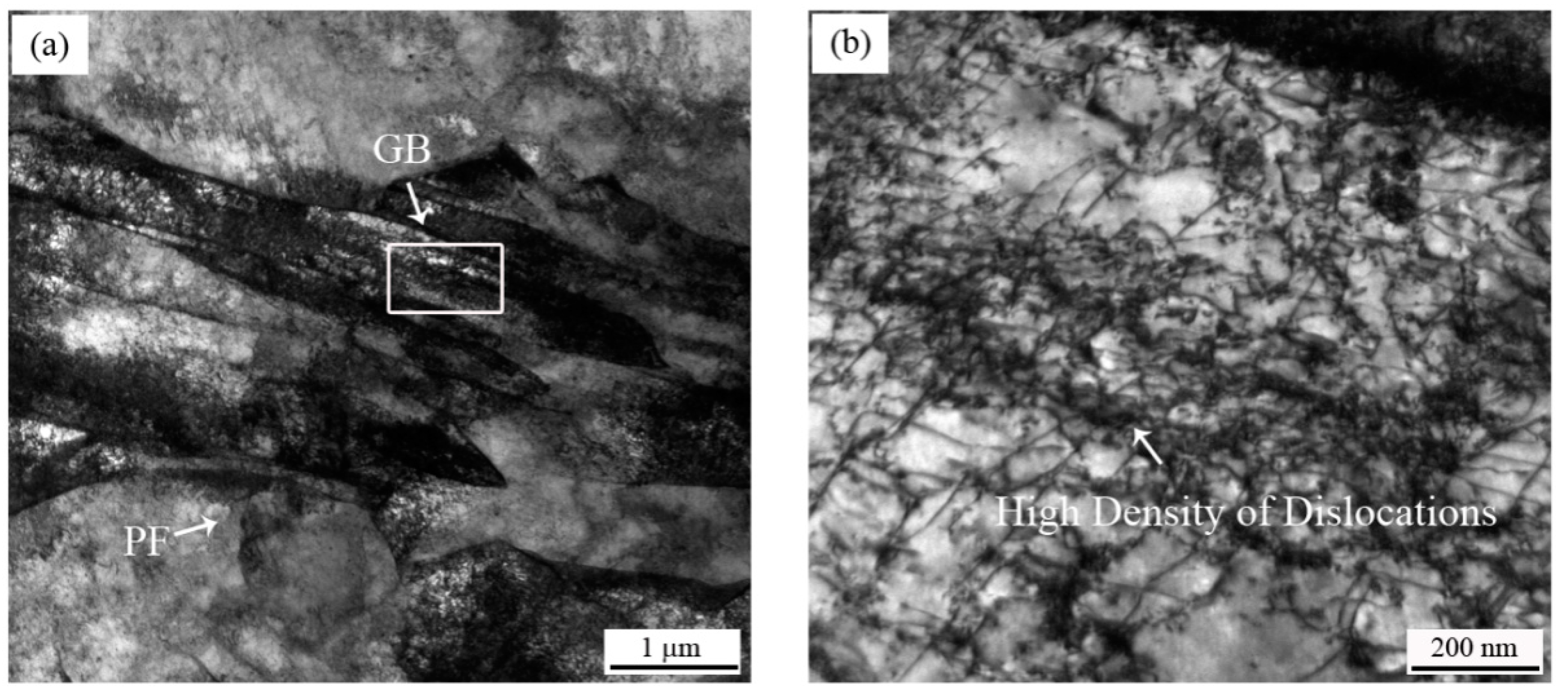
Due to the high solidification rate of the CASTRIP® process, it was almost difficult to observe the MnS inclusions. The alloying elements had no time to diffuse and precipitate into inclusions. As a result, the corrosion resistance of the material was improved. Additionally, the grain boundaries and phase boundaries also had a great influence on the grain boundary corrosion and pitting corrosion involving precipitation and element segregation at the grain boundaries. It has been reported that the increase of appropriate dislocation density shortened the migration and diffusion paths alloying elements, promoting the rapid formation of protective rust layers [29,30]. However, the influence of grain boundaries on corrosion behavior of steel was complex and controversial. In our work, when the microstructure of PF changes to GB + BF, a large number of dislocations were formed, resulting in a large number of element aggregation at the dislocations, as indicated by Figure 8. Therefore, high density dislocations formed local corrosive galvanic cells and accelerated the corrosion rate, resulting in poor corrosion resistance.
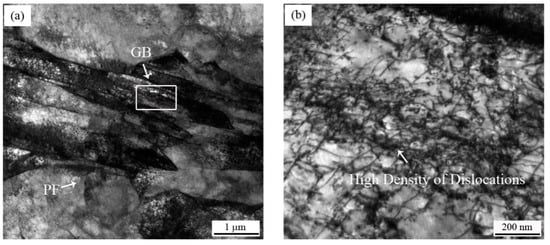
Figure 8.
(a) Morphology of PF and GB in UCS-Q235BRE(R) steel; (b) high magnification of dislocations in GB, framed by a white box.
Given the correlations between the regions of high dislocation density and the formation of chemical heterogeneities known to degrade corrosion performance, our findings demonstrated the impact of the microstructural state on the resistance of uniform and localized corrosion.
5. Conclusions
The microstructure produced by cast strip steel mainly consisted of homogeneous polygonal ferrite with a small amount of pearlite, while multiple alloying led to the emergence of granular bainite and bainitic ferrite. Rare earth elements strongly promoted the homogenization and refinement of the microstructure.
Potentiodynamic polarization, EIS, and weight loss under wet–dry immersion test formed an agreement, confirming that the corrosion behavior was significantly improved by the addition of rare earth elements. UCS-Q235BRE(R) steel provided the best set of corrosion properties and was promising for future use.
The corrosion resistance of the homogeneous single-phase microstructure was proven to be better than that of the multiphase microstructure. Rare earth elements had remarkable influence on improving corrosion resistance when experimental steels processed a single-phase microstructure.
Author Contributions
Writing—original draft preparation, X.L.; conceptualization, H.Z.; investigation, L.C. and T.L.; methodology, Q.F.; resources, H.L.; funding, Y.S.; supervision, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Jiangsu Shagang Iron and Steel Research Institute.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maleki, A.; Taherizadeh, A.; Hosseini, N. Twin roll casting of steels: An overview. ISIJ Int. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Campbell, P.; Blejde, W.; Mahapatra, R.; Wechsler, R.; Gillen, G. The castrip® process—Direct casting of steel sheet at nucor crawfordsville. Iron Steel Technol. 2005, 2, 56–62. [Google Scholar]
- Choo, D.K.; Moon, H.K.; Kang, T.W.; Lee, S.H. Analysis and prevention of cracking during strip casting of AISI 304 stainless steel. Metall. Mater. Trans. A 2001, 32, 2249–2258. [Google Scholar] [CrossRef]
- Lindenberg, H.; Henrion, J.; Schwaha, K.; Vespasiani, G. Eurostrip-State of the art of strip casting. Rev. Métallurgie 2002, 99, 615–627. [Google Scholar] [CrossRef]
- Campbell, P.; Mahapatra, R.; Blejde, W.; Wechsler, R.; Gillen, G. The CASTRIP® Process: Progress towards commercial strip casting at Nucor Crawfordsville. Rev. Metall.-Cah. D Inf. Tech. 2006, 103, 25–31. [Google Scholar] [CrossRef]
- Killmore, C.R.; Edelman, D.G.; Carpenter, K.R.; Kaul, H.R.; Williams, J.G.; Campbell, P.C.; Blejde, W.N. Recent Product Developments with Ultra-Thin Cast Strip Products Produced by the CASTRIP® Process. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2010; Volume 654–656, pp. 19–201. [Google Scholar]
- Killmore, C.R.; Phillips, A.; Kaul, H.R.; Williams, J.G.; Creely, H.; Campbell, P.C.; Schueren, M.A.; Blejde, W.N. Development of ultrathin cast strip products by the CASTRIP® process. Iron Steel Technol. 2007, 4, 90–105. [Google Scholar]
- Duan, H.; Shan, Y.Y.; Yang, K.; Shi, X.B.; Yan, W.; Ren, Y. Effect of Rare Earth and Cooling Process on Microstructure and Mechanical Properties of an Ultra-Cleaned X80 Pipeline Steel. Acta Metall. Sin. Engl. 2021, 34, 639–648. [Google Scholar] [CrossRef]
- Deng, X.X.; Jiang, M.; Wang, X.H. Mechanisms of inclusion evolution and intra-granular acicular ferrite formation in steels containing rare earth elements. Acta Metall. Sin. Engl. 2012, 25, 241–248. [Google Scholar]
- Ha, H.Y.; Park, C.J.; Kwon, H.S. Effects of misch metal on the formation of non-metallic inclusions and the associated resistance to pitting corrosion in 25% Cr duplex stainless steels. Scr. Mater. 2006, 55, 991–994. [Google Scholar] [CrossRef]
- Wang, L.M.; Lin, Q.; Ji, J.W.; Lan, D.N. New study concerning development of application of rare earth metals in steels. J. Alloys Compd. 2006, 408–412, 384–386. [Google Scholar] [CrossRef]
- Liu, H.L.; Liu, C.J.; Jiang, M.F. Effects of Rare Earths on the Austenite Recrystallization Behavior in X80 Pipeline Steel. Adv. Mater. Res. 2010, 129–131, 542–546. [Google Scholar] [CrossRef]
- Wu, Y.M.; Wang, L.M.; Du, T. Thermodynamics of rare earth elements in liquid iron. J. Less-Common Met. 1985, 110, 187–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Liang, J.H.; Pei, W.; Liu, P.F.; Zhang, W.; Zhao, A.M. Effect of rare earth elements on the segregation behavior and microstructure of super austenitic stainless steel. J. Mater. Res. Technol. 2022, 19, 20–29. [Google Scholar] [CrossRef]
- Carpenter, K.R.; Killmore, C.R. The Effect of Nb on the Continuous Cooling Transformation Curves of Ultra-Thin Strip CASTRIP© Steels. Metals 2015, 5, 1857–1877. [Google Scholar] [CrossRef]
- Zapuskalov, N. Comparison of Continuous Strip Casting with Conventional Technology. ISIJ Int. 2003, 43, 1115–1127. [Google Scholar] [CrossRef]
- Ge, S.; Isac, M.; Guthrie, R.I.L. Progress of strip casting technology for steel; Historical developments. ISIJ Int. 2012, 52, 2109–2122. [Google Scholar] [CrossRef]
- Lian, X.T.; Chen, L.; Fan, Z.W.; Liu, T.S.; Xu, D.X.; Dong, H. Effects of modified inclusions and precipitates alloyed by rare earth element on corrosion and impact properties in low alloy steel. Acta Metall. Sin. (Engl. Lett.) 2022, 35, 1719–1730. [Google Scholar] [CrossRef]
- Liu, Z.; Lian, X.T.; Liu, T.S.; Yang, Y.D.; Zhu, J.N.; Dong, H. Effects of rare earth elements on corrosion behaviors of low-carbon steels and weathering steels. Mater. Corros. 2020, 71, 258–266. [Google Scholar] [CrossRef]
- Wen, J.; Cui, H.; Wei, N.; Song, X.; Zhang, G.; Wang, C.; Song, Q. Effect of phase composition and microstructure on the corrosion resistance of Ni-Al intermetallic compounds. J. Alloys Compd. 2017, 695, 2424–2433. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Carpenter, K.; Chen, Z.X.; Killmore, C. The effect of cooling rate and coiling temperature on the niobium retention in Ultra-Thin Cast Strip steel. Mater. Sci. Eng. A 2017, 700, 234–240. [Google Scholar] [CrossRef]
- Wits, J.J.; Kop, T.A.; Leeuwen, Y.M.; Sietsma, J.; Zwaag, S. A study on the austenite-to-ferrite phase transformation in binary substitutional iron alloys. Mater. Sci. Eng. A 2000, 283, 234–241. [Google Scholar] [CrossRef]
- Mecozzi, M.G.; Sietsma, J.; Zwaag, S. Analysis of γ → α transformation in a Nb micro-alloyed C–Mn steel by phase field modelling. Acta Mater. 2006, 54, 1431–1440. [Google Scholar] [CrossRef]
- Fu, H.G.; Xiao, Q.; Li, Y.X. A study of the microstructures and properties of Fe-V-W-Mo alloy modified by rare earth. Mater. Sci. Eng. A. 2005, 395, 281–287. [Google Scholar] [CrossRef]
- Yue, L.J.; Wang, L.M.; Han, J.S. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Cai, G.J.; Li, C.S. Effects of Ce on Inclusions, Microstructure, Mechanical Properties, and Corrosion Behavior of AISI 202 Stainless Steel. J. Mater. Eng. Perform. 2015, 24, 3989–4009. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tanaka, R.; Minamigawa, M.; Kandori, K.; Tanaka, H.; Nakayama, T. Assessment of rust layers formed on weathering steel in saline environment by gas adsorption. Mater. Corros. 2015, 66, 1460–1466. [Google Scholar] [CrossRef]
- Almeida, E.; Morcillo, M.; Rosales, B.; Marrocos, M. Atmospheric corrosion of mild steel. Part I-Rural and urban atmospheres. Mater. Corros. 2000, 51, 859–864. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Jin, J.; Liu, M.; Li, X.G. Optimizing the nickel content in weathering steels to enhance their corrosion resistance in acidic atmospheres. Corros. Sci. 2017, 115, 135–142. [Google Scholar] [CrossRef]
- Avci, R.; Davis, B.H.; Wolfenden, M.L.; Beech, I.B.; Lucas, H.; Paul, D. Mechanism of MnS-mediated pit initiation and propagation in carbon steel in an anaerobic sulfidogenic media. Corros. Sci. 2013, 76, 267–274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).