Thermodynamics of the Formation of Non-Metallic Inclusions during the Deoxidation of GCr15 Bearing Steel
Abstract
1. Introduction
2. Research Method
3. Deoxidation Equilibrium Calculation for a Single Element
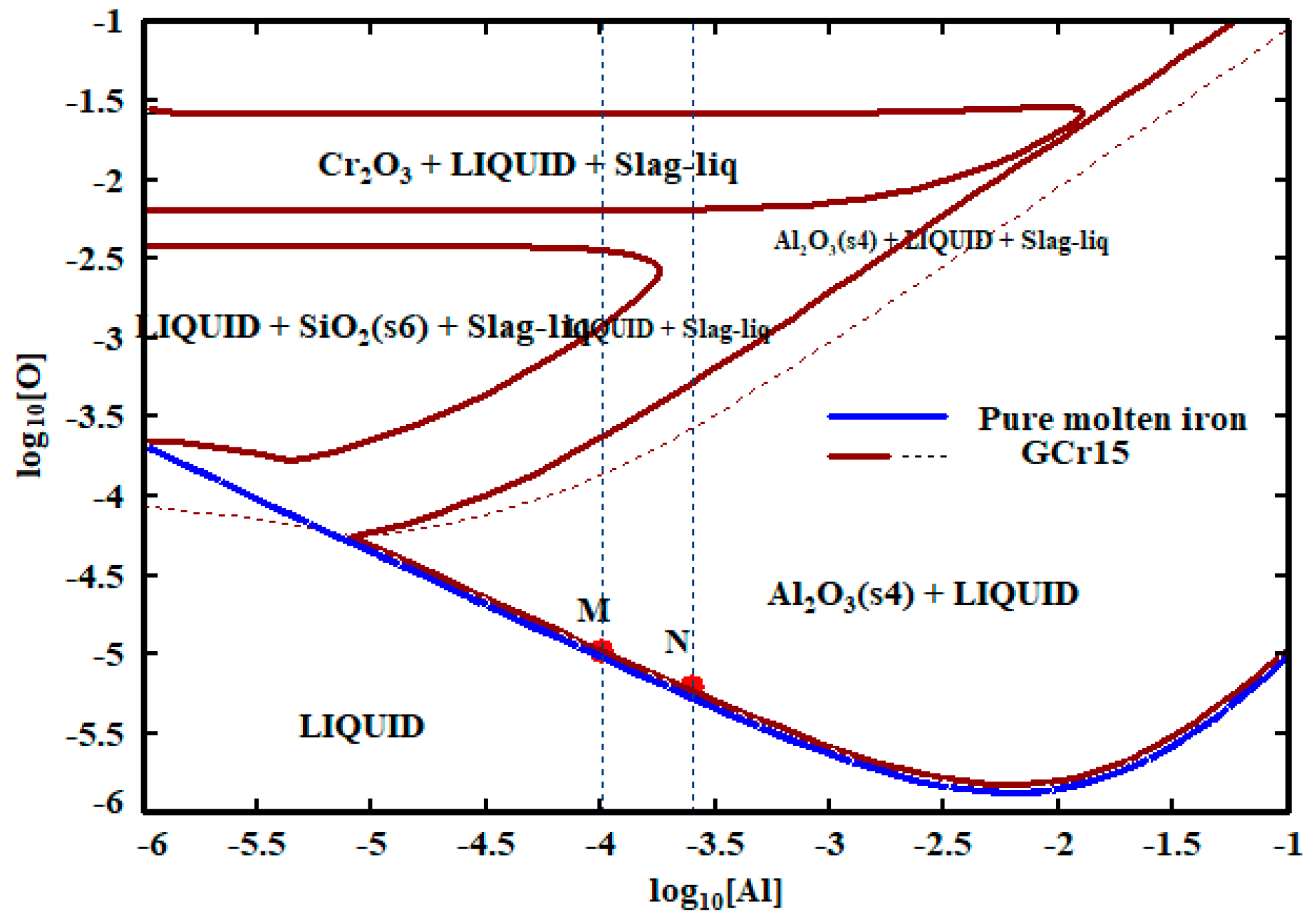
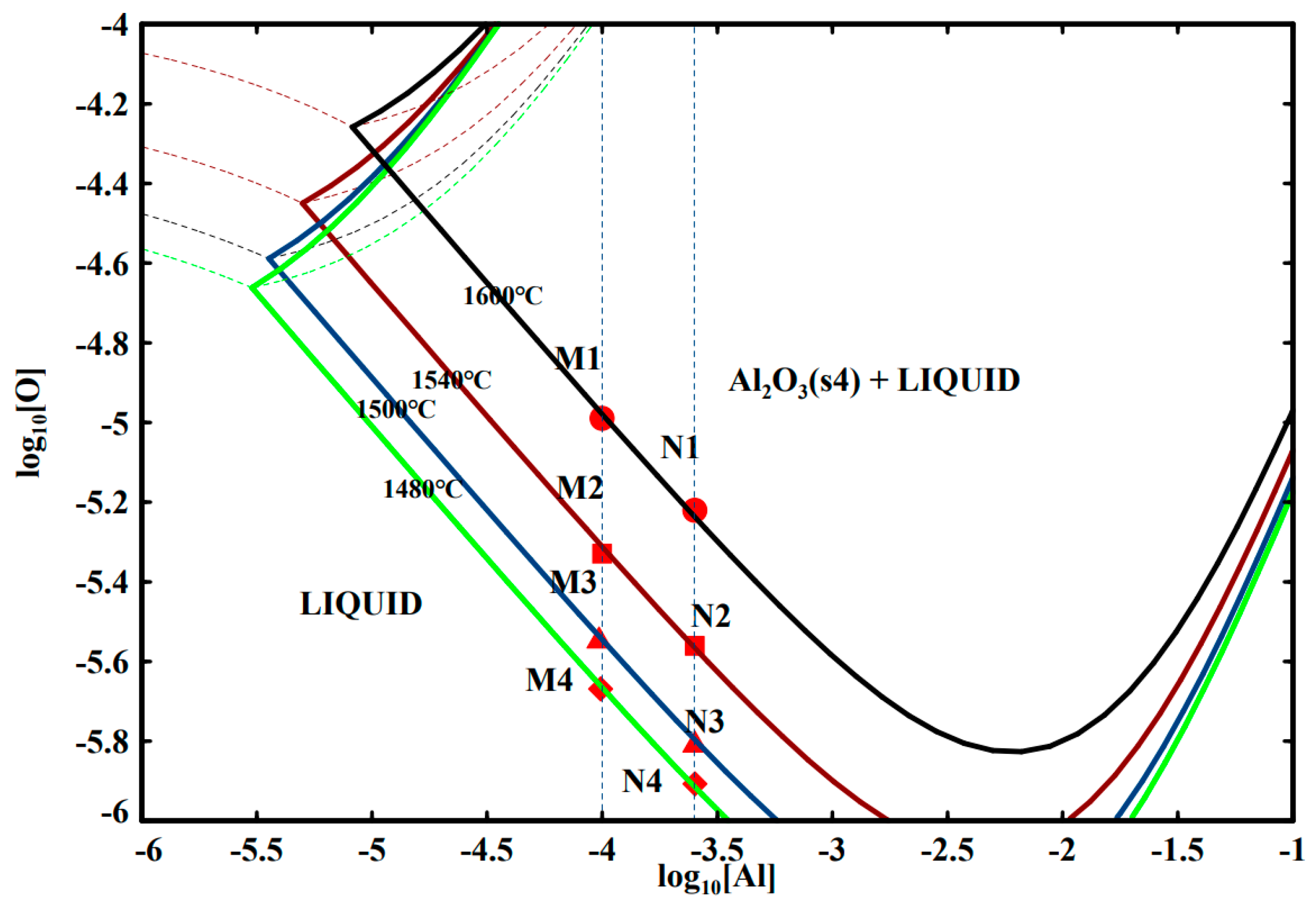
3.1. Al-O Equilibrium of GCr15 Bearing Steel
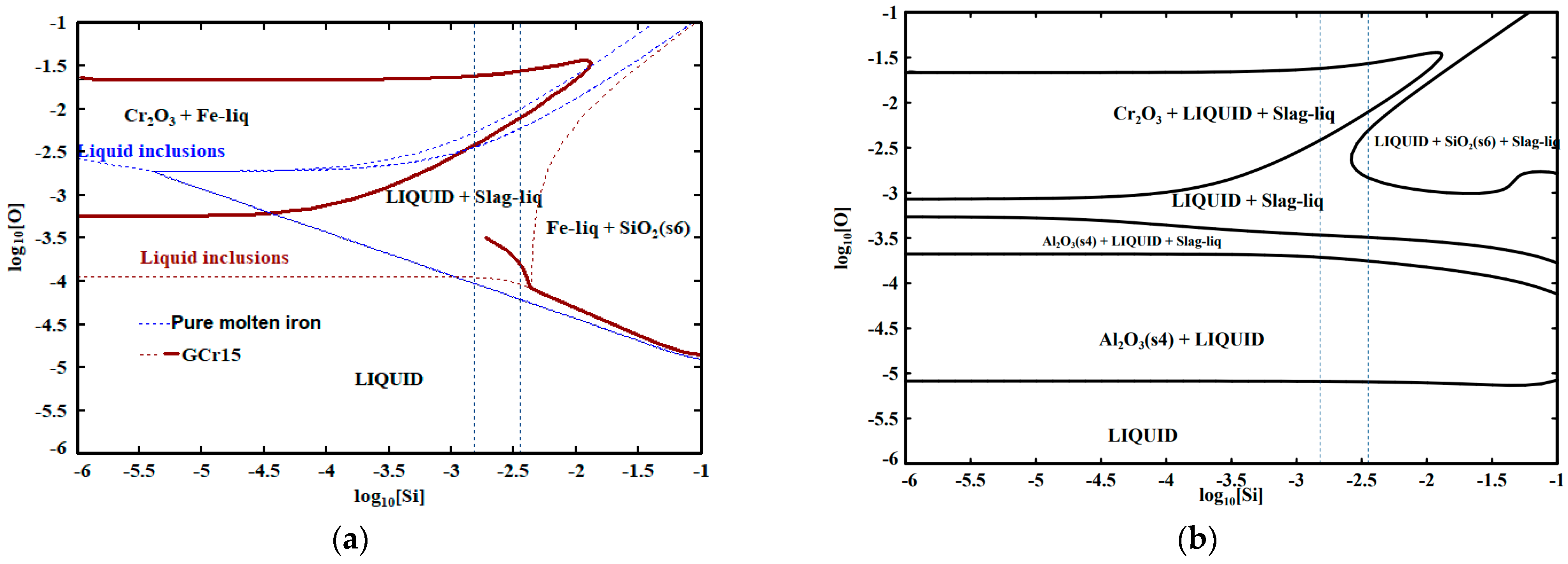
3.2. Si-O Equilibrium of GCr15 Bearing Steel
3.3. Mn-O Equilibrium of GCr15 Bearing Steel
4. Thermodynamic Analysis of the Deoxidation of Different Binary Combinations
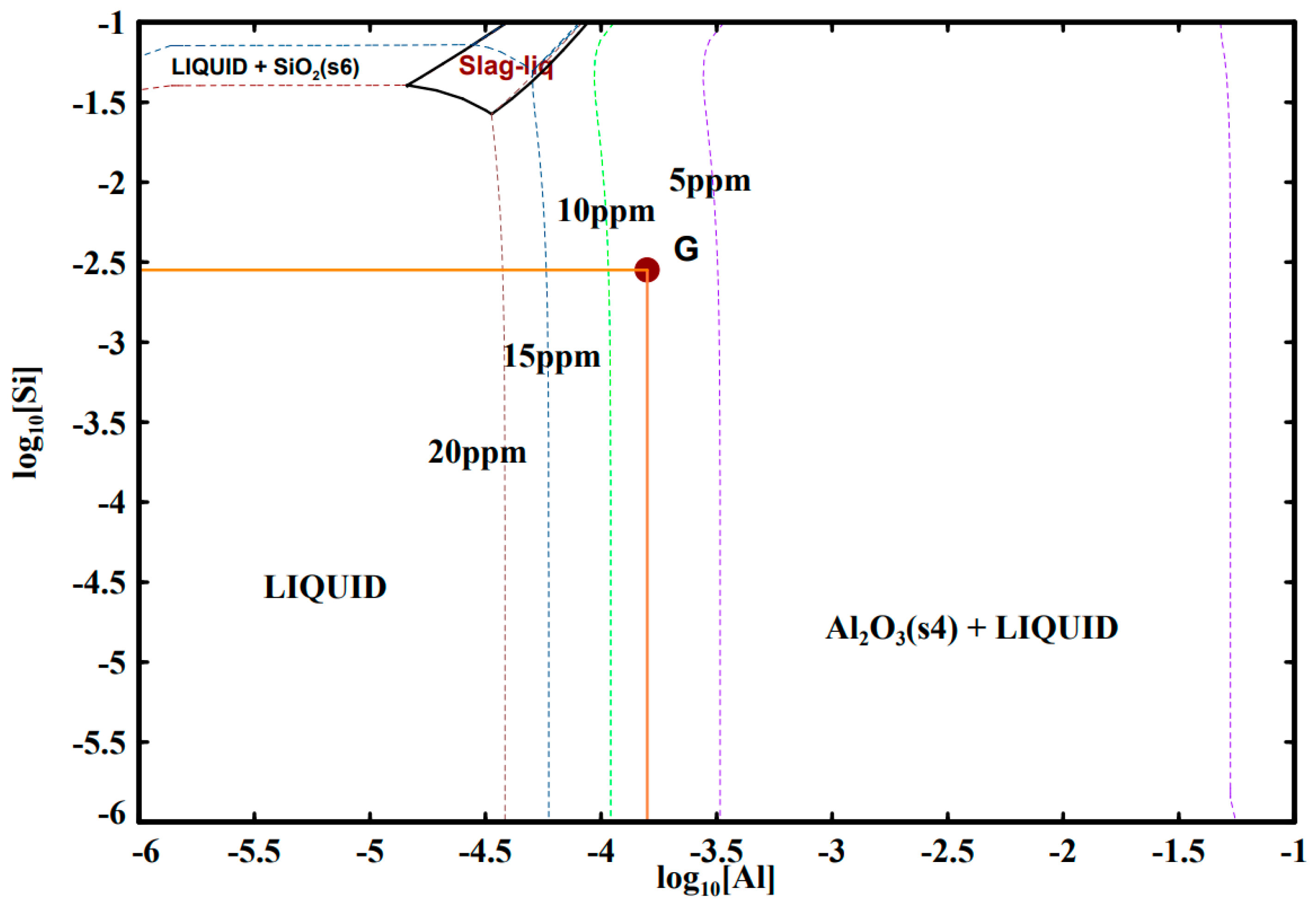
4.1. Al-Si Deoxidation Equilibrium
4.2. Si-Mn Deoxidation Equilibrium
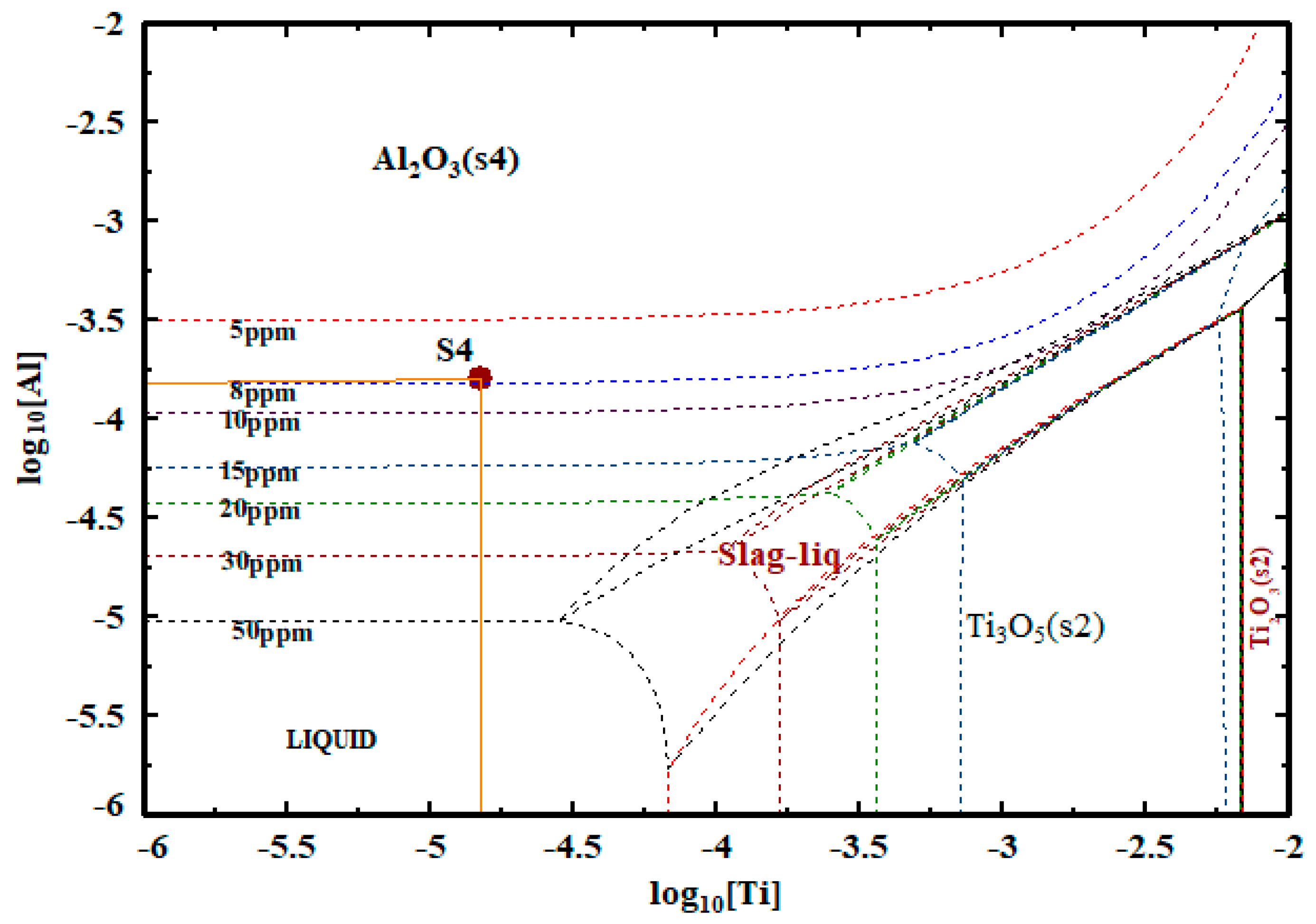
4.3. Al-Ti Deoxidation Equilibrium
5. Thermodynamic Analysis of Inclusion Modifications in GCr15 Bearing Steel by Rare-Earth Ce
6. Conclusions
- (1)
- Based on thermodynamic calculations with FactSage8.1, the single deoxidization curves of Al, Si, and Mn and the binary compound deoxidization curves of Al-Si and Si-Mn in pure iron conditions and GCr15 bearing steel production conditions were quite different. When using the FactSage 8.1 thermodynamic software, database selection is very important. Different databases may lead to different calculation results. The calculation results of the FactSage thermodynamic software can be used as a reference, and the test results need to be corrected and verified.
- (2)
- Temperature has a great influence on the Al-O equilibrium curve of GCr15 bearing steel. With the decrease in temperature, the Al-O equilibrium curve moves downward. With the same Al content, the equilibrium oxygen content decreases. When T = 1873 K, the [Al] in GCr15 bearing steel is in the range of 0.010–0.025% and the [O] content can reach 6–10 ppm; when the temperature is reduced to 1753 K, the [O] content can reach 1.23–2.14 ppm.
- (3)
- The Al-Ti equilibrium curve of GCr15 bearing steel shows that when the [Ti] content of GCr15 bearing steel is controlled at 0.0015% and the [O] content in the molten steel is reduced to less than 50 ppm, there is no titanium oxide in the molten steel, and the deoxidation product is mainly Al2O3.
- (4)
- When Ce was added to Al deoxidized bearing steel at T = 1873 K, the evolution path of Al2O3 was Al11O18Ce→CeAlO3→Ce2O3. When Ce was used to modify the inclusions in Si-Mn deoxidized bearing steel at T = 1873 K, the stable oxide type in the molten steel was closely related to the [O] content in the molten steel. CeCrO3 appeared when [O] > 50 ppm and disappeared when [O] < 50 ppm. When [O] = 10 ppm, [Ce] = 0.012% was the critical control point. When [Ce] > 0.012%, CeS inclusions appeared in the steel, and the final equilibrium product was CeS + Ce2O3. When [Ce] < 0.012%, CeS was not produced in the steel, and only Ce2O3 existed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, C.; Bao, Y.-P.; Gan, P.; Wang, M.; He, J.-S. Effect of main inclusions on crack initiation in bearing steel in the very high cycle fatigue regime. Int. J. Miner. Met. Mater. 2018, 25, 623–629. [Google Scholar] [CrossRef]
- Arakere, N.K. Gigacycle rolling contact fatigue of bearing steels: A review. Int. J. Fatigue 2016, 93, 238–249. [Google Scholar] [CrossRef]
- Shiozawa, K.; Lu, L. Very high-cycle fatigue behavior of shot-peened high-carbon–chromium bearing steel. Fatigue Fract. Eng. Mater. Struct. 2010, 25, 813–822. [Google Scholar] [CrossRef]
- Krupp, U.; Koschella, K.; Gierltler, A. The significance of grain size, segregations and inclusions for the very high cycle fatigue (VHCF) behavior of tempered martensitic steels. Procedia Struct. Integr. 2019, 23, 517–522. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Guo, S.-Q.; Qiao, M.-R.; Qin, L.-B.; Zou, X.-J.; Ren, Z.-M. Study on the modification of inclusions by Ca treatment in GCr18Mo bearing steel. Adv. Manuf. 2019, 7, 438–447. [Google Scholar] [CrossRef]
- Ma, W.J.; Bao, Y.P.; Wang, M.; Zhao, D.W. Influence of slag composition on bearing steel cleanness. Ironmak. Steelmak. 2014, 41, 26–30. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, M.; Bao, Y. The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Sili-con-Deoxidization Bearing Steel. Metals 2019, 9, 812. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Liu, Y.; Wang, F.; Wang, Q. Cerium Addition Effect on Modification of Inclusions, Primary Carbides and Microstructure Refinement of H13 Die Steel. ISIJ Int. 2021, 61, 1850–1859. [Google Scholar] [CrossRef]
- Dong, Z.; Qian, D.; Yin, F.; Wang, F. Enhanced Impact Toughness of Previously Cold Rolled High-Carbon Chromium Bearing Steel with Rare Earth Addition. J. Mater. Eng. Perform. 2021, 30, 8178–8187. [Google Scholar] [CrossRef]
- Ma, C.; Lv, M.; Gao, X.; Wang, H.; Wei, H.; Gao, S. High-throughput search for RE (La, Ce and Y) contained stoichiometric compound in steels. Mater. Res. Express 2021, 8, 046514. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, X.; Ren, Y.; Liu, X.; Shan, Q. Characteristics of Inclusions in Low Carbon Al-Killed Steel during Ladle Furnace Refining and Calcium Treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Duan, H.; Yang, W.; Sun, L. Stability Diagram of Mg-Al-O System Inclusions in Molten Steel. Met. Mater. Trans. B 2015, 46, 1809–1825. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Yang, W.; Duan, H. Formation and Thermodynamics of Mg-Al-Ti-O Complex Inclusions in Mg-Al-Ti-Deoxidized Steel. Met. Mater. Trans. B 2014, 45, 2057–2071. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, L.; Guo, Z.; Qiu, G. Thermodynamics and Nucleation Kinetics Model of Nonalloyed Carbon Deoxidation of Bearing Steel. Steel Res. Int. 2023, 94, 2200269. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, L.; Guo, Z.; Qiu, G. Thermodynamic model and mechanism of non-alloyed hydrogen deoxidation of GCr15 bear-ing steel. Ironmak. Steelmak. 2023, 50, 360–369. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fujimatsu, T.; Tsunekage, N.; Hiraoka, K.; Kida, K.; Santos, E.C. Effect of inclusion/matrix interface cavities on internal-fracture-type rolling contact fatigue life. Mater. Des. 2011, 32, 4980–4985. [Google Scholar] [CrossRef]
- Neishi, Y.; Makino, T.; Matsui, N.; Matsumoto, H.; Higashida, M.; Ambai, H. Influence of the Inclusion Shape on the Rolling Contact Fatigue Life of Carburized Steels. Met. Mater. Trans. A 2013, 44, 2131–2140. [Google Scholar] [CrossRef]
- Xiao, W.; Bao, Y.-P.; Gu, C.; Wang, M.; Liu, Y.; Huang, Y.-S.; Sun, G.-T. Ultrahigh cycle fatigue fracture mechanism of high-quality bearing steel obtained through different deoxidation methods. Int. J. Miner. Met. Mater. 2021, 28, 804–815. [Google Scholar] [CrossRef]
- Guo, X.; Tan, M.; Li, T.; Ju, L.; Dang, J.; Guo, H. Formation Mechanisms and Three-dimensional Characterization of Composite Inclusions in Low Aluminum Steel Deoxidized by Silicon. ISIJ Int. 2023, 63, 338–345. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Jo, J.-O.; Lee, C.-O.; Kim, D.-S.; Pak, J.-J. Thermodynamic Relation between Aluminum and Titanium in Liquid Iron. ISIJ Int. 2008, 48, 17–22. [Google Scholar] [CrossRef][Green Version]
- Ruby-Meyer, F.; Lehmann, J.; Gaye, H. Thermodynamic analysis of inclusions in Ti-deoxidised steels. Scand. J. Metall. 2000, 29, 206–212. [Google Scholar] [CrossRef]
- Matsuura, H.; Wang, C.; Wen, G.H.; Sridhar, S. The Transient Stages of Inclusion Evolution during Al and/or Ti Additions to Molten Iron. ISIJ Int. 2007, 47, 1265–1274. [Google Scholar] [CrossRef]
- Van Ende, M.-A.; Guo, M.; Dekkers, R.; Burty, M.; Van Dyck, J.; Jones, P.T.; Blanpain, B.; Wollants, P. Formation and Evolution of Al–Ti Oxide Inclusions during Secondary Steel Refining. ISIJ Int. 2009, 49, 1133–1140. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Zhu, F.; Zhao, H.; Qin, J.; Zhong, L. Effects of rare-earth micro-alloying on microstructures, carbides, and internal friction of 51CrV4 steels. J. Alloys Compd. 2020, 824, 153849. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Zhang, F.; Fu, X.; Li, H.; Yang, C. Experimental and DFT study on cerium inclusions in clean steels. J. Rare Earths 2021, 39, 477–486. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.-C.; Ren, X.; Zhang, S.-H.; Wang, S.-B. Evolution of Al2O3 inclusions by cerium treatment in low carbon high manganese steel. J. Iron Steel Res. Int. 2017, 24, 925–934. [Google Scholar] [CrossRef]
- Yang, C.; Luan, Y.; Li, D.; Li, Y. Effects of rare earth elements on inclusions and impact toughness of high-carbon chromium bearing steel. J. Mater. Sci. Technol. 2019, 35, 1298–1308. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C. Effect of Cerium and Magnesium Addition on Evolution and Particle Size of Inclusions in Al-killed Molten Steel. ISIJ Int. 2022, 62, 1852–1861. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Dai, Q.; Chai, S.; Li, K. Evolution of inclusions with cerium addition and effects of C-containing rare earth inclusions on the toughness of ultra-high-strength structural steel. Ironmak. Steelmak. 2023, 50, 184–1996. [Google Scholar] [CrossRef]
- Katsumata, A.; Todoroki, H. Effect of Rare Earth Metal on Inclusion Composition in Molten Stainless Steel. ISS Trans. Iron Steelmak. 2002, 29, 51–57. [Google Scholar]




| Elements | C | Si | Mn | P | S | Cr | Al | Ti |
|---|---|---|---|---|---|---|---|---|
| Components | 1 | 0.28 | 0.35 | 0.001 | 0.0003 | 1.5 | 0.015 | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Zhu, L.; Zhao, R.; Guo, Z.; Qiu, G. Thermodynamics of the Formation of Non-Metallic Inclusions during the Deoxidation of GCr15 Bearing Steel. Metals 2023, 13, 1680. https://doi.org/10.3390/met13101680
Cao L, Zhu L, Zhao R, Guo Z, Qiu G. Thermodynamics of the Formation of Non-Metallic Inclusions during the Deoxidation of GCr15 Bearing Steel. Metals. 2023; 13(10):1680. https://doi.org/10.3390/met13101680
Chicago/Turabian StyleCao, Lei, Liguang Zhu, Ruihua Zhao, Zhihong Guo, and Guoxing Qiu. 2023. "Thermodynamics of the Formation of Non-Metallic Inclusions during the Deoxidation of GCr15 Bearing Steel" Metals 13, no. 10: 1680. https://doi.org/10.3390/met13101680
APA StyleCao, L., Zhu, L., Zhao, R., Guo, Z., & Qiu, G. (2023). Thermodynamics of the Formation of Non-Metallic Inclusions during the Deoxidation of GCr15 Bearing Steel. Metals, 13(10), 1680. https://doi.org/10.3390/met13101680






