Effect of Pt Addition on the Oxidation and Corrosion Resistance of Al0.25CoCrFeNi High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
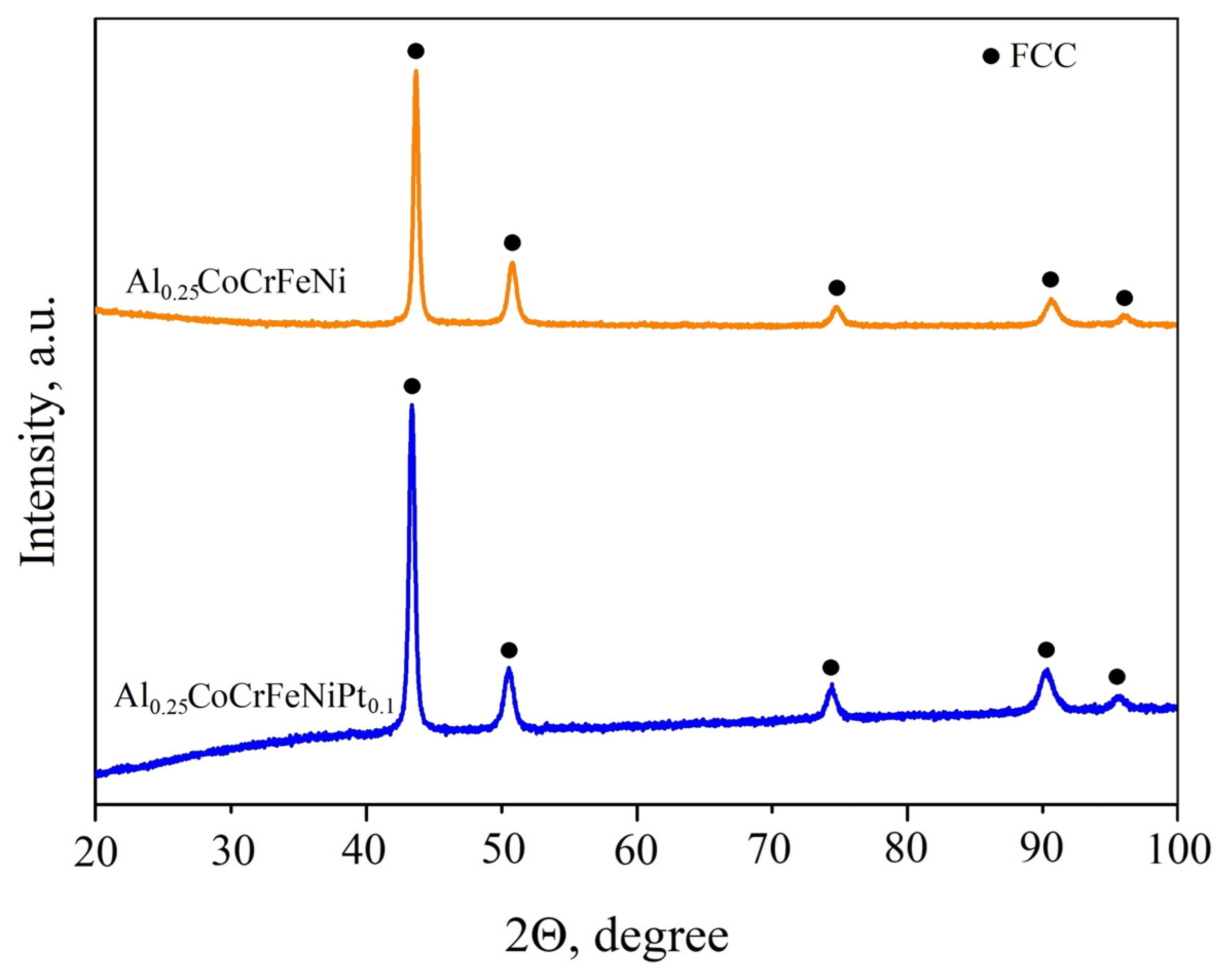
3.1. Microstructure and Phase Composition
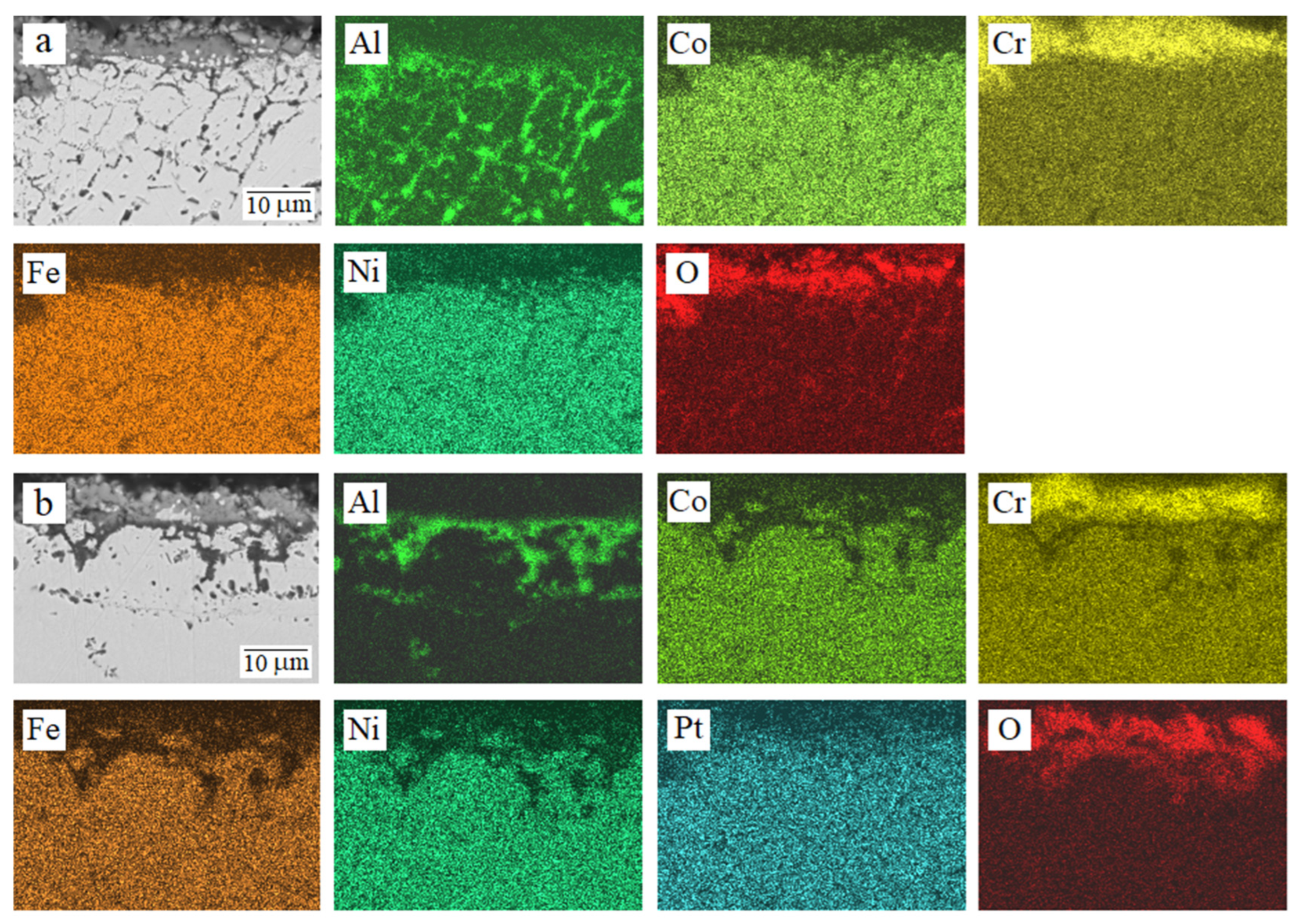
3.2. High-Temperature Oxidation
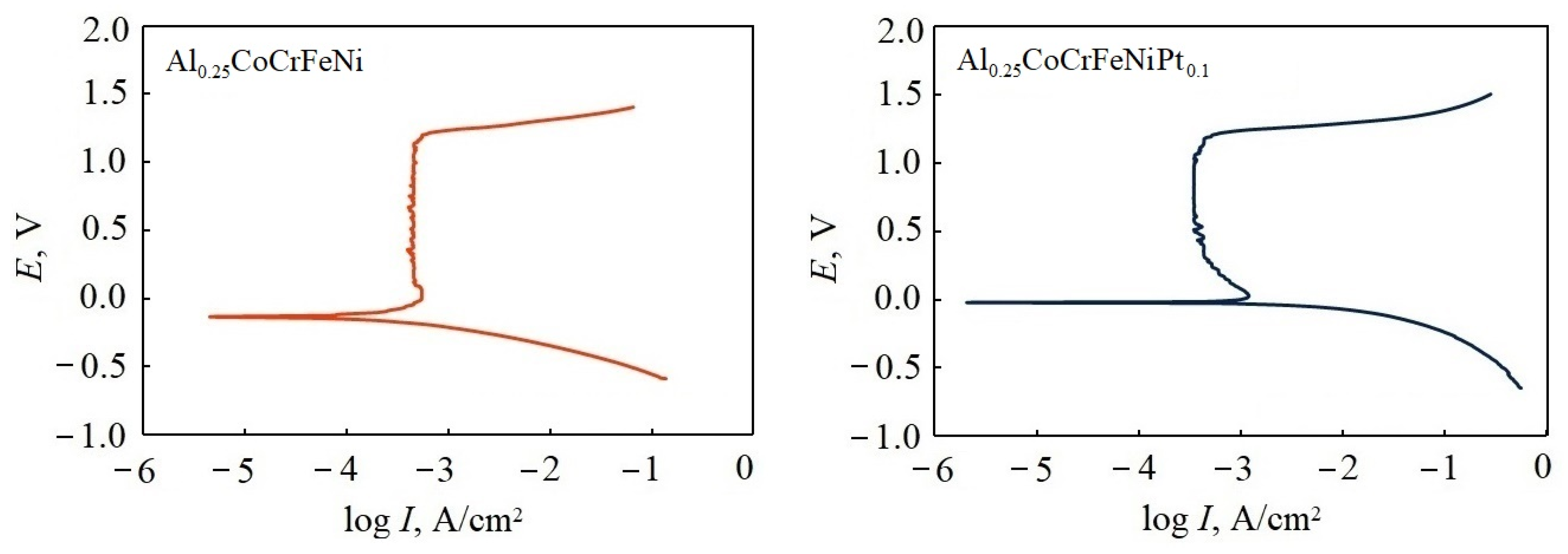
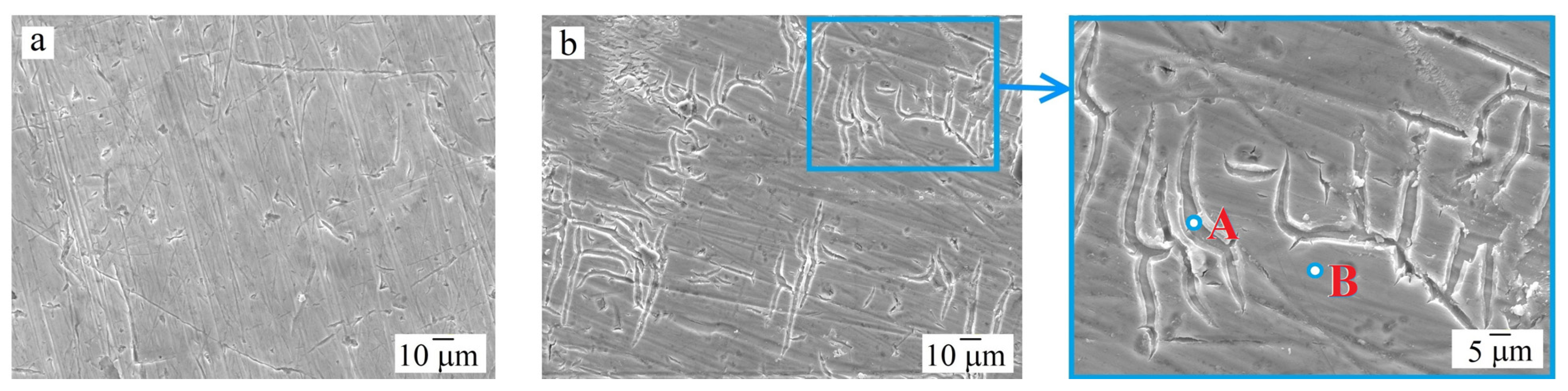
3.3. Corrosion Resistance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—A review. Materials 2021, 14, 5814. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Yang, L.; Guo, Y.; Wang, Y. High hardness and wear resistance in AlCrFeNiV high-entropy alloy induced by dual-phase body-centered cubic coupling effects. Materials 2022, 15, 6896. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Luan, J.H.; Han, B.; Wei, J.; Kai, J.J.; Liu, C.T. Nanoparticles-strengthened high-entropy alloys for cryogenic applications showing an exceptional strength-ductility synergy. Scr. Mater. 2019, 164, 30–35. [Google Scholar] [CrossRef]
- Cho, F.Y.; Tung, S.W.; Ouyang, F.Y. High temperature oxidation behavior of high entropy alloy Al4Co3Cr25Cu10Fe25Ni33 in oxygen-containing atmospheres. Mater. Chem. Phys. 2022, 278, 125678. [Google Scholar] [CrossRef]
- Kumar, P.; Lam, T.-N.; Tripathi, P.K.; Singh, S.S.; Liaw, P.K.; Huang, E.-W. Recent progress in oxidation behavior of high-entropy alloys: A review. APL Mater. 2022, 10, 120701. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. NPJ Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal spray high-entropy alloy coatings: A review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.; Hao, J. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Samoilova, O.; Shaburova, N.; Samodurova, M.; Pashkeev, K.; Ostovari Moghaddam, A.; Trofimov, E. Microstructure and wear behavior of Al0.25CoCrFeNiSi0.6 high-entropy alloy coating deposited on stainless steel by detonation spraying. J. Therm. Spray Technol. 2023, 32, 1220–1229. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Tan, Z.; He, D.; Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. High temperature oxidation behavior of Al0.6CrFeCoNi and Al0.6CrFeCoNiSi0.3 high entropy alloys. J. Alloys Compd. 2018, 764, 845–852. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Shaburova, N.A.; Sudarikov, M.V.; Veselkov, S.N.; Samoilova, O.V.; Trofimov, E.A. High temperature oxidation resistance of Al0.25CoCrFeNiMn and Al0.45CoCrFeNiSi0.45 high entropy alloys. Vacuum 2021, 192, 110412. [Google Scholar] [CrossRef]
- Shaburova, N.A.; Ostovari Moghaddam, A.; Veselkov, S.N.; Sudarikov, M.V.; Samoilova, O.V.; Trofimov, E.A. High-temperature oxidation behavior of AlxCoCrFeNiM (M = Cu, Ti, V) high-entropy alloys. Phys. Mesomech. 2021, 24, 653–662. [Google Scholar] [CrossRef]
- Yang, J.-J.; Kuo, C.-M.; Lin, P.-T.; Liu, H.-C.; Huang, C.-Y.; Yen, H.-W.; Tsai, C.-W. Improvement in oxidation behavior of Al0.2Co1.5CrFeNi1.5Ti0.3 high-entropy superalloys by minor Nb addition. J. Alloys Compd. 2020, 825, 153983. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Mroczka, K.; Berent, K.; Kulik, T.; Danielewski, M. Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x = 0; 0.5; 1). Intermetallics 2017, 84, 52–61. [Google Scholar] [CrossRef]
- Pratskova, S.; Samoilova, O.; Ageenko, E.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. Corrosion resistance of AlxCoCrFeNiM (M = Ti, V, Si, Mn, Cu) high entropy alloys in NaCl and H2SO4 solutions. Metals 2022, 12, 352. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, Z.; Xu, H.; Sun, Y. Studies on the microstructure and properties of AlxCoCrFeNiTi1-x high entropy alloys. J. Alloys Compd. 2018, 741, 826–833. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Fabijanic, D.; Barlow, A.J.; Fraser, H.L.; Birbilis, N. Microstructural evolution, electrochemical and corrosion properties of AlxCoCrFeNiTiyhigh entropy alloys. Mater. Des. 2019, 170, 107698. [Google Scholar] [CrossRef]
- Li, B.Y.; Peng, K.; Hu, A.P.; Zhou, L.P.; Zhu, J.J.; Li, D.Y. Structure and properties of FeCoNiCrCu0.5Alxhigh-entropy alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 735–741. [Google Scholar] [CrossRef]
- Qiu, X.W. Microstructure and properties of AlCrFeNiCoCu high entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2013, 555, 246–249. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, Z.M.; Fu, H.M.; Han, E.-H.; Zhang, H.F.; Wang, J.Q. Microstructure and corrosion behavior of AlCoCrFeNiSi0.1 high-entropy alloy. Intermetallics 2019, 114, 106599. [Google Scholar] [CrossRef]
- Samoilova, O.; Pratskova, S.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. Corrosion resistance of Al0.5CoCrFeNiCuxAgy(x = 0.25, 0.5; y = 0, 0.1) high-entropy alloys in 0.5M H2SO4 solution. Materials 2023, 16, 3585. [Google Scholar] [CrossRef]
- Swalin, R.A. Thermodynamics of Solids, 2nd ed.; Wiley: New York, NY, USA, 1972; 400p. [Google Scholar]
- Wang, W.-R.; Wang, W.-L.; Yeh, J.-W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Ogura, M.; Fukushima, T.; Zeller, R.; Dederichs, P.H. Structure of the high-entropy alloy AlxCrFeCoNi: Fcc versus bcc. J. Alloys Compd. 2017, 715, 454–459. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, S.; Jin, Y.; Xu, L.; Xu, X.; Yin, C.; Jia, Y. High-temperature oxidation behaviours of AlCoCrFeNi high-entropy alloy at 1073–1273 K. Oxid. Met. 2020, 94, 265–281. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Pakseresht, A.; Omidvar, H.; Shafiei, A. Investigation of the high-temperature oxidation behavior of the Al0.5CoCrFeNi high entropy alloy. Surf. Interf. 2020, 21, 100724. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Z.; Chen, Y.Z.; Shi, J.C.; Wang, Z.Y.; Wang, S.; Liu, F. Effect of Al content on high temperature oxidation resistance of AlxCoCrCuFeNi high entropy alloys (x = 0, 0.5, 1, 1.5, 2). Vacuum 2019, 169, 108837. [Google Scholar] [CrossRef]
- Mohanty, A.; Sampreeth, J.K.; Bembalge, O.; Hascoet, J.Y.; Marya, S.; Immanuel, R.J.; Panigrahi, S.K. High temperature oxidation study of direct laser deposited AlXCoCrFeNi (X=0.3, 0.7) high entropy alloys. Surf. Coat. Technol. 2019, 380, 125028. [Google Scholar] [CrossRef]
- Kai, W.; Li, C.C.; Cheng, F.P.; Chu, K.P.; Huang, R.T.; Tsay, L.W.; Kai, J.J. Air-oxidation of FeCoNiCr-based quinary high-entropy alloys at 700–900 °C. Corros. Sci. 2017, 121, 116–125. [Google Scholar] [CrossRef]
- Lussana, D.; Baldissin, D.; Massazza, M.; Baricco, M. Thermodynamic and kinetics aspects of high temperature oxidation on a 304L stainless steel. Oxid. Met. 2014, 81, 515–528. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.B.; Liu, Y.D.; Zhang, W.L.; Wang, T.G.; Jiang, S.M.; Gong, J.; Sun, C. The role of Re in effecting isothermal oxidation behavior of β-(Ni,Pt)Al coating on a Ni-based single crystal superalloy. Corros. Sci. 2020, 176, 108892. [Google Scholar] [CrossRef]
- Baskaran, T.; Esakkiraja, N.; Samartha, C.; Kumar, P.; Jayaram, V.; Paul, A. Effect of addition of Pt, Pd and Ir to β-NiAl-bond coat on oxidation resistance and growth of interdiffusion zone. Surf. Coat. Technol. 2021, 426, 127766. [Google Scholar] [CrossRef]
- Bai, M.; Chen, Y.; Xiao, P. Investigations on the diffusion of platinum between CMSX-4 superalloy and platinum-enriched bond coat. Coatings 2021, 11, 441. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Peng, H.; Zheng, L.; Guo, H.B.; Gong, S.K. Thermal cycling performance of La2Ce2O7/YSZ TBCs with Pt/Dy co-doped NiAl bond coat on single crystal superalloy. Rare Met. 2021, 40, 2568–2578. [Google Scholar] [CrossRef]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Moxin aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Xiao, D.H.; Zhou, P.F.; Wu, W.Q.; Diao, H.Y.; Gao, M.C.; Song, M.; Liaw, P.K. Microstructure, mechanical and corrosion behaviors of AlCoCuFeNi-(Cr,Ti) high entropy alloys. Mater. Des. 2017, 116, 438–447. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of aluminium content of AlxCrFe1.5MnNi0.5 high–entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]




| Alloy | Al | Co | Cr | Fe | Ni | Pt | ΔSmix, J∙mole−1∙K−1 |
|---|---|---|---|---|---|---|---|
| Al0.25CoCrFeNi | 5.86 | 23.47 | 23.88 | 23.21 | 23.58 | – | 1.53R |
| Al0.25CoCrFeNiPt0.1 | 5.64 | 22.93 | 23.10 | 22.96 | 23.05 | 2.32 | 1.60R |
| Alloy | kp | Alloy | kp |
|---|---|---|---|
| Al0.25CoCrFeNi | 22 × 10−13 | Al0.2Co1.5CrFeNi1.5Ti0.3Nb0.05 [19] | 78.5 × 10−13 |
| Al0.25CoCrFeNiPt0.1 | 5.62 × 10−13 | Al0.5CoCrFeNi [33] | 8.91 × 10−13 |
| Al0.6CrFeCoNi [16] | 11.8 × 10−13 | FeCoNiCrAl [36] | 4.9 × 10−13 |
| Al0.2Co1.5CrFeNi1.5Ti0.3 [19] | 280 × 10−13 | AISI 304L [37] | 23 × 10−13 |
| Alloy | Al | Co | Cr | Fe | Ni | Pt | O | |
|---|---|---|---|---|---|---|---|---|
| Al0.25CoCrFeNi | Av | 8.99 | 4.54 | 23.92 | 4.30 | 4.75 | – | 53.50 |
| LC | 23.86 | 3.20 | 13.88 | 3.15 | 3.11 | – | 52.80 | |
| RC | 0.06 | 5.18 | 31.36 | 4.37 | 5.52 | – | 53.51 | |
| Al0.25CoCrFeNiPt0.1 | Av | 14.11 | 4.21 | 19.19 | 4.17 | 3.37 | 0.21 | 54.74 |
| LC | 22.71 | 4.17 | 11.25 | 4.30 | 3.38 | 0.27 | 53.92 | |
| RC | 1.02 | 3.83 | 33.03 | 3.37 | 2.93 | 0.08 | 55.74 | |
| Alloy | Ecorr, V | Icorr, μA/cm2 | Rp, kΩcm2 | Ipas, μA/cm2 | ΔE | Eb, V |
|---|---|---|---|---|---|---|
| Al0.25CoCrFeNi | −0.132 ± 0.003 | 6.67 ± 0.05 | 1.12 ± 0.03 | 417 | 1.262 | 1.195 |
| Al0.25CoCrFeNiPt0.1 | −0.018 ± 0.002 | 3.93 ± 0.02 | 6.79 ± 0.05 | 356 | 1.222 | 1.218 |
| Al0.25CoCrFeNiCu [22] | −0.164 ± 0.002 | 607 ± 3 | 0.088 ±0.001 | 271 | 0.077 | – |
| Al0.5CoCrFeNiTi0.5 [23] | −0.400 | 320 | – | 24.8 | 0.650 | 0.980 |
| FeCoNiCrCu0.5Al0.5 [25] | −0.112 | 4.190 | – | 3000 | 0.550 | – |
| AlCoCrFeNiSi0.1 [27] | −0.453 | 304.20 | – | 19.35 | 1.350 | 0.925 |
| SS 304 [27] | −0.438 | 105.12 | – | 4.47 | 1.261 | 0.925 |
| Al0.5CoCrFeNiCu0.25 [28] | −0.112 ± 0.002 | 3.52 ± 0.02 | 7.81 ± 0.05 | 21.5 | 0.967 | – |
| Al0.5CoCrFeNiCu0.25Ag0.1 [28] | −0.143 ± 0.002 | 5.21 ± 0.05 | 8.47 ± 0.05 | 28.8 | 1.282 | – |
| Al0.25CoCrFeNi [42] | −0.095 | 16.7 | – | 7.1 | 1.080 | – |
| SS 304 [42] | −0.185 | 45.3 | – | 19.1 | 0.750 | – |
| Co1.5CrFeNi1.5Ti0.5 [43] | −0.092 | 30 | – | 9 | 1.130 | 1.089 |
| Co1.5CrFeNi1.5Ti0.5Mo0.1 [43] | −0.071 | 78 | – | 9 | 1.112 | 1.089 |
| AlCoCuFeNiCr [44] | −0.075 | 5.09 | 14.450 | – | 0.650 | – |
| AlCoCuFeNiCrTi [44] | −0.256 | 39.59 | 1.558 | – | 0.940 | – |
| Al0.3CrFe1.5MnNi0.5 [45] | −0.194 | 2390 | – | 73.9 | 1.176 | – |
| Alloy | Al | Co | Cr | Fe | Ni | Pt | O | S | |
|---|---|---|---|---|---|---|---|---|---|
| Al0.25CoCrFeNi | Av | 5.66 | 21.88 | 23.17 | 21.54 | 22.03 | – | 5.64 | 0.08 |
| Al0.25CoCrFeNiPt0.1 | Av | 4.10 | 19.91 | 21.45 | 19.56 | 19.50 | 5.60 | 8.94 | 0.94 |
| A | 2.40 | 19.68 | 24.64 | 18.11 | 19.36 | 2.62 | 12.08 | 1.11 | |
| B | 4.53 | 20.45 | 20.72 | 20.56 | 20.64 | 6.91 | 5.77 | 0.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilova, O.; Pratskova, S.; Suleymanova, I.; Shaburova, N.; Ostovari Moghaddam, A.; Trofimov, E. Effect of Pt Addition on the Oxidation and Corrosion Resistance of Al0.25CoCrFeNi High-Entropy Alloy. Metals 2023, 13, 1709. https://doi.org/10.3390/met13101709
Samoilova O, Pratskova S, Suleymanova I, Shaburova N, Ostovari Moghaddam A, Trofimov E. Effect of Pt Addition on the Oxidation and Corrosion Resistance of Al0.25CoCrFeNi High-Entropy Alloy. Metals. 2023; 13(10):1709. https://doi.org/10.3390/met13101709
Chicago/Turabian StyleSamoilova, Olga, Svetlana Pratskova, Ilsiya Suleymanova, Nataliya Shaburova, Ahmad Ostovari Moghaddam, and Evgeny Trofimov. 2023. "Effect of Pt Addition on the Oxidation and Corrosion Resistance of Al0.25CoCrFeNi High-Entropy Alloy" Metals 13, no. 10: 1709. https://doi.org/10.3390/met13101709






