The Influence of Homogenisation Parameters on the Microstructure and Hardness of AlMnFeMgSi(Zr) Wrought Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigated Aluminium Alloys
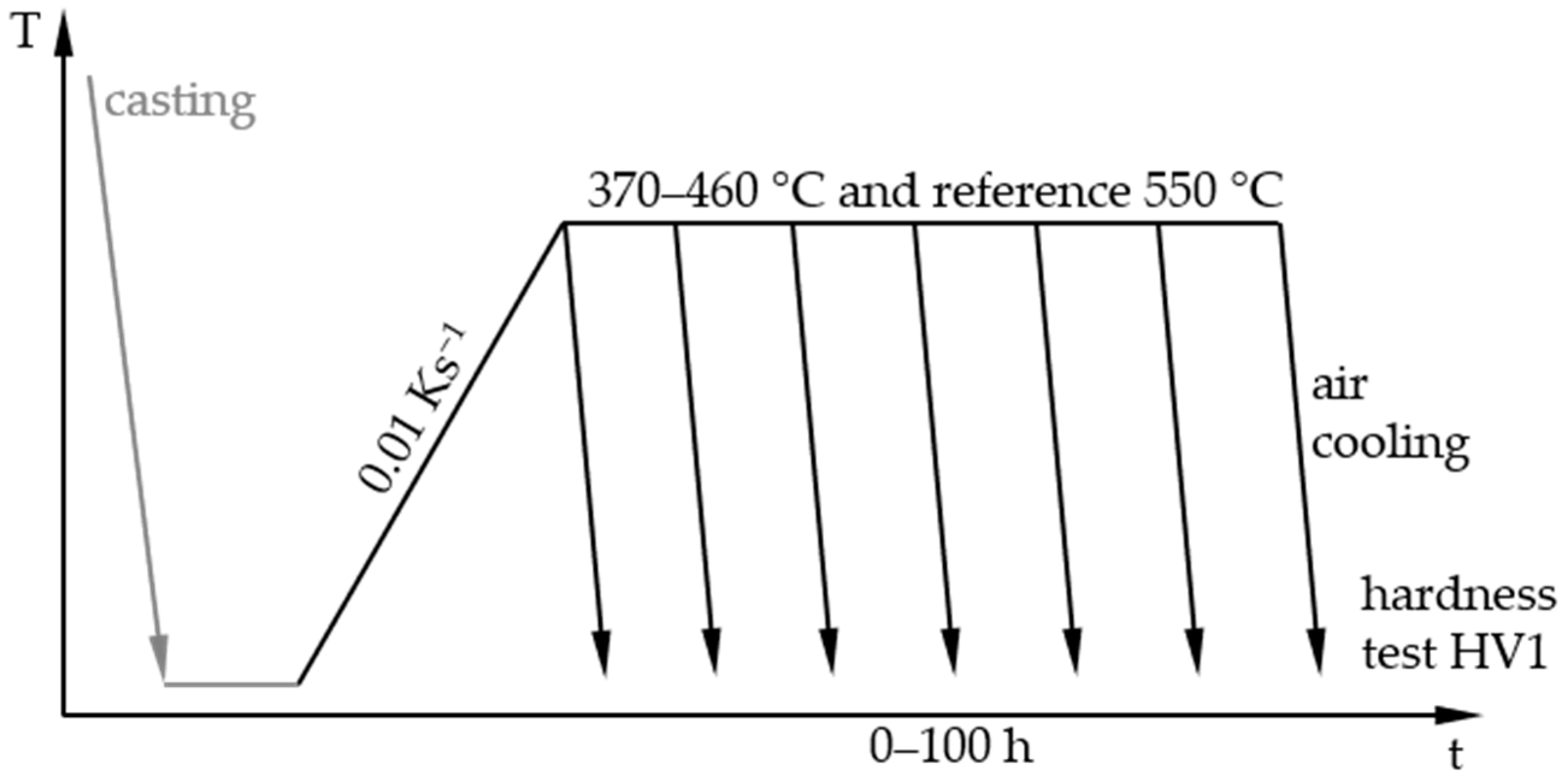
2.2. Heat Treatments and Hardness Tests
2.3. Micro- and Nanostructure Analysis
3. Results and Discussion
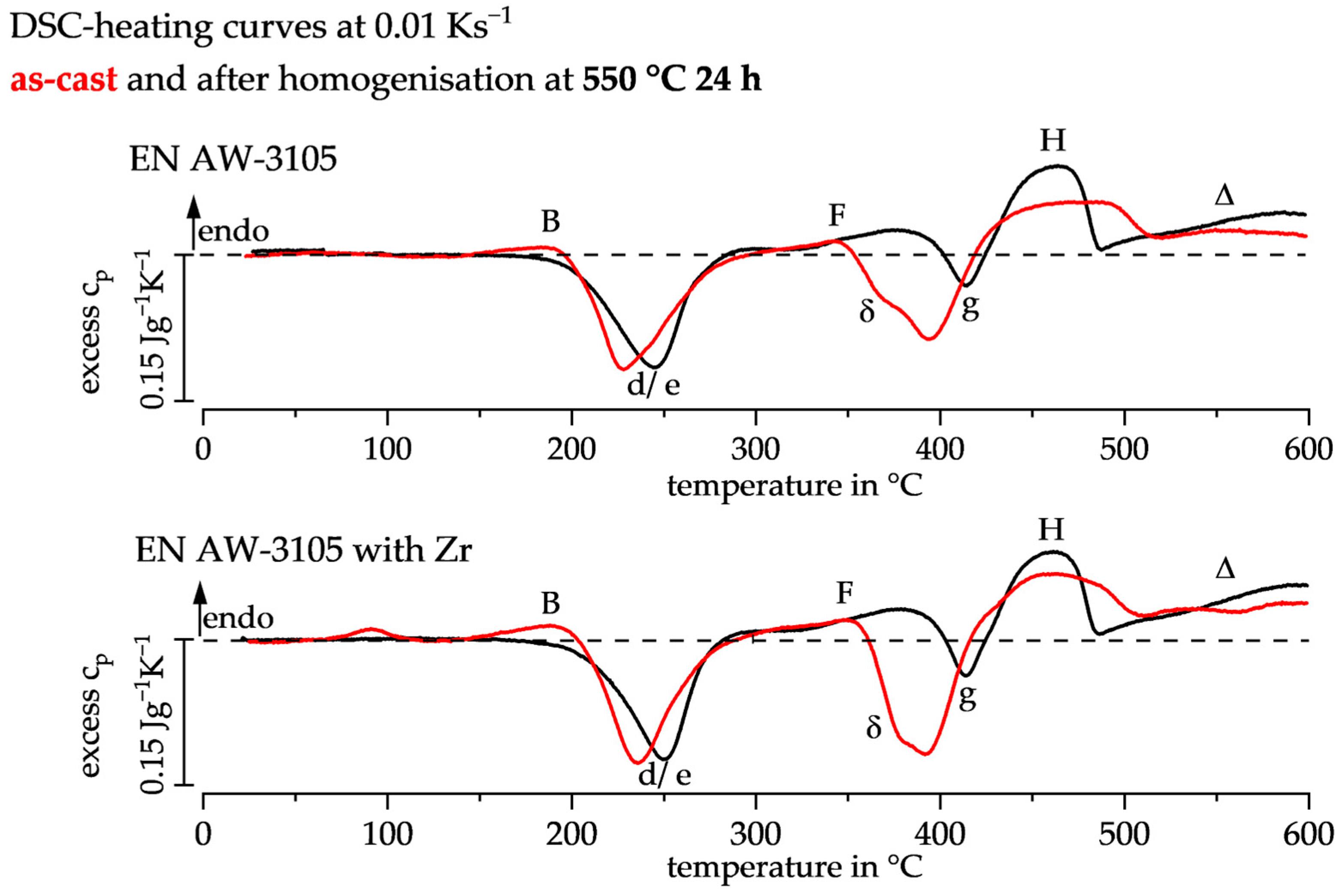
3.1. DSC Heating Curves of the As-Cast and Homogenised State
3.2. Hardness after Homogenisation
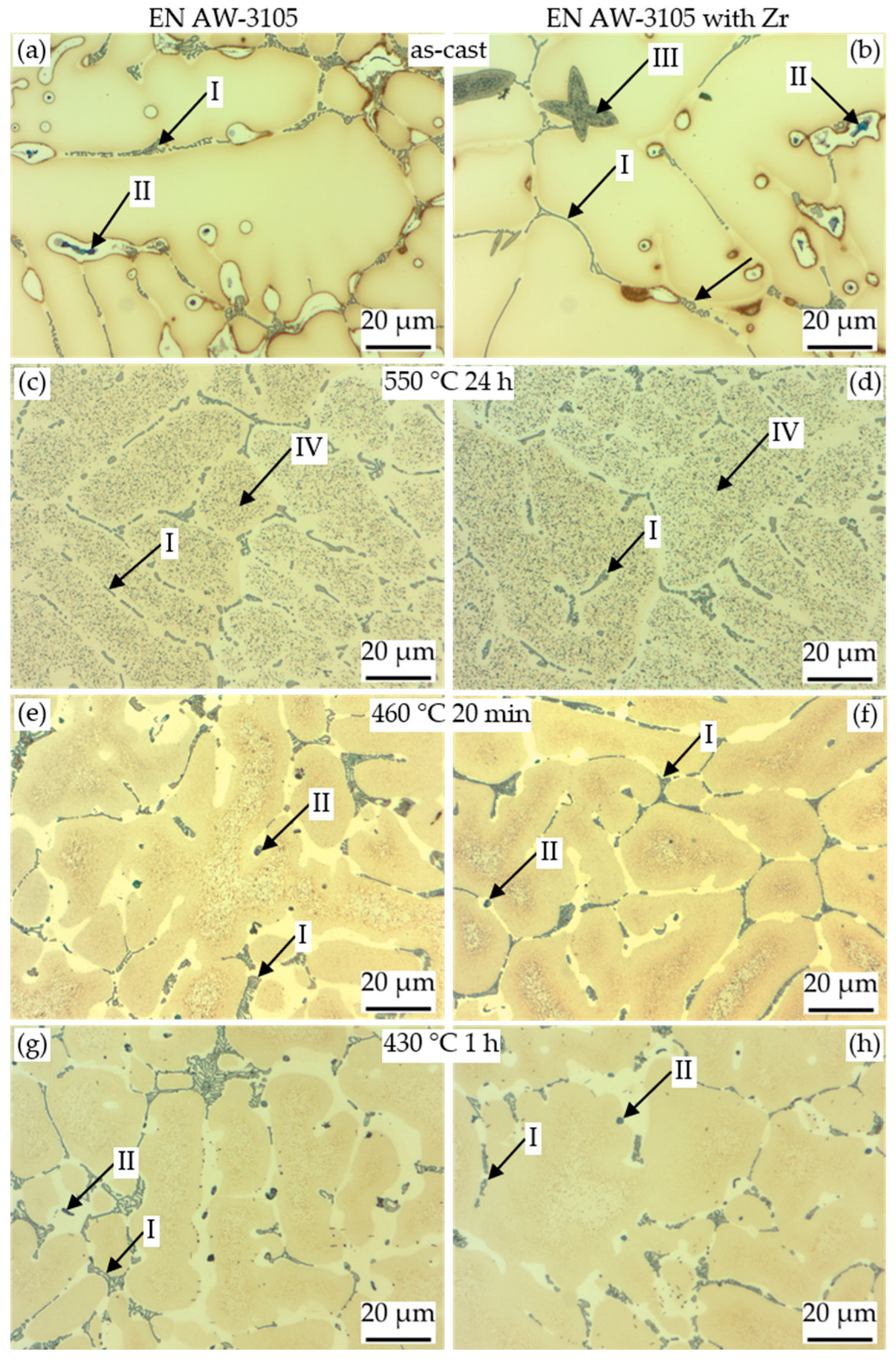
3.3. Microstructure of the Alloys in As-Cast and Homogenised State
3.3.1. Light Microscopy and SEM Analysis
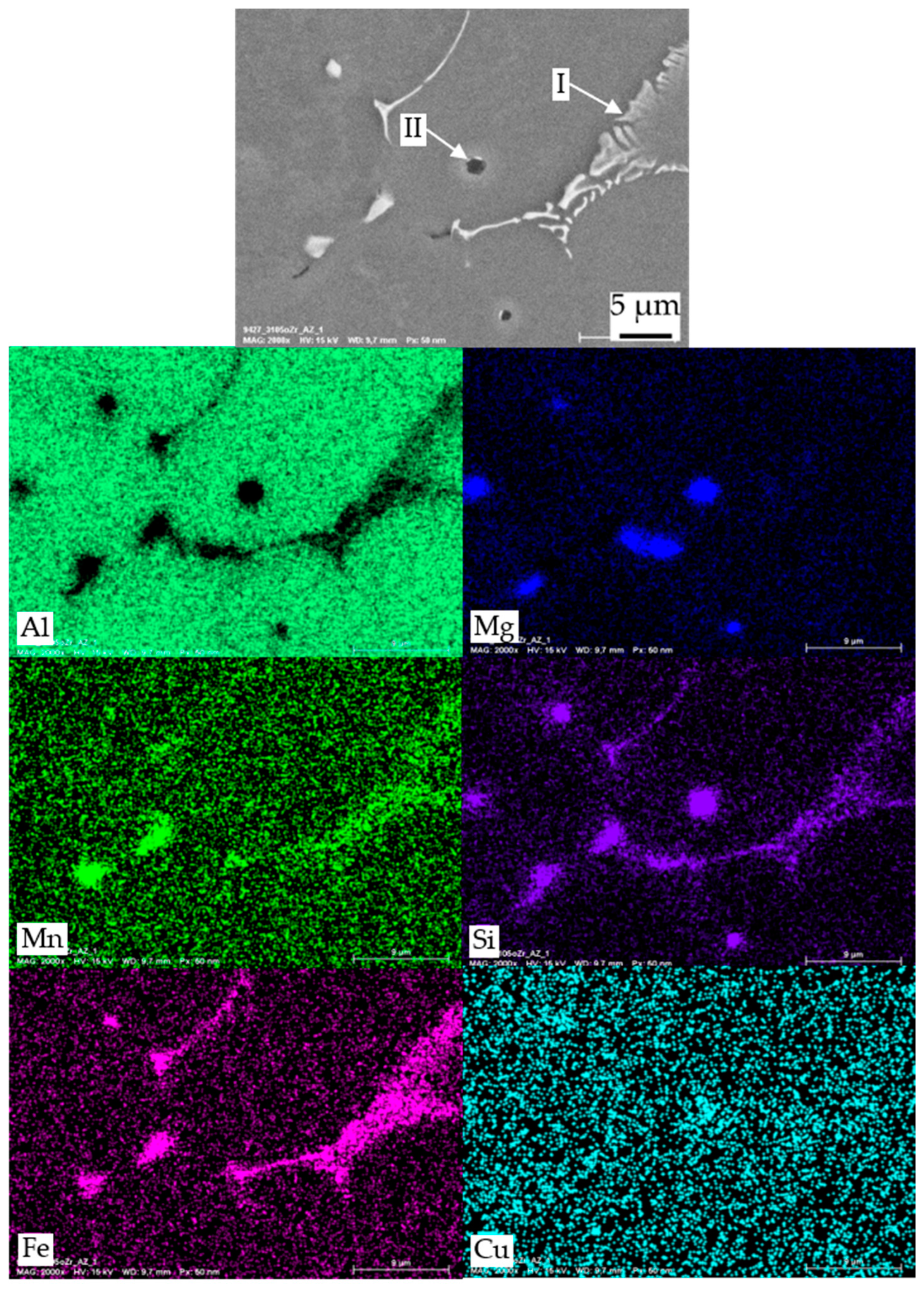
3.3.2. TEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, D.L.; Kang, S.B.; Koo, H.S. Characteristics of morphology and crystal structure of α-phase in two Al-Mn-Mg alloys. Mater. Chem. Phys. 2000, 63, 37–43. [Google Scholar] [CrossRef]
- Li, Y.; Arnberg, L. Evolution of eutectic intermetallic particles in DC-cast AA3003 alloy during heating and homogenization. Mater. Sci. Eng. A 2003, 347, 130–135. [Google Scholar] [CrossRef]
- Alexander, D.; Greer, A.L. Solid-state intermetallic phase tranformations in 3XXX aluminium alloys. Acta Mater. 2002, 50, 2571–2583. [Google Scholar] [CrossRef]
- Pettersen, T.; Li, Y.J.; Furu, T.; Marthinsen, K. Effect of Changing Homogenization Treatment on the Particle Structure in Mn-Containing Aluminium Alloys. Mater. Sci. Forum 2007, 558–559, 301–306. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.-G. Effect of Metastable Mg2Si and Dislocations on α-Al(MnFe)Si Dispersoid Formation in Al-Mn-Mg 3xxx Alloys. Met. Mater. Trans. A 2018, 49, 5799–5814. [Google Scholar] [CrossRef]
- Lodgaard, L.; Ryum, N. Precipitation of dispersoids containing Mn and/or Cr in Al–Mg–Si alloys. Mater. Sci. Eng. A 2000, 283, 144–152. [Google Scholar] [CrossRef]
- Kemsies, R.H.; Milkereit, B.; Wenner, S.; Holmestad, R.; Kessler, O. In situ DSC investigation into the kinetics and microstructure of dispersoid formation in Al-Mn-Fe-Si(-Mg) alloys. Mater. Des. 2018, 146, 96–107. [Google Scholar] [CrossRef]
- Pettersen, T.; Furu, T.; Håkonsen, A. Effect of Changing Homogenisation Treatment for the Alloys AA3102 and AA3103. Mater. Sci. Forum 2002, 396–402, 1067–1072. [Google Scholar] [CrossRef]
- Osten, J.; Milkereit, B.; Schick, C.; Kessler, O. Dissolution and precipitation behaviour during continuous heating of Al-Mg-Si alloys in a wide range of heating rates. Materials 2015, 8, 2830–2848. [Google Scholar] [CrossRef]
- Fröck, H.; Reich, M.; Milkereit, B.; Kessler, O. Scanning rate extension of conventional DSCs through indirect measurements. Materials 2019, 12, 1085. [Google Scholar] [CrossRef]
- Milkereit, B.; Österreich, M.; Schuster, P.; Kirov, G.; Mukeli, E.; Kessler, O. Dissolution and Precipitation Behavior for Hot Forming of 7021 and 7075 Aluminum Alloys. Metals 2018, 8, 531. [Google Scholar] [CrossRef]
- Kemsies, R.H. Dispersoidbildung und Dispersoidstabilität in Aluminium-Mangan-Legierungen. Ph.D. Thesis, Rostock University, Rostock, Germany, 2019. Available online: https://rosdok.uni-rostock.de/resolve/id/rosdok_disshab_0000002154 (accessed on 27 August 2023).
- Vlach, M.; Stulíková, I.; Smola, B.; Kudrnova, H.; Kekule, T.; Malek, J.; Ocenasek, V. Phase Transformations and Recrystallization in Cold-Rolled Al–Mn, Al–Sc–Zr and Al–Mn–Sc–Zr Alloy. Defect Diffus. Forum 2014, 354, 93–100. [Google Scholar] [CrossRef]
- Algendy, A.Y.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.-G. Effect of Sc and Zr Additions on Dispersoid Microstructure and Mechanical Properties of Hot-Rolled AA5083. In Light Metals 2023; Broek, S., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 1229–1237. ISBN 978-3-031-22531-4. [Google Scholar]
- Pan, S.; Wang, Z.; Li, C.; Wan, D.; Chen, X.; Chen, K.; Li, Y. Achieving superior dispersion-strengthening effect in an AA5xxx Al-Mg-Mn alloy by mico-alloying. Mater. Des. 2023, 226, 111647. [Google Scholar] [CrossRef]
- Elasheri, A.; Elgallad, E.M.; Parson, N.; Chen, X.-G. Evolution of Zr-Bearing Dispersoids during Homogenization and Their Effects on Hot Deformation and Recrystallization Resistance in Al-0.8%Mg-1.0%Si Alloy. J. Mater. Eng. Perform. 2021, 30, 7851–7862. [Google Scholar] [CrossRef]
- Iakoubovskii, K.; Mitsuishi, K.; Nakayama, Y.; Furuya, K. Thickness measurements with electron energy loss spectroscopy. Microsc. Res. Tech. 2008, 71, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Rebello, J.M.A.; Acselrad, O.; Dille, J.; Delplancke, J.L. Identification of Precipitates in 6013 Aluminum Alloy (Al–Mg–Si–Cu). Z. Met. 2002, 93, 208–211. [Google Scholar] [CrossRef]
- Ohmori, Y.; Doan, L.C.; Matsuura, Y.; Kobayashi, S.; Nakai, K. Morphology and Crystallography of β-Mg2Si Precipitation in Al-Mg-Si Alloys. Mater. Trans. 2001, 42, 2576–2583. [Google Scholar] [CrossRef]
- Li, Y.; Arnberg, L. Quantitative study on the precipitation behavior of dispersoids in DC-cast AA3003 alloy during heating and homogenization. Acta Mater. 2003, 51, 3415–3428. [Google Scholar] [CrossRef]
- Elasheri, A.; Elgallad, E.M.; Parson, N.; Chen, X.-G. Nucleation and transformation of Zr-bearing dispersoids in Al–Mg–Si 6xxx alloys. J. Mater. Res. 2023, 38, 696–707. [Google Scholar] [CrossRef]
- Lypchanskyi, O.; Rigas, N.; Korpała, G.; Merklein, M.; Prahl, U. Ex-situ and in-situ investigations of the microstructural evolution of AA6082 aluminum alloy during heat treatment. Mater. Sci. Eng. A 2023, 870, 144828. [Google Scholar] [CrossRef]
- Wong, K.M.; Daud, A.R.; Jalar, A. Microhardness and Tensile Properties of a 6XXX Alloy Through Minor Additions of Zr. J. Mater. Eng. Perform. 2009, 18, 62–65. [Google Scholar] [CrossRef]





| Alloy | Mass Fraction in % | ||||||
|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Ti | Zr | |
| EN AW-3105 | 0.53 | 0.49 | 0.17 | 0.73 | 0.59 | 0.014 | 0.002 |
| EN AW-3105 with Zr | 0.55 | 0.46 | 0.17 | 0.73 | 0.58 | 0.016 | 0.195 |
| DIN EN 573-3 | ≤0.6 | ≤0.7 | ≤0.3 | 0.3–0.8 | 0.2–0.8 | ≤0.1 | ≤0.05 |
| Alloy | EN AW-3105 | EN AW-3105 with Zr | ||
|---|---|---|---|---|
| State | 460 °C 20 min | 430 °C 1 h | 460 °C 20 min | 430 °C 1 h |
| analysed sample volume in µm3 | 0.66 | 1.16 | 0.75 | 0.65 |
| Peak | Dissolution Reaction | Precipitation Reaction |
|---|---|---|
| B | GP-zones [18] | |
| d/e | β″/MgSiCu precursor [18] | |
| F | β″/MgSiCu precursor | |
| δ | α-Al(Mn,Fe)Si [5] | |
| g | β-Mg2Si [19] | |
| H | β-Mg2Si | |
| Δ | α-Al(Mn,Fe)Si [20] |
| EN AW-3105 | EN AW-3105 with Zr | ||
|---|---|---|---|
| Temperature | Duration | Hardness ± std | Hardness ± std |
| 550 °C | 0 h | 46 ± 1 HV1 | 51 ± 2 HV1 |
| 460 °C | 0.33 h | 63 ± 2 HV1 | 70 ± 3 HV1 |
| 430 °C | 3 h | 62 ± 2 HV1 | 71 ± 2 HV1 |
| 400 °C | 10 h | 64 ± 1 HV1 | 70 ± 2 HV1 |
| 370 °C | 30 h | 61 ± 2 HV1 | 70 ± 1 HV1 |
| Particle Type | Contained Elements | Assumed Phase |
|---|---|---|
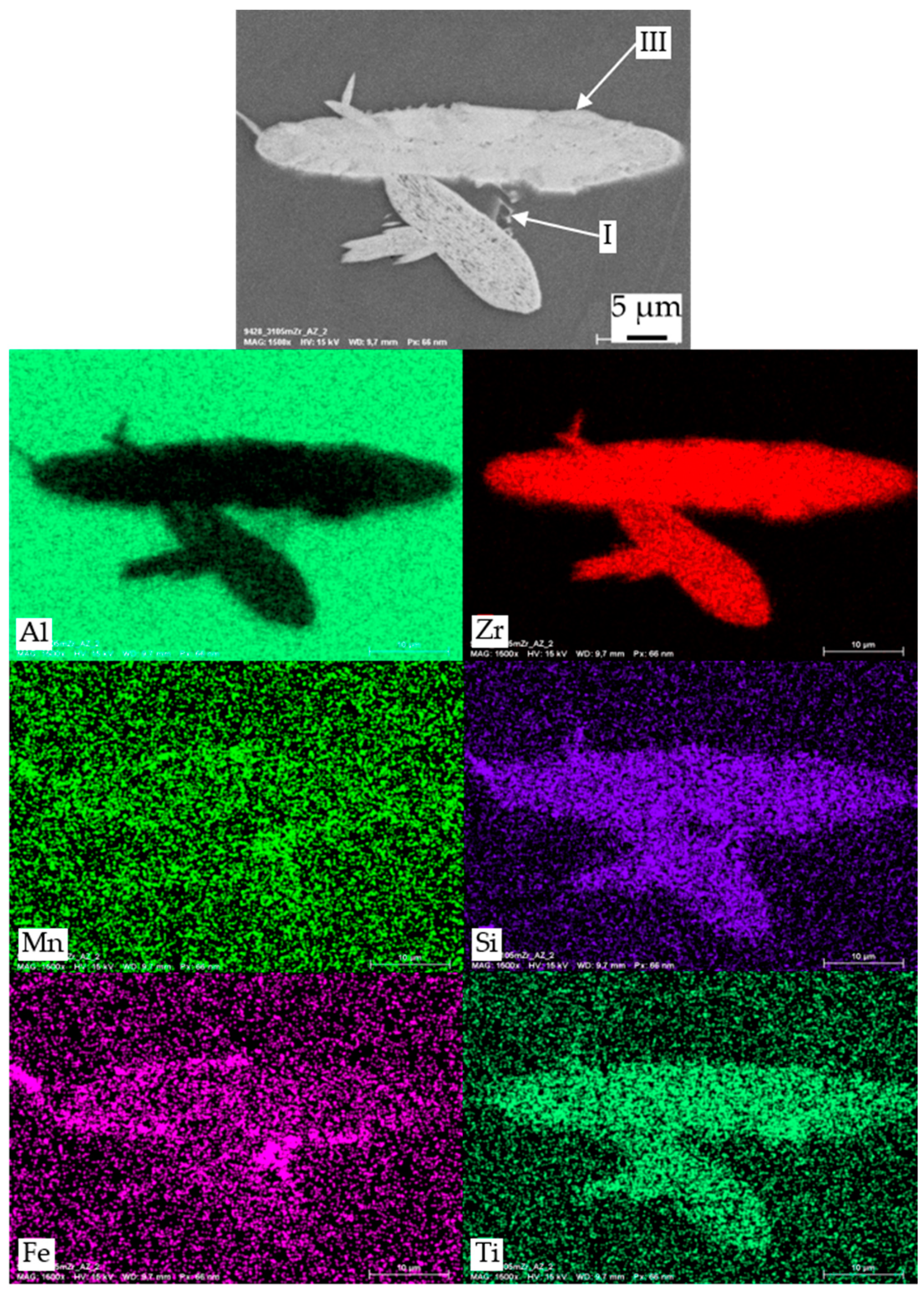
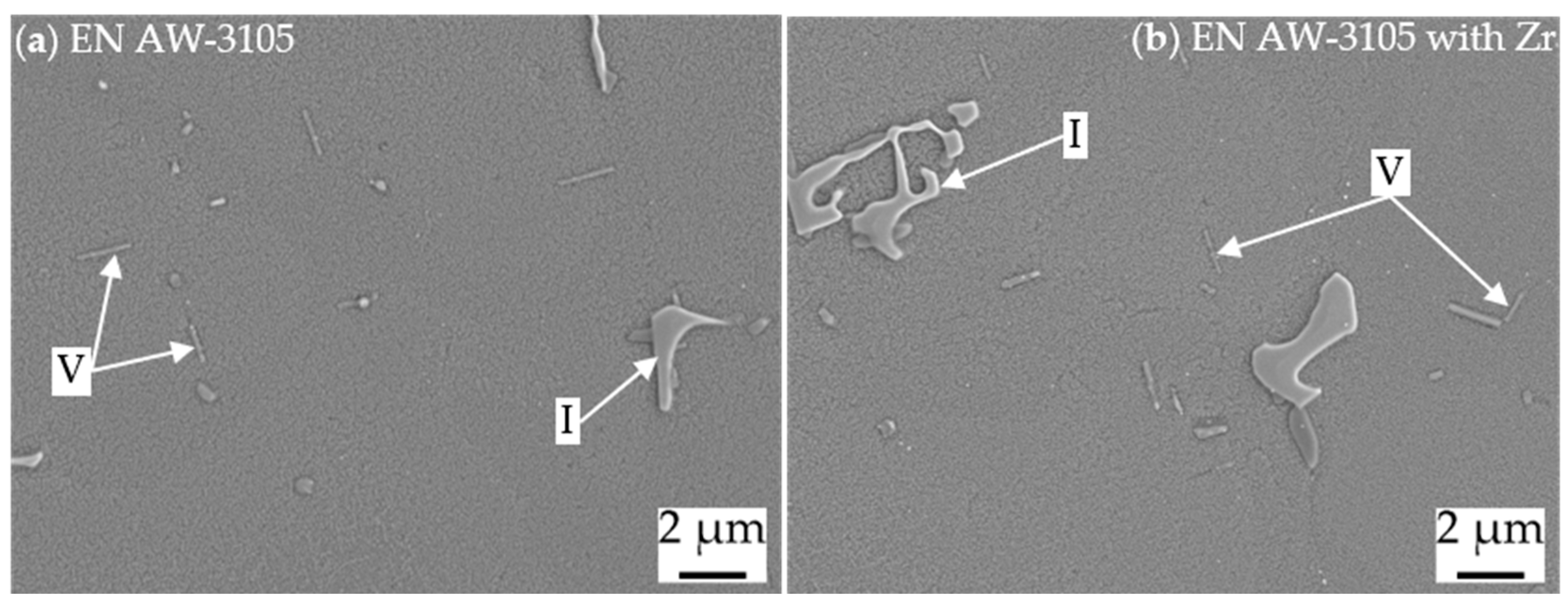
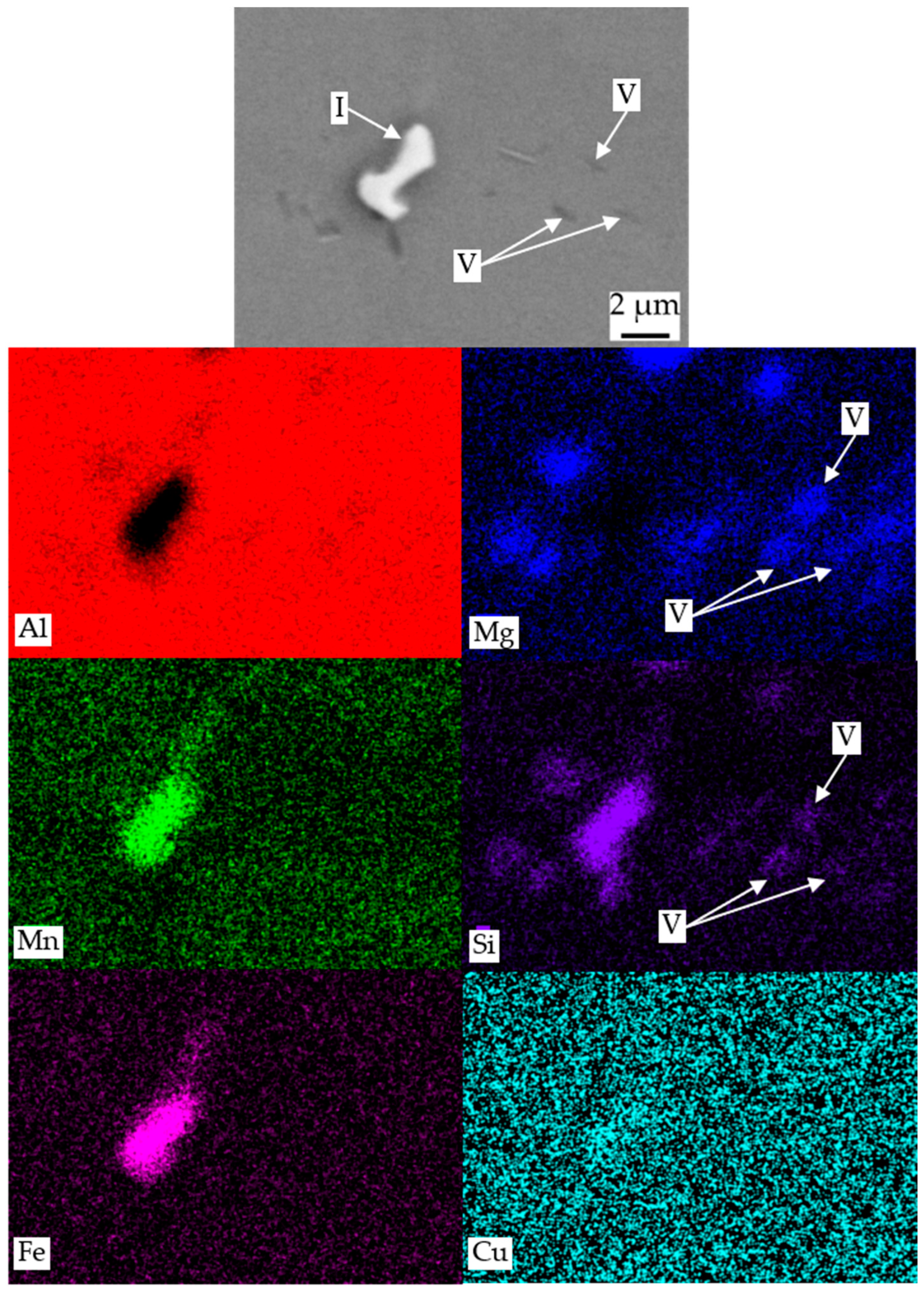
| I | Al, Mn, Si, Fe | primary α-Al(Mn,Fe)Si |
| II | Mg, Si, (Cu) | primary β-Mg2Si or Q-MgSiCu |
| III | Zr, Si, Ti, (Al) | primary Al3Zr |
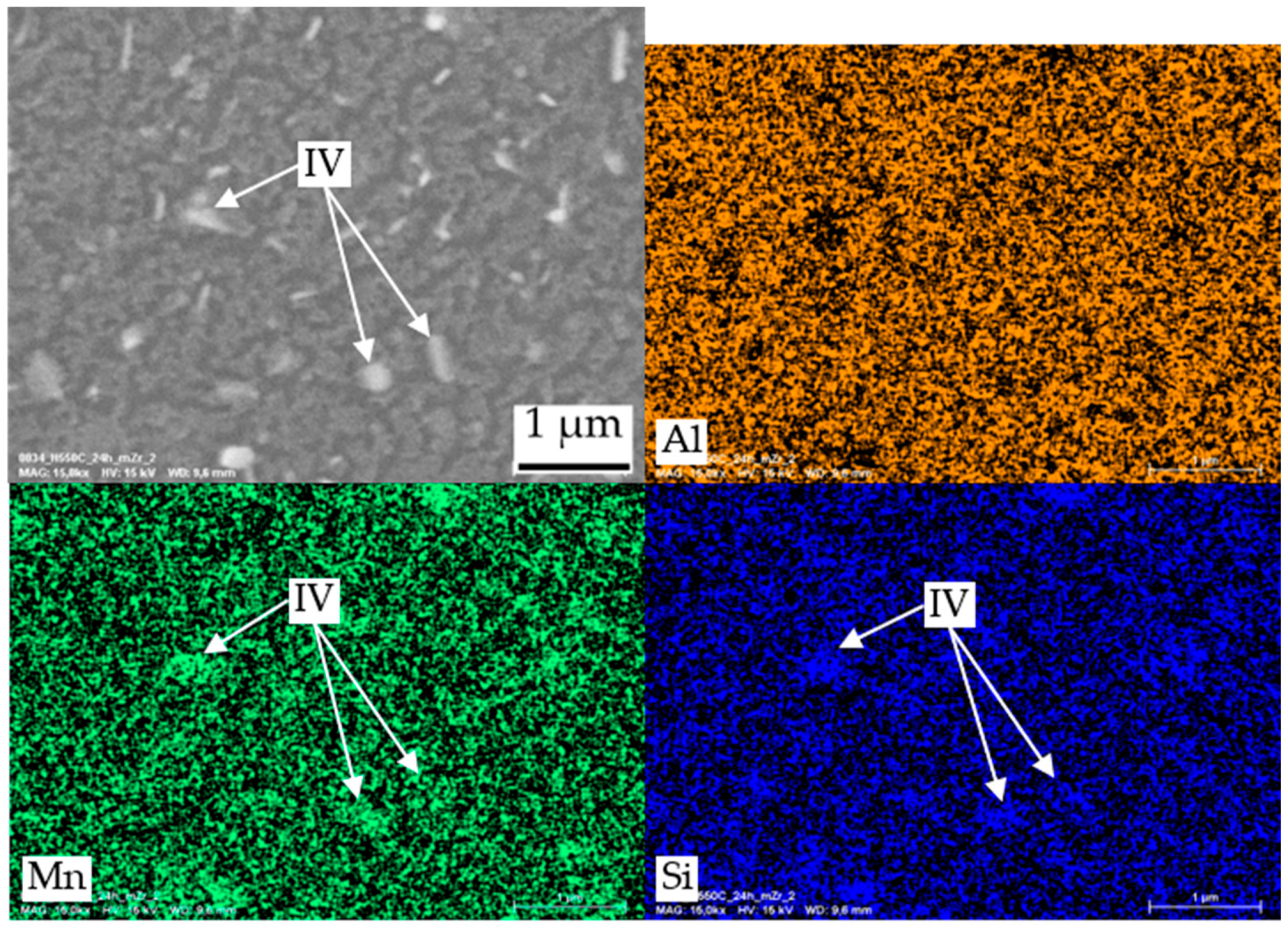
| IV | Mn, Si | dispersoids α-Al(Mn,Fe)Si |
| V | Mg, Si | secondary β or β′ [22] |
| Feret Diameter in nm | Sum of Frequency in % | |||
|---|---|---|---|---|
| EN AW-3105 | EN AW-3105 with Zr | |||
| 430 °C 1 h | 460 °C 20 min | 430 °C 1 h | 460 °C 20 min | |
| <10 | 4.1 | 5.0 | 42.6 | 46.2 |
| 10–30 | 49.8 | 50.0 | 32.1 | 32.8 |
| 30–60 | 28.3 | 39.7 | 20.4 | 16.4 |
| >60 | 17.8 | 5.3 | 4.9 | 4.6 |
| Alloy | EN AW-3105 | EN AW-3105 with Zr | ||
|---|---|---|---|---|
| State | 460 °C 20 min | 430 °C 1 h | 460 °C 20 min | 430 °C 1 h |
| Number density per µm3 | 1057 | 505 | 2783 | 2348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broer, J.; Mallow, S.; Oldenburg, K.; Milkereit, B.; Kessler, O. The Influence of Homogenisation Parameters on the Microstructure and Hardness of AlMnFeMgSi(Zr) Wrought Alloys. Metals 2023, 13, 1706. https://doi.org/10.3390/met13101706
Broer J, Mallow S, Oldenburg K, Milkereit B, Kessler O. The Influence of Homogenisation Parameters on the Microstructure and Hardness of AlMnFeMgSi(Zr) Wrought Alloys. Metals. 2023; 13(10):1706. https://doi.org/10.3390/met13101706
Chicago/Turabian StyleBroer, Jette, Sina Mallow, Kevin Oldenburg, Benjamin Milkereit, and Olaf Kessler. 2023. "The Influence of Homogenisation Parameters on the Microstructure and Hardness of AlMnFeMgSi(Zr) Wrought Alloys" Metals 13, no. 10: 1706. https://doi.org/10.3390/met13101706





