Improving Corrosion and Stress Corrosion Cracking Performance of Machined Biodegradable Alloy ZX20 by HF-Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Specimens
2.3. HF-Treatment
2.4. Mechanical Testing
2.5. Microscopy
2.6. X-ray Diffraction
2.7. Immersion Testing
2.8. Cell Viability
2.8.1. Cell Culture
2.8.2. Extract Preparation
2.8.3. Antiproliferative Assay
3. Results
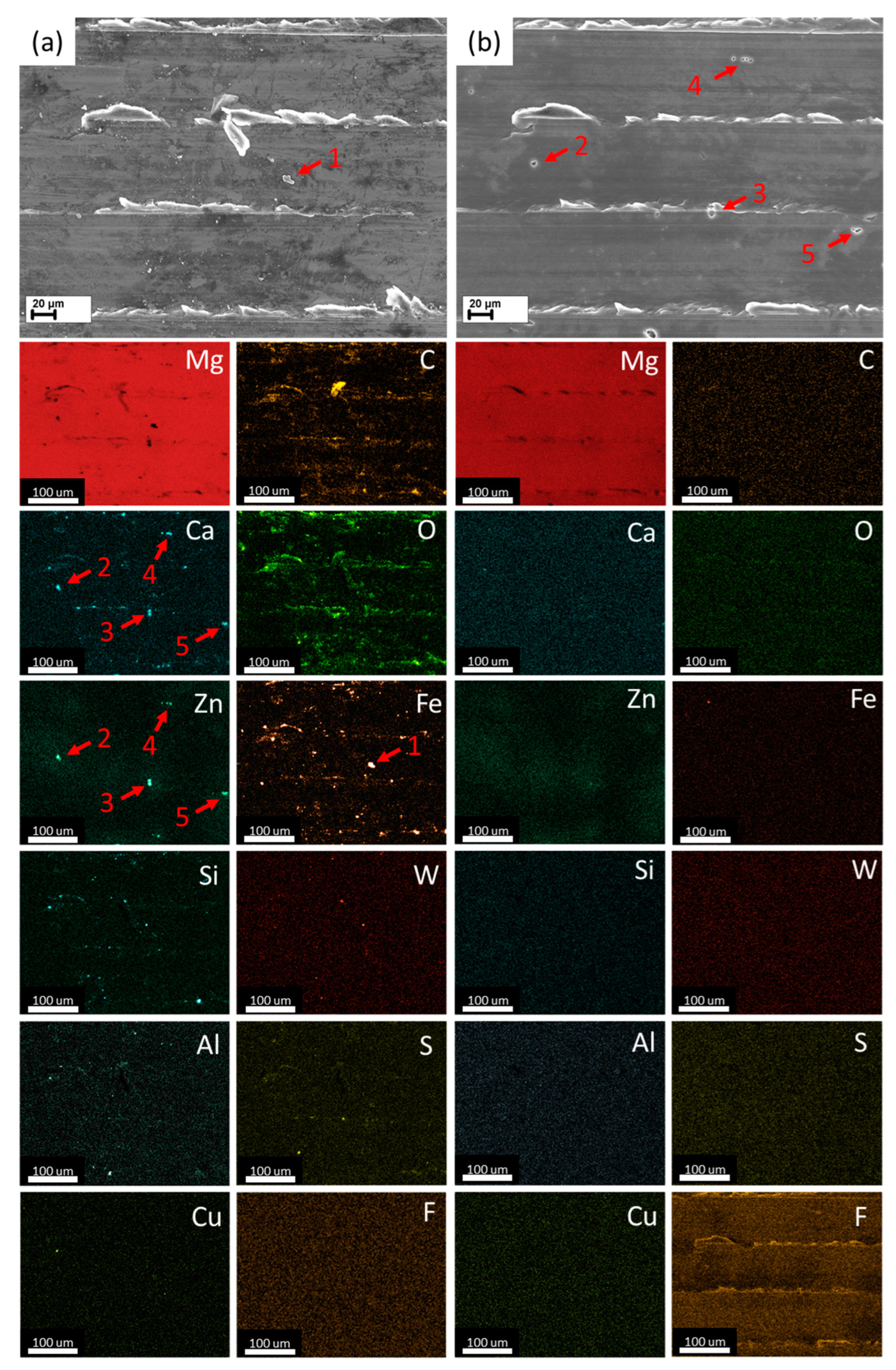
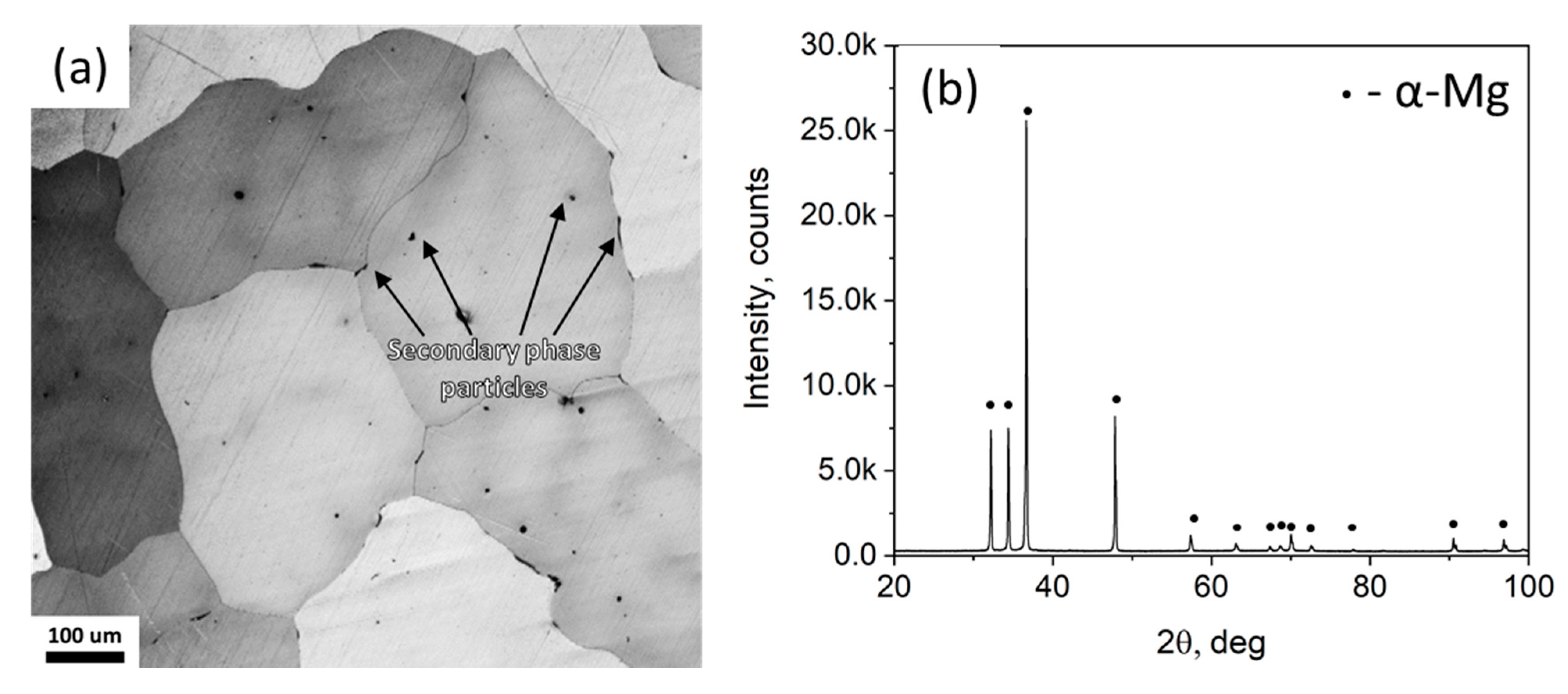
3.1. Effect of the HF-Treatment on the Side Surface and Microstructure
3.2. Cell Viability
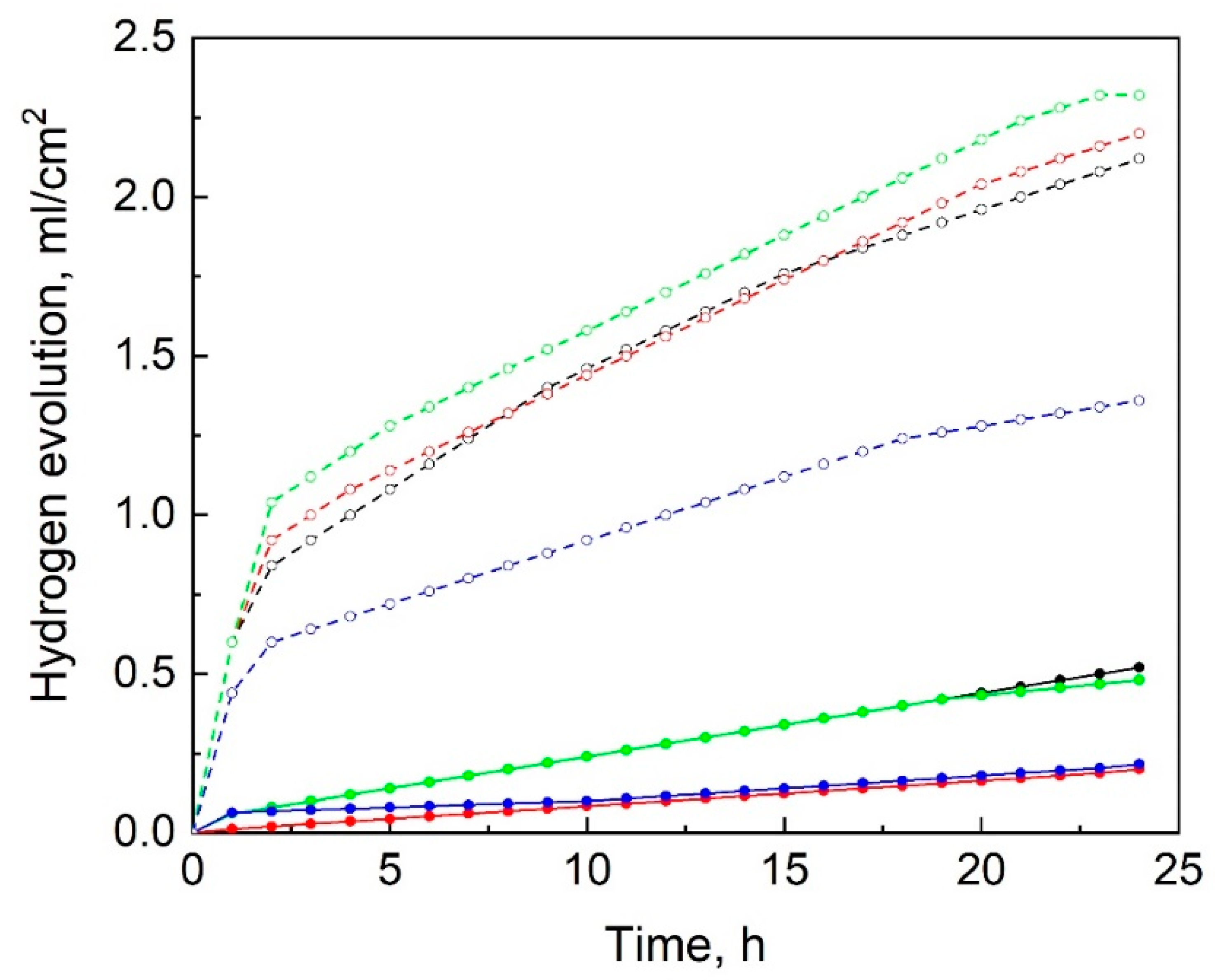
3.3. Immersion Testing
3.4. Mechanical Testing
3.5. Microscopic Examination of the Tensile-Tested Specimens
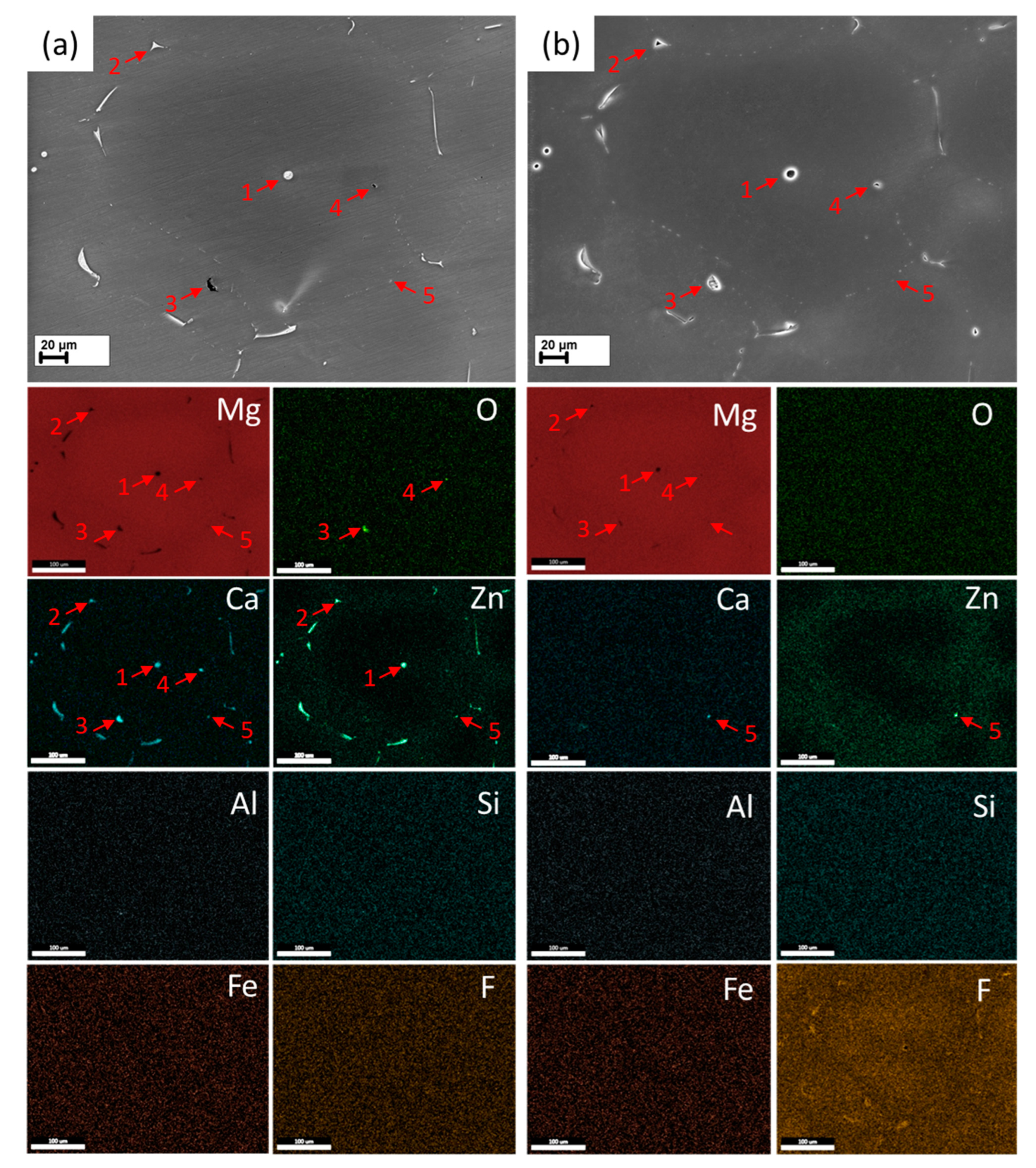
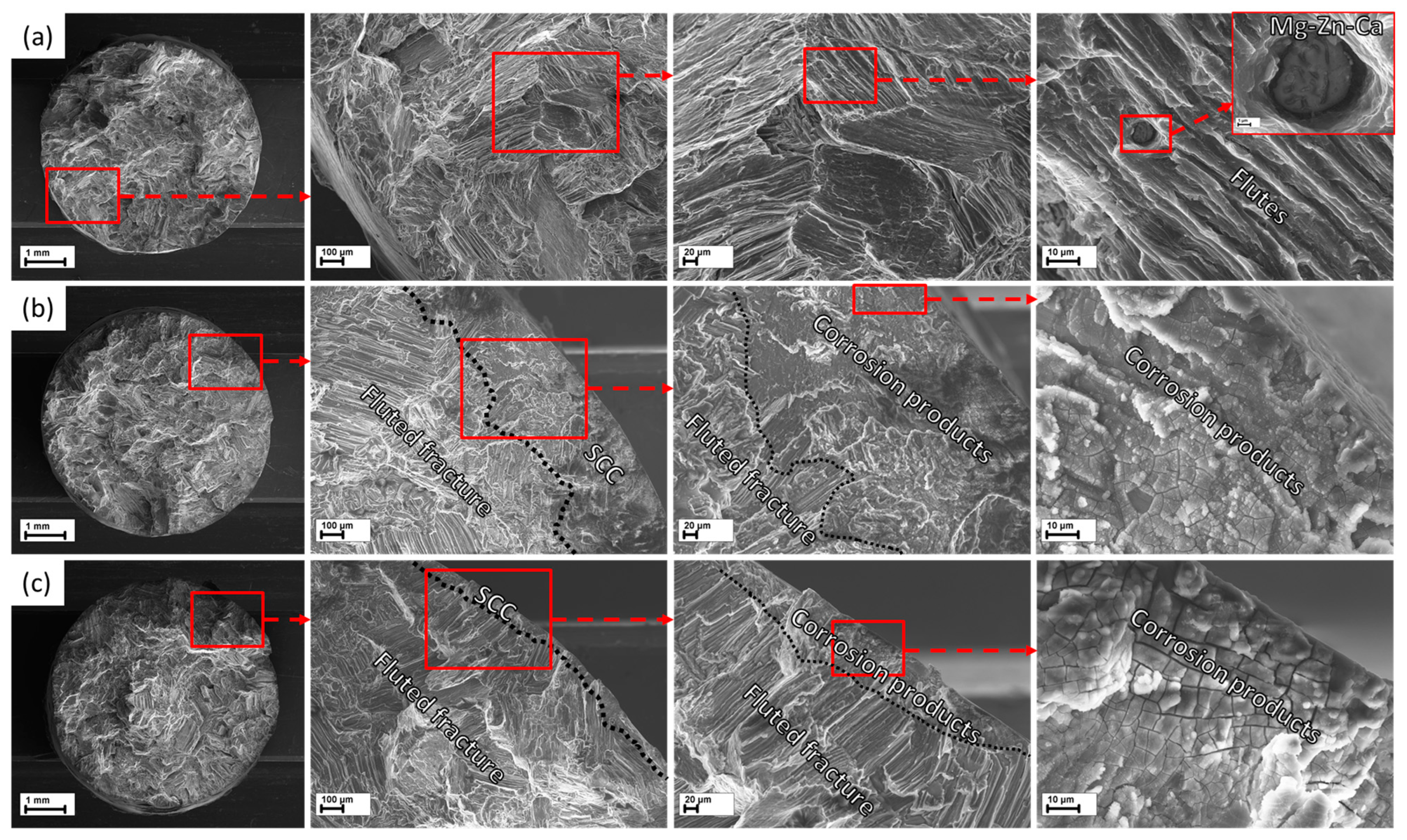
3.5.1. Fracture Surface
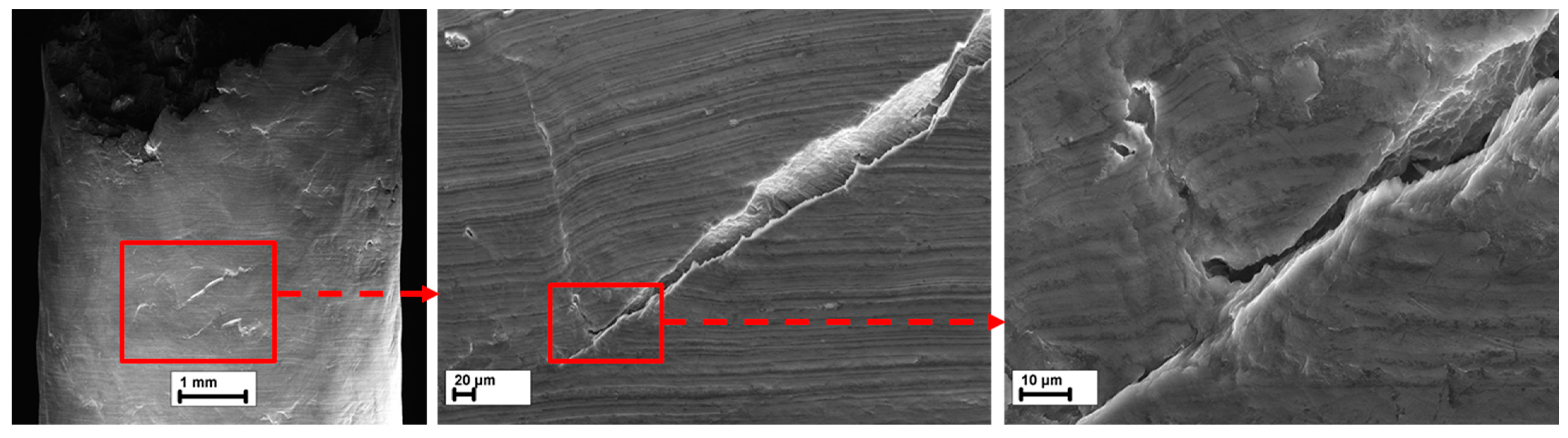
3.5.2. Side Surface
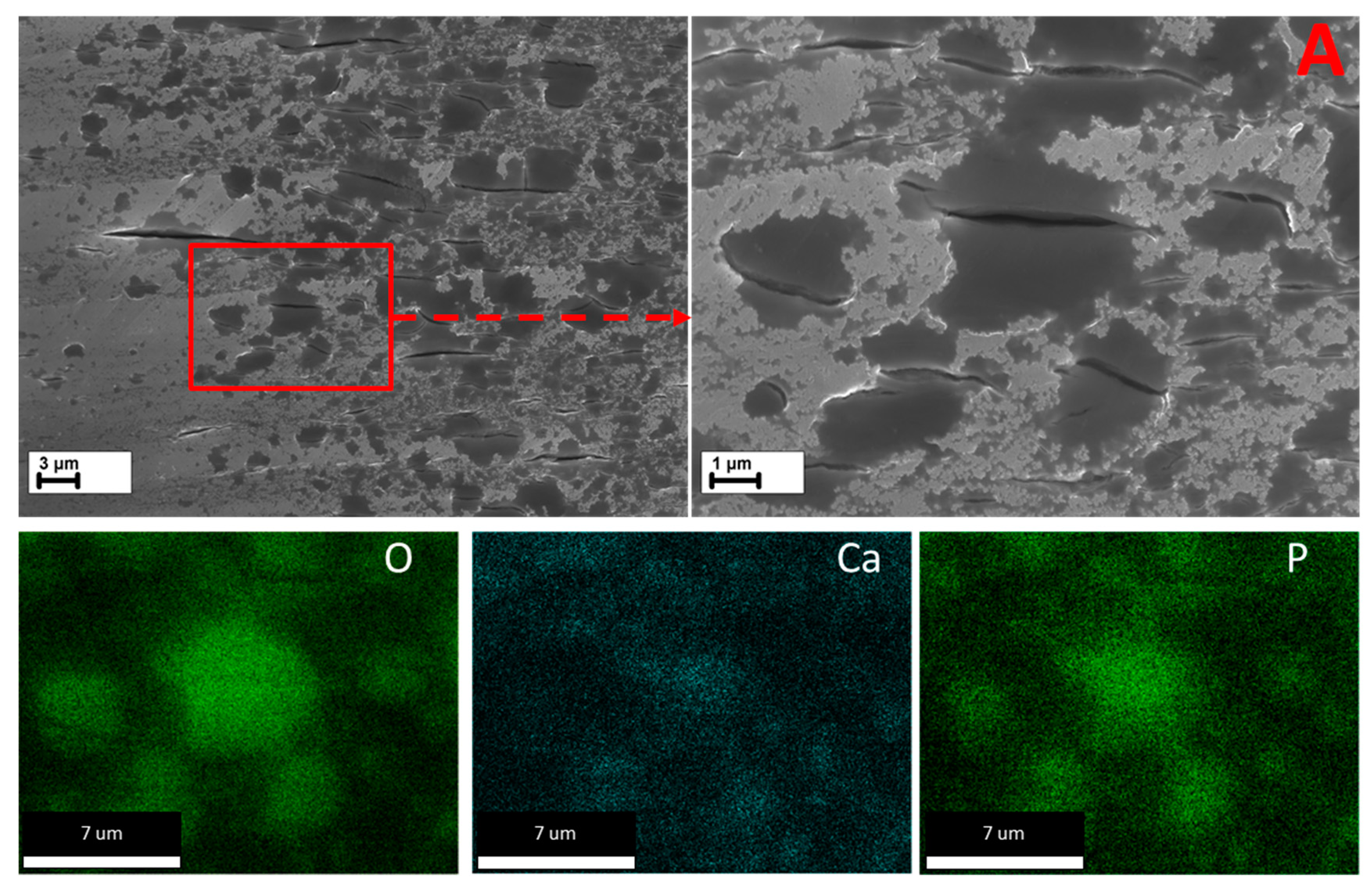
3.5.3. Cross-Sections
4. Discussion
4.1. Effect of HF-Treatment on the Corrosion Process
4.2. Effect of HF-Treatment on Stress Corrosion Cracking
4.3. The Methodological Aspects That Need Attention
5. Conclusions
- The immersion and SSRT testing showed that ultrasonic 15-min-long HF-treatment does not affect the cytotoxicity and significantly improves corrosion and SCC resistance of the machined specimens of the ZX20 alloy, as is witnessed by the decrease in their corrosion rate from 2.7 ± 0.3 down to 0.8 ± 0.1 mm/year and by reduction in their ductility and strength loss from 53 ± 2 to 36 ± 5% and from 36 ± 3 to 20 ± 3% respectively.
- According to the side surface and microstructure examinations, the observed increase in corrosion and SCC performance is associated with the three effects induced by the HF-treatment, namely the following: (1) formation of the MgF2 protective film, (2) removal of the surficial contaminating particles and films originated from the sample preparation procedures such as machining, and (3) removal of the surficial secondary phase particles including binary (Mg, Zn)2Ca and ternary Ca2Mg6Zn3 ones.
- In both the reference and HF-treated specimens, the mechanism of SCC was similar in that the cracks originated in the side surface corrosion products layer at the bottom of corrosion pits and propagated across the bodies and boundaries of the grains and secondary phase particles with no pronounced influence from the latter on the path and origination points of the crack.
- The increase in the SCC resistance of the HF-treated specimens is chiefly attributed to their increased corrosion resistance, providing fewer corrosion pits and products and, thus, reducing potential nucleation sites for stress corrosion cracks.
- Exerting no cytotoxic effect, the HF-treatment has been proven to be used as an efficient finishing preparation procedure prior to corrosion or SCC testing as well as before sterilization or coating of the biodegradable implants made of Mg alloys.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-Based Composites and Alloys for Medical Applications: A Review of Mechanical and Corrosion Properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.L.; Chen, X.; Pan, F. Review of Mg Alloy Corrosion Rates. J. Magnes. Alloys 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Shi, Z.; Soltan, A.; Johnston, S.; Dargusch, M.S. Understanding the Corrosion of Mg and Mg Alloys; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128098943. [Google Scholar]
- Song, Y.; Fu, H.; Wang, Z.; Yang, P. Stress Corrosion Cracking of Magnesium Alloys: Mechanism, Influencing Factors, and Prevention Technology. Mater. Rep. 2019, 33, 834–840. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A Critical Review of the Stress Corrosion Cracking (SCC) of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Singh Raman, R.K. A Review of Stress-Corrosion Cracking and Corrosion Fatigue of Magnesium Alloys for Biodegradable Implant Applications. JOM 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Gao, C.; Li, S.; Liu, L.; Bin, S.; Yang, Y.; Peng, S.; Shuai, C. Dual Alloying Improves the Corrosion Resistance of Biodegradable Mg Alloys Prepared by Selective Laser Melting. J. Magnes. Alloys 2021, 9, 305–316. [Google Scholar] [CrossRef]
- Kiani, F.; Lin, J.; Vahid, A.; Munir, K.; Wen, C.; Li, Y. Microstructures, Mechanical Properties, Corrosion, and Biocompatibility of Extruded Mg-Zr-Sr-Ho Alloys for Biodegradable Implant Applications. J. Magnes. Alloys 2023, 11, 110–136. [Google Scholar] [CrossRef]
- Cao, X.; Li, C.; Wu, B.; Liang, M. Research of Dynamic Corrosion Behavior, Microstructure, and Biocompatibility of Mg–Zn–Ca–Zr Alloys in Simulated Body Fluid Solution Induced by Zn Element Addition. Adv. Eng. Mater. 2023, 25, 2201402. [Google Scholar] [CrossRef]
- Zhu, G.; Lyu, S.; Shen, G.; Tian, L.; Chen, M. Effect of the Microstructure Parameters on the Mechanical Properties and Corrosion Behavior of Mg Alloys with High Zn or Ca Content. J. Mater. Sci. 2023, 58, 12373–12390. [Google Scholar] [CrossRef]
- Cihova, M.; Martinelli, E.; Schmutz, P.; Myrissa, A.; Schäublin, R.; Weinberg, A.M.; Uggowitzer, P.J.; Löffler, J.F. The Role of Zinc in the Biocorrosion Behavior of Resorbable Mg–Zn–Ca Alloys. Acta Biomater. 2019, 100, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.J.; Park, J.; Lee, S.H.; Kim, D.H.; Lee, G.C.; Jeon, H.; Chae, M.; Lee, K.S.; Sun, J.Y.; Lee, D.H.; et al. Optimization of the Clinically Approved Mg-Zn Alloy System through the Addition of Ca. Biomater. Res. 2022, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Munteanu, C.; Bălțatu, M.S.; Cimpoeșu, R.; Ioanid, N. Microstructural and Electrochemical Influence of Zn in MgCaZn Biodegradable Alloys. Materials 2023, 16, 2487. [Google Scholar] [CrossRef]
- Shiri, M.; Jafari, H. Effect of Extrusion Temperature and Extrusion Ratio on Microstructure and Biodegradation Behavior of Mg-4.5Zn Binary Alloy. JOM 2019, 71, 4705–4714. [Google Scholar] [CrossRef]
- Incesu, A.; Gungor, A. Mechanical Properties and Biodegradability of Mg–Zn–Ca Alloys: Homogenization Heat Treatment and Hot Rolling. J. Mater. Sci. Mater. Med. 2020, 31, 123. [Google Scholar] [CrossRef]
- Peron, M.; Skaret, P.C.; Fabrizi, A.; Varone, A.; Montanari, R.; Roven, H.J.; Ferro, P.; Berto, F.; Torgersen, J. The Effect of Equal Channel Angular Pressing on the Stress Corrosion Cracking Susceptibility of AZ31 Alloy in Simulated Body Fluid. J. Mech. Behav. Biomed. Mater. 2020, 106, 103724. [Google Scholar] [CrossRef]
- Kulyasova, O.B.; Khudododova, G.D.; Dyakonov, G.S.; Zheng, Y.; Valiev, R.Z. Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys. Materials 2022, 15, 6749. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Alawad, M.O.; Aljohani, T.A.; El-Garaihy, W.H. Influence of Ultrafine-Grained Microstructure and Texture Evolution of ECAPed ZK30 Magnesium Alloy on the Corrosion Behavior in Different Corrosive Agents. Materials 2022, 15, 5515. [Google Scholar] [CrossRef]
- Bin, S.J.B.; Fong, K.S.; Chua, B.W.; Gupta, M. Development of Biocompatible Bulk MgZnCa Metallic Glass with Very High Corrosion Resistance in Simulated Body Fluid. Materials 2022, 15, 8989. [Google Scholar] [CrossRef]
- Shaban, M.; Alateyah, A.I.; Alsharekh, M.F.; Alawad, M.O.; BaQais, A.; Kamel, M.; Alsunaydih, F.N.; El-Garaihy, W.H.; Salem, H.G. Influence of ECAP Parameters on the Structural, Electrochemical and Mechanical Behavior of ZK30: A Combination of Experimental and Machine Learning Approaches. J. Manuf. Mater. Process. 2023, 7, 52. [Google Scholar] [CrossRef]
- Heidari, E.; Esmaeili, Y.; Boutorabi, S.M.A. Effect of Solution Treatment on the Microstructure and Bio-Corrosion Behavior of Magnesium Cast Alloy. Int. J. Met. 2023, 1–11. [Google Scholar] [CrossRef]
- Popa, M.; Stefan, L.M.; Prelipcean, A.M.; Drob, S.I.; Anastasescu, M.; Calderon Moreno, J.M. Inhibition of Mg Corrosion in Physiological Fluids by Carbonate Coating. Corros. Sci. 2022, 209, 110775. [Google Scholar] [CrossRef]
- Chiu, C.; Lu, C.-T.; Chen, S.H.; Ou, K.L. Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy. Materials 2017, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg-Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Popescu, D.; Zapciu, A.; Antoniac, A.; Miculescu, F.; Moldovan, H. Magnesium Filled Polylactic Acid (PLA) Material for Filament Based 3D Printing. Materials 2019, 12, 719. [Google Scholar] [CrossRef]
- Gaur, S.; Singh Raman, R.K.; Khanna, A.S. In Vitro Investigation of Biodegradable Polymeric Coating for Corrosion Resistance of Mg-6Zn-Ca Alloy in Simulated Body Fluid. Mater. Sci. Eng. C 2014, 42, 91–101. [Google Scholar] [CrossRef]
- Moreno, L.; Mohedano, M.; Arrabal, R.; Matykina, E. Development and Screening of (Ca-P-Si-F)-PEO Coatings for Biodegradability Control of Mg-Zn-Ca Alloys. J. Magnes. Alloys 2022, 10, 2220–2237. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Lv, Y.; Dong, Z.; Yang, L.; Zhang, E.; Hashimoto, T.; Zhou, X. PEO Coating on Mg-Ag Alloy: The Incorporation and Release of Ag Species. J. Magnes. Alloys 2022, 11, 2182–2195. [Google Scholar] [CrossRef]
- Yao, X.; Tang, J.; Zhou, Y.; Atrens, A.; Dargusch, M.S.; Wiese, B.; Ebel, T.; Yan, M. Surface Modification of Biomedical Mg-Ca and Mg-Zn-Ca Alloys Using Selective Laser Melting: Corrosion Behaviour, Microhardness and Biocompatibility. J. Magnes. Alloys 2021, 9, 2155–2168. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Gu, X.; Jiang, B.; Liang, J. Effect of Severe Shot Peening on Corrosion Behavior of AZ31 and AZ91 Magnesium Alloys. J. Alloys Compd. 2019, 770, 500–506. [Google Scholar] [CrossRef]
- Santos, V.; Uddin, M.; Hall, C. Mechanical Surface Treatments for Controlling Surface Integrity and Corrosion Resistance of Mg Alloy Implants: A Review. J. Funct. Biomater. 2023, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Liu, Y.; Zhang, M.; Tian, Y.; Chen, L. Research Progress on Corrosion Behaviors and Improvement Methods of Medical Degradable Mg−Based Alloys. Metals 2022, 13, 71. [Google Scholar] [CrossRef]
- Wei, L.; Gao, Z. Recent Research Advances on Corrosion Mechanism and Protection, and Novel Coating Materials of Magnesium Alloys: A Review. RSC Adv. 2023, 13, 8427–8463. [Google Scholar] [CrossRef]
- Zhai, C.Y.; Dai, C.Y.; Lv, X.; Shi, B.; Li, Y.R.; Yang, Y.; Fan, D.; Lee, E.S.; Sun, Y.; Jiang, H.B. Fluoride Coatings on Magnesium Alloy Implants. Bioinorg. Chem. Appl. 2022, 2022, 7636482. [Google Scholar] [CrossRef]
- Dai, C.Y.; Gao, X.; Zhai, C.; Jia, Q.; Zhao, B.C.; Shi, H.; Gao, Q.; Cai, H.; Lee, E.S.; Jiang, H.B. Corrosion Evaluation of Pure Mg Coated by Fluorination in 0.1 M Fluoride Electrolyte. Scanning 2021, 2021, 5574946. [Google Scholar] [CrossRef]
- Qi, Y.; Peng, Z.; Wang, L.; Zhou, J.; Wang, P.; Liang, J. Fluoride-Dominated Coating on Mg Alloys Fabricated by Plasma Electrolytic Process in Ambient Non-Aqueous Electrolyte. Surf. Eng. 2021, 37, 360–364. [Google Scholar] [CrossRef]
- Sun, J.; Jin, S.; Zhao, B.C.; Cai, H.; Sun, L.; Xuan, X.; Oh, J.R.; Jiang, H.B. Enhanced Corrosion Resistance of Biodegradable Mg Alloys via Ultrasonically Treated Fluoride Coating. Surf. Topogr. Metrol. Prop. 2019, 7, 025009. [Google Scholar] [CrossRef]
- Salman, S.A.; Gouda, M.K. Characterization and Corrosion Behavior of Vanadium-Based Conversion Coating on AZ31 Magnesium Alloy. Mater. Today Proc. 2022, 62, 611–614. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.W.; Peuster, M. Development and Biocompatibility of a Novel Corrodible Fluoride-Coated Magnesium-Calcium Alloy with Improved Degradation Kinetics and Adequate Mechanical Properties for Cardiovascular Applications. J. Biomed. Mater. Res. Part A 2010, 93, 763–775. [Google Scholar] [CrossRef]
- Li, Z.; Shizhao, S.; Chen, M.; Fahlman, B.D.; Debao, L.; Bi, H. In Vitro and in Vivo Corrosion, Mechanical Properties and Biocompatibility Evaluation of MgF2-Coated Mg-Zn-Zr Alloy as Cancellous Screws. Mater. Sci. Eng. C 2017, 75, 1268–1280. [Google Scholar] [CrossRef]
- Saliza Azlina, O.; Shafiq Ruba’Ai, M.; Kurniawan, D. Effect of Magnesium Fluoride Coating on Corrosion Behaviour of Magnesium Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 694, 012049. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Tailoring the Composition of Fluoride Conversion Coatings to Achieve Better Corrosion Protection of Magnesium for Biomedical Applications. J. Mater. Chem. B 2014, 2, 3365–3382. [Google Scholar] [CrossRef] [PubMed]
- Panemangalore, D.B.; Shabadi, R.; Gupta, M.; Ji, G. Effect of Fluoride Coatings on the Corrosion Behavior of Mg–Zn–Er Alloys. Surf. Interfaces 2019, 14, 72–81. [Google Scholar] [CrossRef]
- Gambaro, S.; Nascimento, M.L.; Shekargoftar, M.; Ravanbakhsh, S.; Sales, V.; Paternoster, C.; Bartosch, M.; Witte, F.; Mantovani, D. Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials 2022, 15, 8245. [Google Scholar] [CrossRef]
- Bita, T.; Antoniac, A.; Ciuca, I.; Miculescu, M.; Cotrut, C.M.; Paltanea, G.; Dura, H.; Corneschi, I.; Antoniac, I.; Carstoc, I.D.; et al. Effect of Fluoride Coatings on the Corrosion Behavior of Mg–Zn–Ca–Mn Alloys for Medical Application. Materials 2023, 16, 4508. [Google Scholar] [CrossRef]
- Gao, C.; Zeng, Z.; Peng, S.; Tan, W.; Shuai, C. A Continuous MgF2 Network Structure Encapsulated Mg Alloy Prepared by Selective Laser Melting for Enhanced Biodegradation Resistance. Adv. Eng. Mater. 2021, 23, 2100389. [Google Scholar] [CrossRef]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. Inhibiting Stress Corrosion Cracking by Removing Corrosion Products from the Mg-Zn-Zr Alloy Pre-Exposed to Corrosion Solutions. Acta Mater. 2021, 205, 116570. [Google Scholar] [CrossRef]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. On the Role of Pre-Exposure Time and Corrosion Products in Stress-Corrosion Cracking of ZK60 and AZ31 Magnesium Alloys. Mater. Sci. Eng. A 2021, 806, 140876. [Google Scholar] [CrossRef]
- Chakrapani, D.G.; Pugh, E.N. Hydrogen Embrittlement in a Mg-Al Alloy. Metall. Trans. A 1976, 7, 173–178. [Google Scholar] [CrossRef]
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Pre-Exposure Embrittlement and Stress Corrosion Cracking of Magnesium Alloy AZ31B in Chloride Solutions. Corrosion 2014, 70, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. Effect of Air Storage on Stress Corrosion Cracking of ZK60 Alloy Induced by Preliminary Immersion in NaCl-Based Corrosion Solution. Materials 2022, 15, 7862. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.; Merson, E.; Myagkikh, P.; Linderov, M.; Brilevsky, A.; Merson, D. Attaining High Functional Performance in Biodegradable Mg-Alloys: An Overview of Challenges and Prospects for the Mg-Zn-Ca System. Materials 2023, 16, 1324. [Google Scholar] [CrossRef] [PubMed]
- Marek, R.; Eichler, J.; Schwarze, U.Y.; Fischerauer, S.; Suljevic, O.; Berger, L.; Löffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. Long-Term in Vivo Degradation of Mg–Zn–Ca Elastic Stable Intramedullary Nails and Their Influence on the Physis of Juvenile Sheep. Biomater. Adv. 2023, 150, 213417. [Google Scholar] [CrossRef]
- Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N.G.; Hohenberger, G.; van den Beucken, J.J.J.P.; Seibert, F.; et al. A Lean Magnesium–Zinc–Calcium Alloy ZX00 Used for Bone Fracture Stabilization in a Large Growing-Animal Model. Acta Biomater. 2020, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, J.; Becker, M.; Martinelli, E.; Weinberg, A.M.; Mingler, B.; Kilian, H.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. High-Strength Low-Alloy (HSLA) Mg-Zn-Ca Alloys with Excellent Biodegradation Performance. JOM 2014, 66, 566–572. [Google Scholar] [CrossRef]
- Barajas, J.D.; Joya, J.C.; Durán, K.S.; Hernández-Barrios, C.A.; Coy, A.E.; Viejo, F. Relationship between Microstructure and Formation-Biodegradation Mechanism of Fluoride Conversion Coatings Synthesised on the AZ31 Magnesium Alloy. Surf. Coat. Technol. 2019, 374, 424–436. [Google Scholar] [CrossRef]
- Casanova, P.Y.; Jaimes, K.J.; Parada, N.J.; Hernández-Barrios, C.A.; Aparicio, M.; Viejo, F.; Coy, A.E. Synthesis and Evaluation of MgF2 Coatings by Chemical Conversion on Magnesium Alloys for Producing Biodegradable Orthopedic Implants of Temporary Use. J. Phys. Conf. Ser. 2013, 466, 012003. [Google Scholar] [CrossRef]
- Schäublin, R.E.; Becker, M.; Cihova, M.; Gerstl, S.S.A.; Deiana, D.; Hébert, C.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. Precipitation in Lean Mg–Zn–Ca Alloys. Acta Mater. 2022, 239, 118223. [Google Scholar] [CrossRef]
- Merson, E.D.; Poluyanov, V.A.; Myagkikh, P.N.; Merson, D.L.; Vinogradov, A.Y. The Effect of Testing Conditions on Stress Corrosion Cracking of Biodegradable Magnesium Alloy ZK60. Lett. Mater. 2022, 12, 177–183. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An Innovative Specimen Configuration for the Study of Mg Corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zander, D.; Zumdick, N.A. Influence of Ca and Zn on the Microstructure and Corrosion of Biodegradable Mg-Ca-Zn Alloys. Corros. Sci. 2015, 93, 222–233. [Google Scholar] [CrossRef]
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Hydrogen Embrittlement of Magnesium and Magnesium Alloys: A Review. J. Electrochem. Soc. 2013, 160, C168–C178. [Google Scholar] [CrossRef]
- Merson, E.; Myagkikh, P.; Poluyanov, V.; Merson, D.; Vinogradov, A. On the Role of Hydrogen in Stress Corrosion Cracking of Magnesium and Its Alloys: Gas-Analysis Study. Mater. Sci. Eng. A 2019, 748, 337–346. [Google Scholar] [CrossRef]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. Effect of Strain Rate and Corrosion Products on Pre-Exposure Stress Corrosion Cracking in the ZK60 Magnesium Alloy. Mater. Sci. Eng. A 2022, 830, 142304. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Dhamotharan, S.; Sathishkumar, G.; Gopalakrishnan, P.; Ravi, K.R. Stress Corrosion Cracking of Biodegradable Mg-4Zn Alloy in Simulated Body Fluid at Different Strain Rates—A Fractographic Investigation. Mater. Sci. Eng. A 2018, 730, 223–231. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Raja, V.S.; Song, G.; Kainer, K.U. Characterisation of Stress Corrosion Cracking (SCC) of Mg–Al Alloys. Mater. Sci. Eng. A 2008, 48, 339–351. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Dietzel, W. Pitting-Induced Hydrogen Embrittlement of Magnesium-Aluminium Alloy. Mater. Des. 2012, 42, 321–326. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.K.S.; Davies, C.H.J.; Hofstetter, J.; Uggowitzer, P.J.; Löffler, J.F. Stress Corrosion Cracking and Corrosion Fatigue Characterisation of MgZn1Ca0.3 (ZX10) in a Simulated Physiological Environment. J. Mech. Behav. Biomed. Mater. 2017, 65, 634–643. [Google Scholar] [CrossRef]






| Mg | Zn | Zr | Al | Fe | Mn | Ni | Cu | Si | Y | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| Balance | 2.0567 | <0.001 | 0.0109 | 0.0038 | 0.0022 | 0.0012 | <0.001 | 0.002 | 0.0059 | 0.0904 |
| NaCl | CaCl2 | KCl | KH2PO4 | MgCl2 · 6 H2O | MgSO4 · 7 H2O | Na2HPO4 · 12 H2O | NaHCO3 | D-Glucose |
|---|---|---|---|---|---|---|---|---|
| 8 | 0.14 | 0.4 | 0.06 | 0.1 | 0.06 | 0.48 | 0.35 | 1 |
| Specimen | Ra, µm | Cell Viability, % | Testing Medium | PW, mm/y | PH, mm/y | EF, % | UTS, MPa | IEFSCC, % | IUTSSCC, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Machined | Polished | |||||||||
| Reference | 0.43 ± 0.01 | 0.23 ± 0.02 | 121 ± 10 | Air | - | - | 18.2 ± 0.2 | 165 ± 1 | - | - |
| Hanks’ | 2.7 ± 0.3 | 2.3 ± 0.4 | 8.5 ± 0.4 | 106 ± 5 | 53 ± 2 | 36 ± 3 | ||||
| HF-treated | 0.84 ± 0.01 | 0.40 ± 0.01 | 103 ± 13 | Air | - | - | 18.9 ± 0.4 | 166 ± 1 | - | - |
| Hanks’ | 0.8 ± 0.1 | 0.4 ± 0.2 | 11.7 ± 1.0 | 132 ± 6 | 36 ± 5 | 20 ± 3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merson, E.D.; Poluyanov, V.A.; Myagkikh, P.N.; Bunev, A.S.; Merson, D.L.; Vinogradov, A. Improving Corrosion and Stress Corrosion Cracking Performance of Machined Biodegradable Alloy ZX20 by HF-Treatment. Metals 2023, 13, 1660. https://doi.org/10.3390/met13101660
Merson ED, Poluyanov VA, Myagkikh PN, Bunev AS, Merson DL, Vinogradov A. Improving Corrosion and Stress Corrosion Cracking Performance of Machined Biodegradable Alloy ZX20 by HF-Treatment. Metals. 2023; 13(10):1660. https://doi.org/10.3390/met13101660
Chicago/Turabian StyleMerson, Evgeniy D., Vitaliy A. Poluyanov, Pavel N. Myagkikh, Alexander S. Bunev, Dmitri L. Merson, and Alexei Vinogradov. 2023. "Improving Corrosion and Stress Corrosion Cracking Performance of Machined Biodegradable Alloy ZX20 by HF-Treatment" Metals 13, no. 10: 1660. https://doi.org/10.3390/met13101660
APA StyleMerson, E. D., Poluyanov, V. A., Myagkikh, P. N., Bunev, A. S., Merson, D. L., & Vinogradov, A. (2023). Improving Corrosion and Stress Corrosion Cracking Performance of Machined Biodegradable Alloy ZX20 by HF-Treatment. Metals, 13(10), 1660. https://doi.org/10.3390/met13101660







