Heat Treatment Induced Structural Transformations and High-Temperature Oxidation Behavior of Al21Co22Cr22Fe13Ni22 High-Entropy Coatings Produced by Non-Vacuum Electron Beam Cladding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Methods of Investigation
3. Results
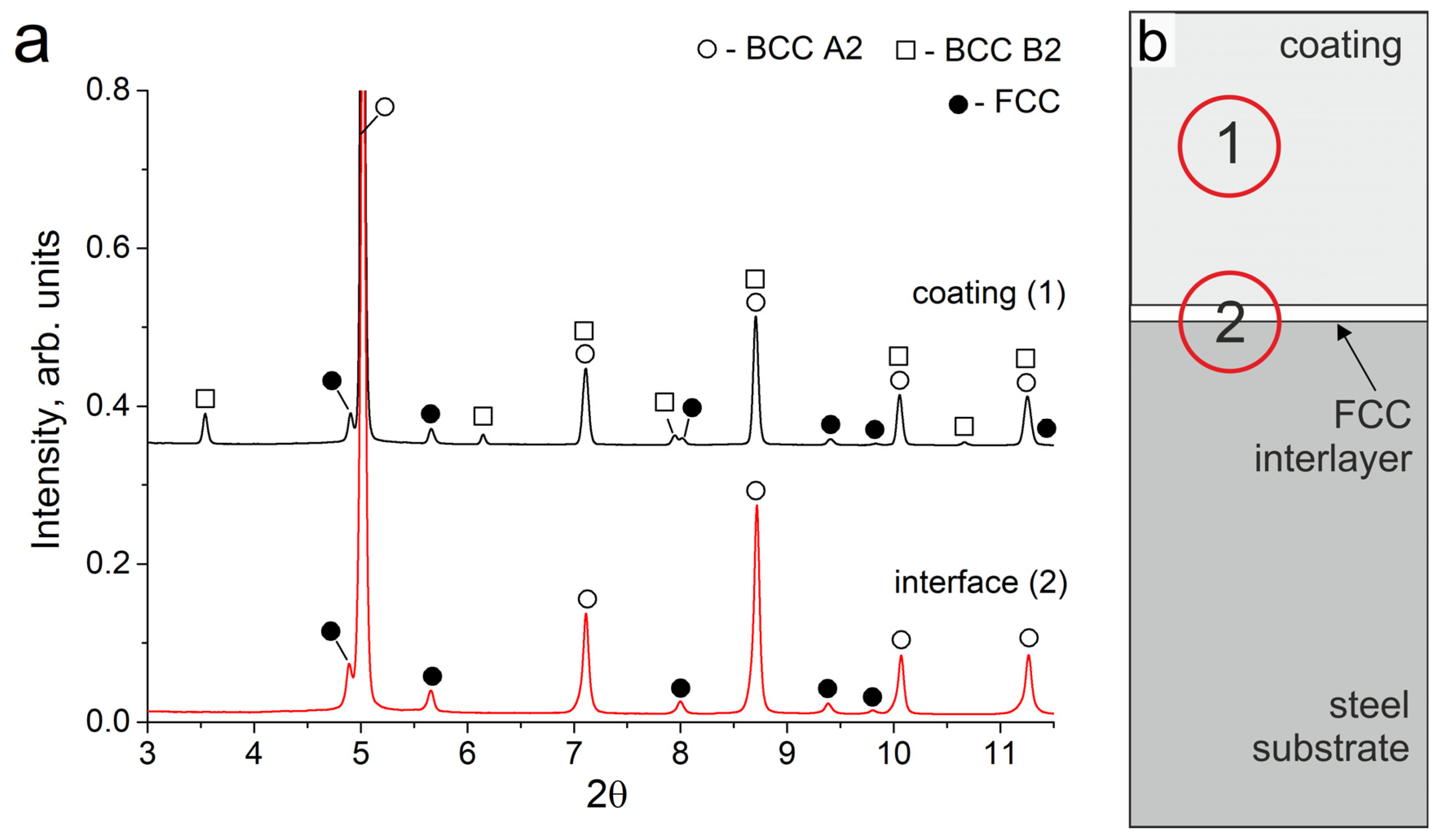
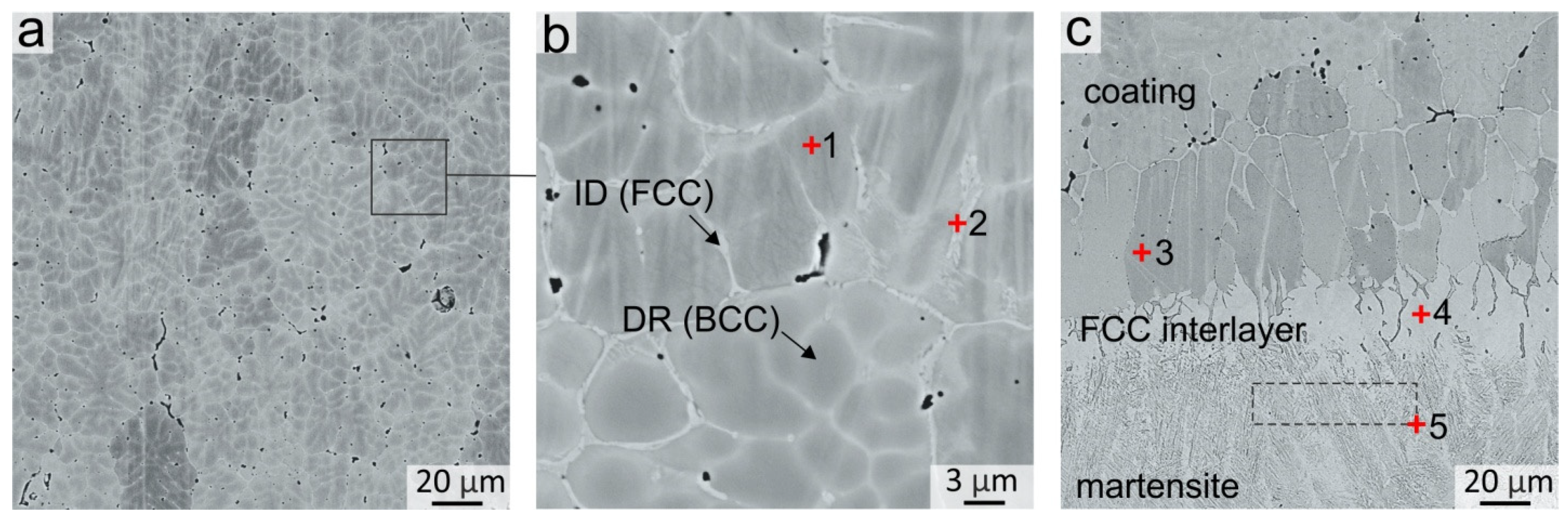
3.1. Structure of the Coatings
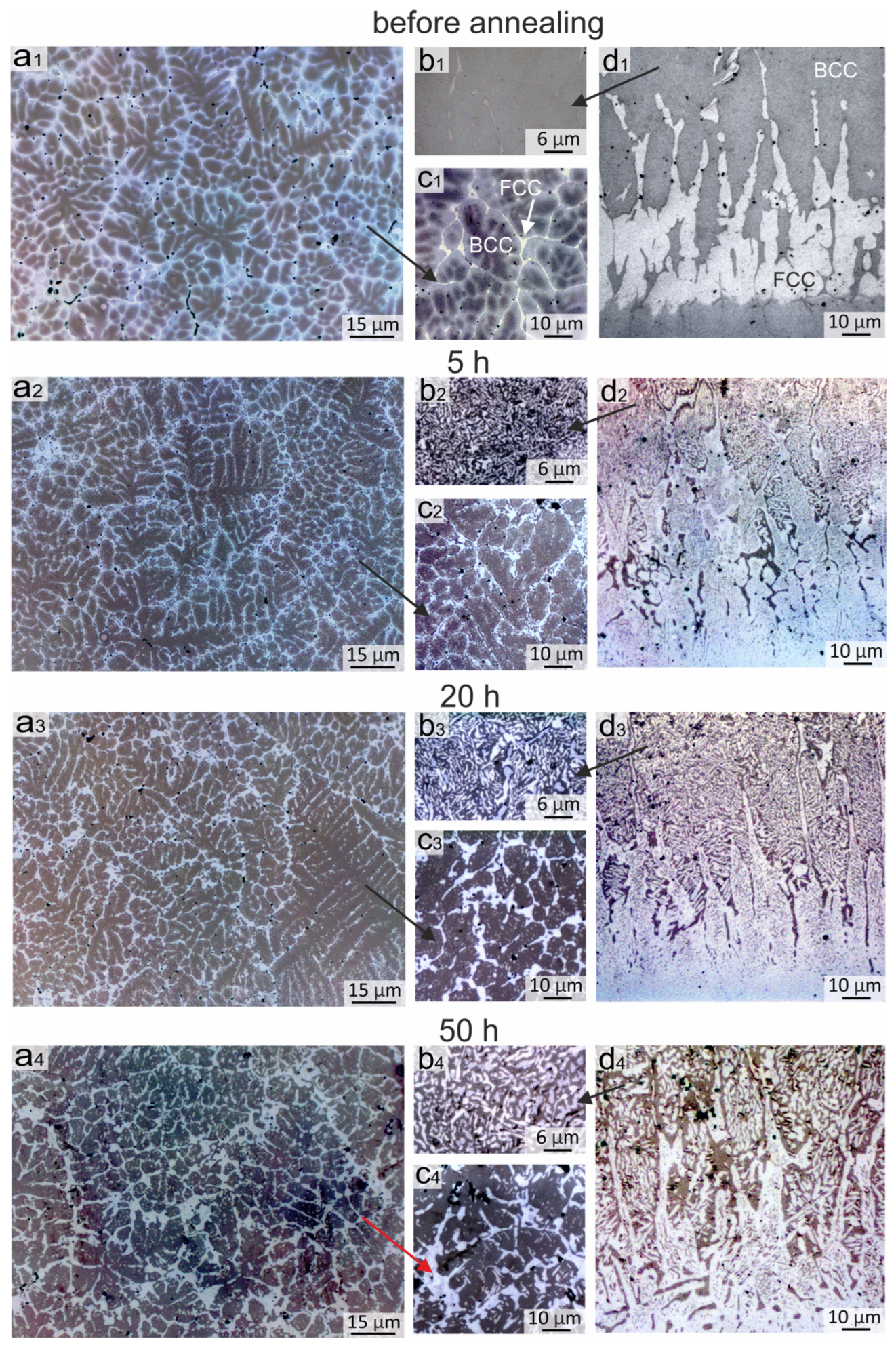
3.2. Evolution of the Coating Structure during Annealing at 900 °C
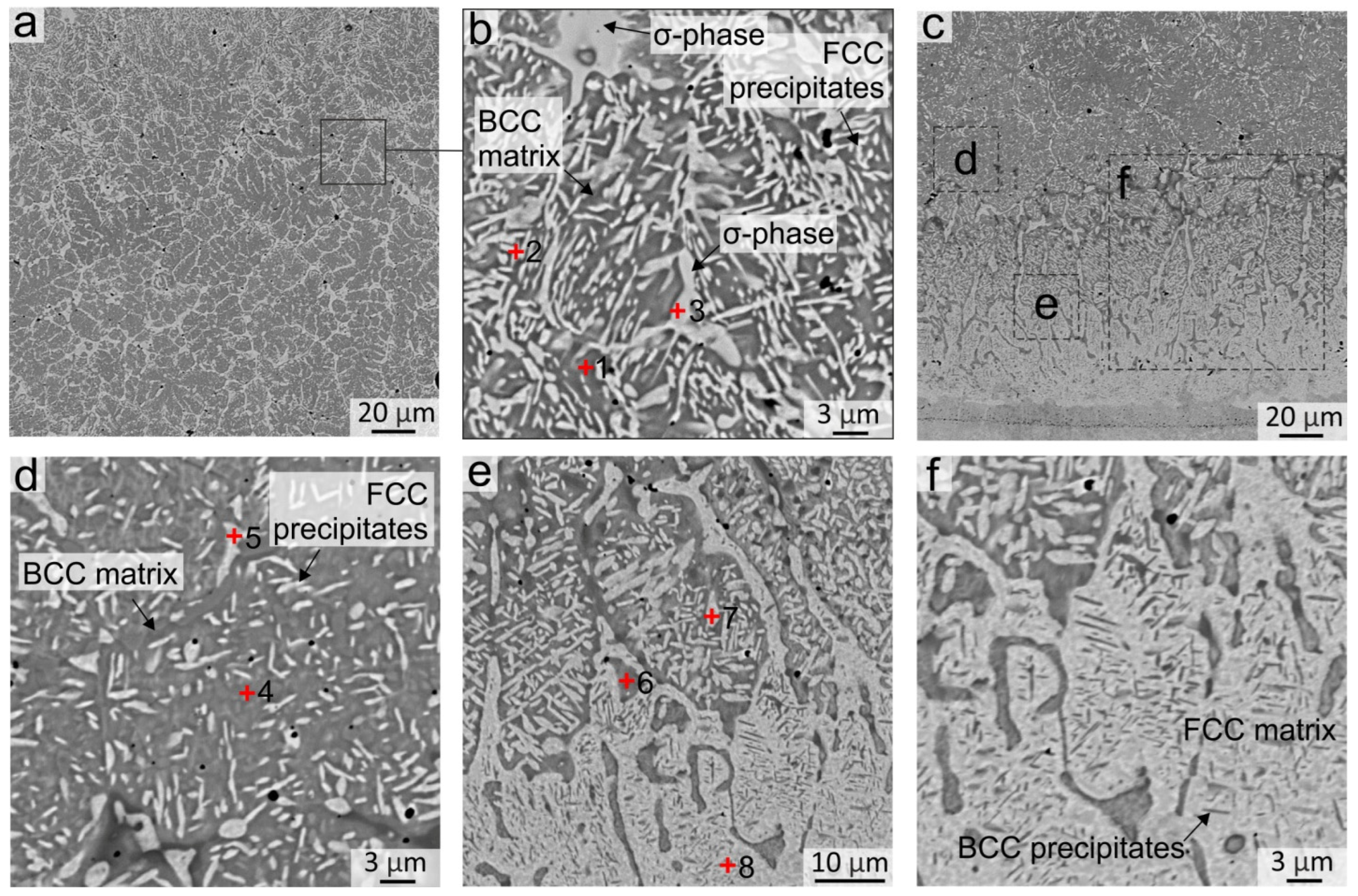
3.2.1. Structural Transformations Occurring in the Top and Middle Part of the Coating
3.2.2. Structural Changes Occurring at the Bottom of the Coating
3.2.3. Structural Transformations Occurring at the “Coating-Substrate” Interface
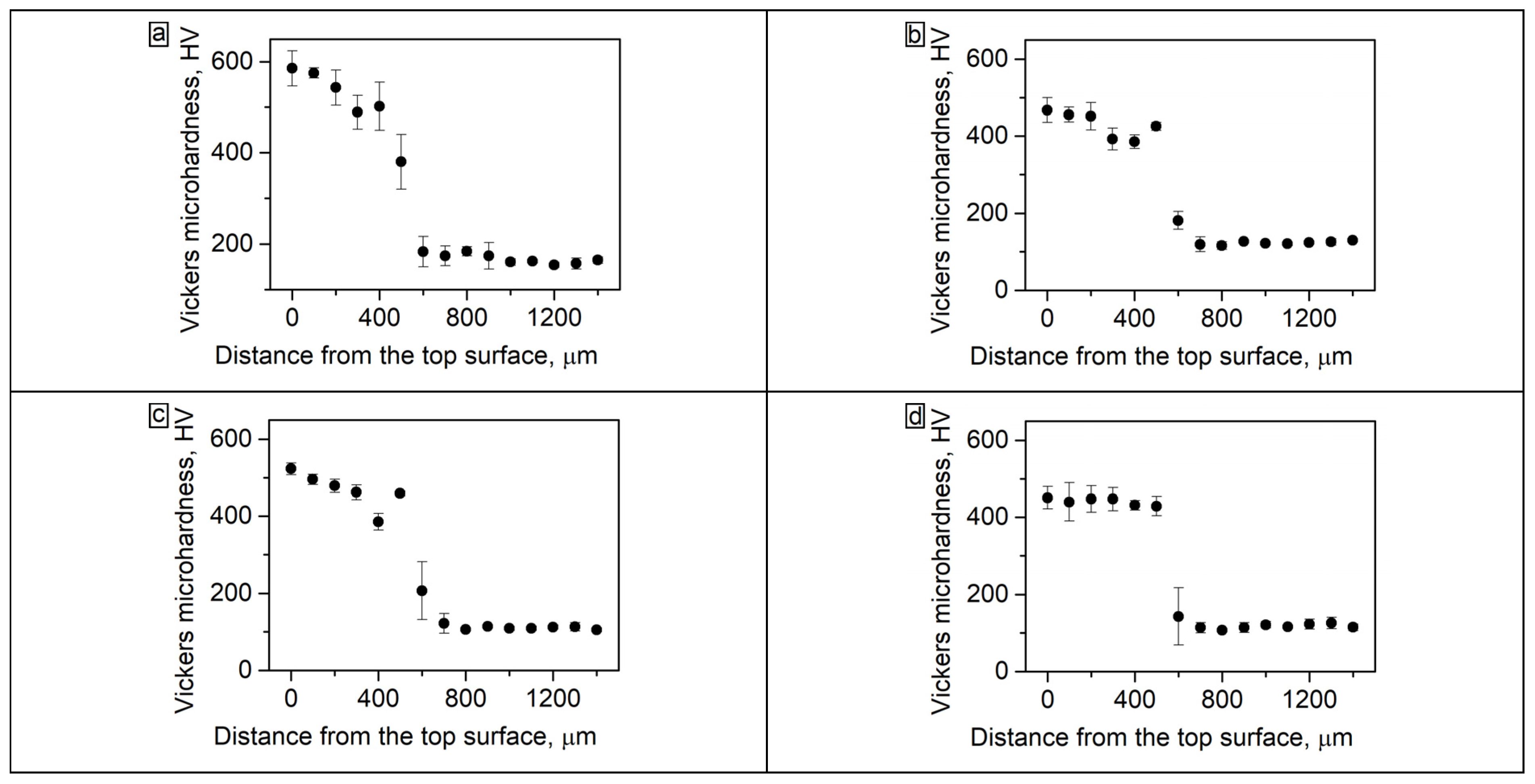
3.3. Microhardness of the Coating before and after Annealing
3.4. Oxidation of Coatings at 900 °C during 50 h
3.4.1. Composition of Oxide Films
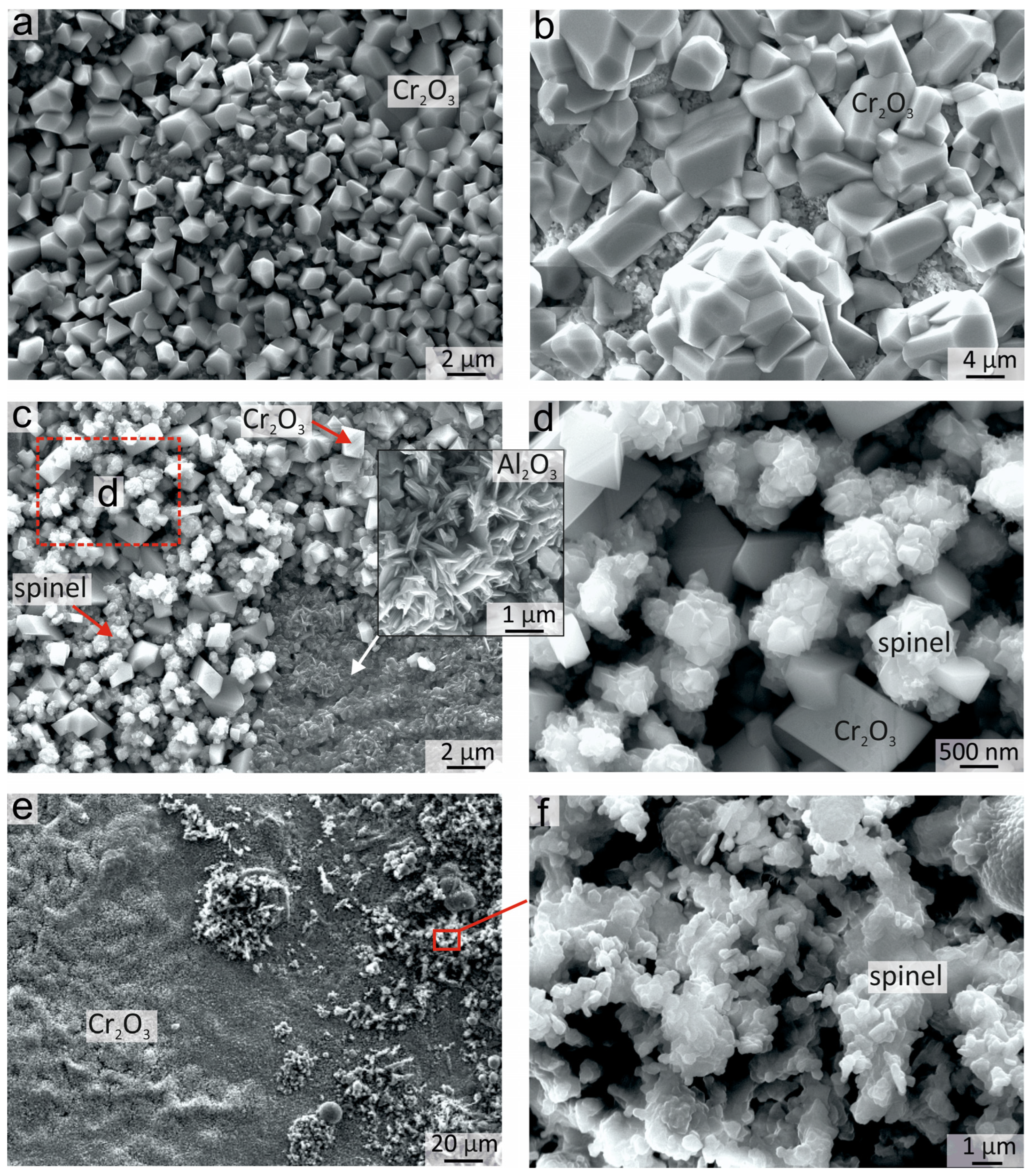
3.4.2. Surface Morphology of Oxide Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured High-entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Wang, S.; Ren, C.; Tian, H.; Yu, J.; Sun, M. MoS2/ZnO van Der Waals Heterostructure as a High-Efficiency Water Splitting Photocatalyst: A First-Principles Study. Phys. Chem. Chem. Phys. 2018, 20, 13394–13399. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, H.; Ren, C.; Yu, J.; Sun, M. Electronic and Optical Properties of Heterostructures Based on Transition Metal Dichalcogenides and Graphene-like Zinc Oxide. Sci. Rep. 2018, 8, 12009. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal Oxides and Metal Organic Frameworks for the Photocatalytic Degradation: A Review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Grilli, M.L. Metal Oxides. Metals 2020, 10, 820. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation Behavior of Arc Melted AlCoCrFeNi Multi-Component High-Entropy Alloys. J. Alloys Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Influence of Annealing on the Microstructures and Oxidation Behaviors of Al8(CoCrFeNi)92, Al15(CoCrFeNi)85, and Al30(CoCrFeNi)70 High-Entropy Alloys. Metals 2016, 6, 222. [Google Scholar] [CrossRef]
- Butler, T.M.; Alfano, J.P.; Martens, R.L.; Weaver, M.L. High-Temperature Oxidation Behavior of Al-Co-Cr-Ni-(Fe or Si) Multicomponent High-Entropy Alloys. Jom 2015, 67, 246–259. [Google Scholar] [CrossRef]
- Veselkov, S.; Samoilova, O.; Shaburova, N.; Trofimov, E. High-Temperature Oxidation of High-Entropic Alloys: A Review. Materials 2021, 14, 595. [Google Scholar] [CrossRef]
- Garg, M.; Grewal, H.S.; Sharma, R.K.; Arora, H.S. Improving the High Temperature Oxidation Resistance of High Entropy Alloy by Surface Modification. Corros. Rev. 2023, 41, 39–56. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Zajusz, M.; Jawańska, M.; Gil, A.; Jedliński, J.; Mroczka, K.; Matsuda, K.; Kulik, T.; et al. Oxidation Behavior of Alx(CoCrFeNi)100−x High-Entropy Alloys Under Thermal-Cycling Conditions. Oxid. Met. 2021, 96, 307–321. [Google Scholar] [CrossRef]
- Shen, Q.; Kong, X.; Chen, X. Significant Transitions of Microstructure and Mechanical Properties in Additively Manufactured Al–Co–Cr–Fe–Ni High-Entropy Alloy under Heat Treatment. Mater. Sci. Eng. A 2021, 815, 141257. [Google Scholar] [CrossRef]
- Niu, S.; Kou, H.; Guo, T.; Zhang, Y.; Wang, J.; Li, J. Strengthening of Nanoprecipitations in an Annealed Al0.5CoCrFeNi High Entropy Alloy. Mater. Sci. Eng. A 2016, 671, 82–86. [Google Scholar] [CrossRef]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Effect of Heat Treatment on the Phase Composition, Microstructure and Mechanical Properties of Al0.6 Crfeconi and Al0.6 Crfeconisi0.3 High-Entropy Alloys. Metals 2018, 8, 974. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Investigation of the Phase Stabilities in AlNiCoCrFe High Entropy Alloys. J. Alloys Compd. 2017, 691, 119–129. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Diao, H.; Gao, M.C.; Tang, Z.; Poplawsky, J.D.; Liaw, P.K. Understanding Phase Stability of Al-Co-Cr-Fe-Ni High Entropy Alloys. Mater. Des. 2016, 109, 425–433. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat Treatment Impacts the Micro-Structure and Mechanical Properties of AlCoCrFeNi High Entropy Alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, K.; Davies, C.; Wu, X. Evolution of Microstructure, Mechanical and Corrosion Properties of AlCoCrFeNi High-Entropy Alloy Prepared by Direct Laser Fabrication. J. Alloys Compd. 2017, 694, 971–981. [Google Scholar] [CrossRef]
- Zimogliadova, T.A.; Bataev, A.A.; Lazurenko, D.V.; Bataev, I.A.; Bataev, V.A.; Golkovskii, M.G.; Saage, H.; Ogneva, T.S.; Ruktuev, A.A. Structural Characterization of Layers Fabricated by Non-Vacuum Electron Beam Cladding of Ni-Cr-Si-B Self-Fluxing Alloy with Additions of Niobium and Boron. Mater. Today Commun. 2022, 33, 104363. [Google Scholar] [CrossRef]
- Ruktuev, A.A.; Golkovski, M.G.; Lazurenko, D.V.; Bataev, V.A.; Ivanov, I.V.; Thömmes, A.; Bataev, I.A. TiTaNb Clads Produced by Electron Beam Surface Alloying in Regular Air at Atmospheric Pressure: Fabrication, Structure, and Properties. Mater. Charact. 2021, 179, 111375. [Google Scholar] [CrossRef]
- Ruktuev, A.A.; Lazurenko, D.V.; Ogneva, T.S.; Kuzmin, R.I.; Golkovski, M.G.; Bataev, I.A. Structure and Oxidation Behavior of CoCrFeNiX (Where X Is Al, Cu, or Mn) Coatings Obtained by Electron Beam Cladding in Air Atmosphere. Surf. Coatings Technol. 2022, 448, 128921. [Google Scholar] [CrossRef]
- Ogneva, T.; Ruktuev, A.; Girsh, A. Non-Vacuum Electron Beam Cladding of Ti-Ni-Al Intermetallics on Titanium Alloy. Mater. Today Proc. 2019, 11, 191–196. [Google Scholar] [CrossRef]
- Ogneva, T.S.; Ruktuev, A.A.; Lazurenko, D.V.; Emurlaev, K.I.; Malyutina, Y.N.; Golkovsky, M.G.; Egoshin, K.D.; Bataev, I.A. Structure and Oxidation Behavior of NiAl-Based Coatings Produced by Non-Vacuum Electron Beam Cladding on Low-Carbon Steel. Metals 2022, 12, 1679. [Google Scholar] [CrossRef]
- Chen, R.; Xie, T.; Wu, B.; Weng, L.; Ali, H.; Yang, S.; Zhao, Y.; Zhao, P.; Zhang, C.; Cao, R.; et al. A General Approach to Simulate the Atom Distribution, Lattice Distortion, and Mechanical Properties of Multi-Principal Element Alloys Based on Site Preference: Using FCC_CoNiV and CoCrNi to Demonstrate and Compare. J. Alloys Compd. 2023, 935, 168016. [Google Scholar] [CrossRef]
- Silvello, A.; Torres Diaz, E.; Rúa Ramirez, E.; Garcia Cano, I. Microstructural, Mechanical and Wear Properties of Atmospheric Plasma-Sprayed and High-Velocity Oxy-Fuel AlCoCrFeNi Equiatomic High-Entropy Alloys (HEAs) Coatings. J. Therm. Spray Technol. 2023, 2023, 520. [Google Scholar] [CrossRef]
- Stryzhyboroda, O.; Witusiewicz, V.T.; Gein, S.; Röhrens, D.; Hecht, U. Phase Equilibria in the Al–Co–Cr–Fe–Ni High Entropy Alloy System: Thermodynamic Description and Experimental Study. Front. Mater. 2020, 7, 270. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Tian, F.; Delczeg, L.; Chen, N.; Varga, L.K.; Shen, J.; Vitos, L. Structural Stability of NiCoFeCrAlx High-Entropy Alloy from Ab Initio Theory. Phys. Rev. B 2013, 88, 85128. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, B.; Li, C.; Wang, Q.; Dong, C.; Liaw, P.K.; Xu, F.; Sun, L. The BCC/B2 Morphologies in AlxNiCoFeCr High-Entropy Alloys. Metals 2017, 7, 57. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Effects of AL Addition on Microstructure and Mechanical Properties of AlxCoCrFeNi High-Entropy Alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Zhang, G.J.; Tian, Q.W.; Yin, K.X.; Niu, S.Q.; Wu, M.H.; Wang, W.W.; Wang, Y.N.; Huang, J.C. Effect of Fe on Microstructure and Properties of AlCoCrFexNi (X = 1.5, 2.5) High Entropy Alloy Coatings Prepared by Laser Cladding. Intermetallics 2020, 119, 722. [Google Scholar] [CrossRef]
- Sistla, H.R.; Newkirk, J.W.; Frank Liou, F. Effect of Al/Ni Ratio, Heat Treatment on Phase Transformations and Microstructure of AlxFeCoCrNi2−x (X = 0.3, 1) High Entropy Alloys. Mater. Des. 2015, 81, 113–121. [Google Scholar] [CrossRef]
- Kuwabara, K.; Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Mechanical and Corrosion Properties of AlCoCrFeNi High-Entropy Alloy Fabricated with Selective Electron Beam Melting. Addit. Manuf. 2018, 23, 264–271. [Google Scholar] [CrossRef]
- Ostrowska, M.; Riani, P.; Bocklund, B.; Liu, Z.-K.; Cacciamani, G. Thermodynamic Modeling of the Al-Co-Cr-Fe-Ni High Entropy Alloys Supported by Key Experiments. J. Alloys Compd. 2022, 897, 162722. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Yeh, J.-W. Phases, Microstructure and Mechanical Properties of AlxCoCrFeNi High-Entropy Alloys at Elevated Temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Strumza, E.; Hayun, S. Comprehensive Study of Phase Transitions in Equiatomic AlCoCrFeNi High-Entropy Alloy. J. Alloys Compd. 2021, 856, 220. [Google Scholar] [CrossRef]
- Liu, K.; Komarasamy, M.; Gwalani, B.; Shukla, S.; Mishra, R.S. Fatigue Behavior of Ultrafine Grained Triplex Al0.3CoCrFeNi High Entropy Alloy. Scr. Mater. 2019, 158, 116–120. [Google Scholar] [CrossRef]
- Hou, J.; Shi, X.; Qiao, J.; Zhang, Y.; Liaw, P.K.; Wu, Y. Ultrafine-Grained Dual Phase Al0.45CoCrFeNi High-Entropy Alloys. Mater. Des. 2019, 180, 910. [Google Scholar] [CrossRef]
- Chen, M.; Lan, L.; Shi, X.; Yang, H.; Zhang, M.; Qiao, J. The Tribological Properties of Al0.6CoCrFeNi High-Entropy Alloy with the σ Phase Precipitation at Elevated Temperature. J. Alloys Compd. 2019, 777, 180–189. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; Lee, M.; Mantri, S.A.; Ren, Y.; Banerjee, R. Optimizing the Coupled Effects of Hall-Petch and Precipitation Strengthening in a Al0.3CoCrFeNi High Entropy Alloy. Mater. Des. 2017, 121, 254–260. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; Choudhuri, D.; Lee, M.; Hwang, J.Y.; Nam, S.J.; Ryu, H.; Hong, S.H.; Banerjee, R. Stability of Ordered L12 and B2 Precipitates in Face Centered Cubic Based High Entropy Alloys—Al0.3CoFeCrNi and Al0.3CuFeCrNi2. Scr. Mater. 2016, 123, 130–134. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J.; Wagemann, B. The Oxidation of NiAl-III. Internal and Intergranular Oxidation. Corros. Sci. 1994, 36, 37–53. [Google Scholar] [CrossRef]
- Pint, B.A.; Treska, M.; Hobbs, L.W. The Effect of Various Oxide Dispersions on the Phase Composition and Morphology of Al2O3 Scales Grown on β-NiAl. Oxid. Met. 1997, 47, 369. [Google Scholar] [CrossRef]
- Miracle, D.B.B. Overview No. 104 The Physical and Mechanical Properties of NiAl. Acta Metall. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]


| Element | Al, at. % | Co, at. % | Cr, at. % | Ni, at. % | Fe, at. % | C, at. % | Si, at. % | Mn, at. % |
|---|---|---|---|---|---|---|---|---|
| Substrate material | - | - | 0.25 | - | 98.72 | 0.2 | 0.28 | 0.55 |
| Powder mixture | 25 | 25 | 25 | 25 | - | - | - | - |
| Mass of Powder Mixture *, g | Mass of Flux *, g | Beam Current, мA | Table Velocity, mm/s | Mass of Powder Mixture per Unit Area **, g/cm2 | Energy Density, kJ/cm2 |
|---|---|---|---|---|---|
| 15.75 | 7.2 | 25 | 15 | 0.45 | 0.47 |
| Point # | Al, at. % | Cr, at. % | Fe, at. % | Co, at. % | Ni, at. % | Si, at. % | Mn, at. % | Corresponding Phases |
|---|---|---|---|---|---|---|---|---|
| Overall | 21.0 | 21.9 | 12.7 | 21.9 | 21.9 | 0.6 | - | bcc + fcc |
| 1 | 27.68 | 13.50 | 11.21 | 19.80 | 27.38 | 0.44 | - | bcc dendritic grains |
| 2 | 9.21 | 35.10 | 17.98 | 20.80 | 16.25 | 0.66 | - | fcc interdendritic |
| 3 | 16.24 | 12.10 | 39.24 | 13.82 | 17.74 | 0.51 | 0.35 | bcc dendritic grains near substrate |
| 4 | 9.83 | 7.58 | 62.78 | 8.31 | 10.41 | 0.66 | 0.42 | fcc interlayer |
| 5 | 4.87 | 3.25 | 86.48 | - | 4.21 | 0.68 | 0.51 | Martensite |
| No. of Zones | Al, at. % | Cr, at. % | Fe, at. % | Co, at. % | Ni, at. % | Si, at. % | Mn, at. % | Corresponding Phase |
|---|---|---|---|---|---|---|---|---|
| 1 | 34.47 | 0.37 | 12.18 | 15.47 | 31.36 | 6.15 | - | bcc dendritic grains |
| 2 | 9.80 | 29.07 | 24.86 | 19.16 | 16.05 | 1.07 | - | fcc interdendritic |
| 3 | 4.26 | 45.43 | 25.01 | 16.64 | 6.86 | 1.80 | - | σ-phase |
| 4 | 28.91 | 5.87 | 20.06 | 16.14 | 28.07 | 0.55 | 0.40 | bcc matrix |
| 5 | 7.07 | 17.97 | 40.96 | 18.44 | 14.71 | 0.86 | - | fcc precipitates |
| 6 | 27.81 | 6.14 | 30.75 | 11.97 | 22.96 | 0.37 | - | bcc matrix |
| 7 | 7.24 | 8.80 | 57.49 | 13.18 | 12.18 | 0.71 | 0.40 | fcc precipitates |
| 8 | 14.75 | 10.04 | 49.83 | 10.02 | 14.44 | 0.49 | 0.44 | fcc interlayer |
| No. of Zone | O, at. % | Al, at. % | Cr, at. % | Fe, at. % | Co, at. % | Ni, at. % | Si, at. % | Mn, at. % | Corresponding Phases |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 68.19 | 13.22 | 5.04 | 6.33 | 5.11 | 0.91 | 0.79 | 0.41 | Cr2O3 + spinels |
| 2 | 66.36 | 26.21 | 6.18 | 0.41 | 0.41 | 0.43 | - | - | Al2O3 |
| 3 | 14.60 | 12.92 | 22.47 | 14.91 | 26.01 | 9.09 | - | - | fcc |
| 4 | 41.52 | 28.15 | 9.46 | 6.12 | 10.96 | 3.80 | - | - | Al2O3 |
| 5 | - | 3.79 | 27.87 | 20.94 | 33.97 | 13.44 | - | - | fcc |
| 6 | - | 32.33 | 9.12 | 10.44 | 23.93 | 24.18 | - | - | bcc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogneva, T.; Emurlaev, K.; Malyutina, Y.; Domarov, E.; Chakin, I.; Ruktuev, A.; Riabinkina, P.; Yurgin, A.; Bataev, I. Heat Treatment Induced Structural Transformations and High-Temperature Oxidation Behavior of Al21Co22Cr22Fe13Ni22 High-Entropy Coatings Produced by Non-Vacuum Electron Beam Cladding. Metals 2023, 13, 1689. https://doi.org/10.3390/met13101689
Ogneva T, Emurlaev K, Malyutina Y, Domarov E, Chakin I, Ruktuev A, Riabinkina P, Yurgin A, Bataev I. Heat Treatment Induced Structural Transformations and High-Temperature Oxidation Behavior of Al21Co22Cr22Fe13Ni22 High-Entropy Coatings Produced by Non-Vacuum Electron Beam Cladding. Metals. 2023; 13(10):1689. https://doi.org/10.3390/met13101689
Chicago/Turabian StyleOgneva, Tatiana, Kemal Emurlaev, Yulia Malyutina, Evgeny Domarov, Ivan Chakin, Alexey Ruktuev, Polina Riabinkina, Aleksandr Yurgin, and Ivan Bataev. 2023. "Heat Treatment Induced Structural Transformations and High-Temperature Oxidation Behavior of Al21Co22Cr22Fe13Ni22 High-Entropy Coatings Produced by Non-Vacuum Electron Beam Cladding" Metals 13, no. 10: 1689. https://doi.org/10.3390/met13101689






