Efficient Sintering of Mo Matrix Composites—A Study of Temperature Dependences and the Use of the Sinter Additive Ni
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
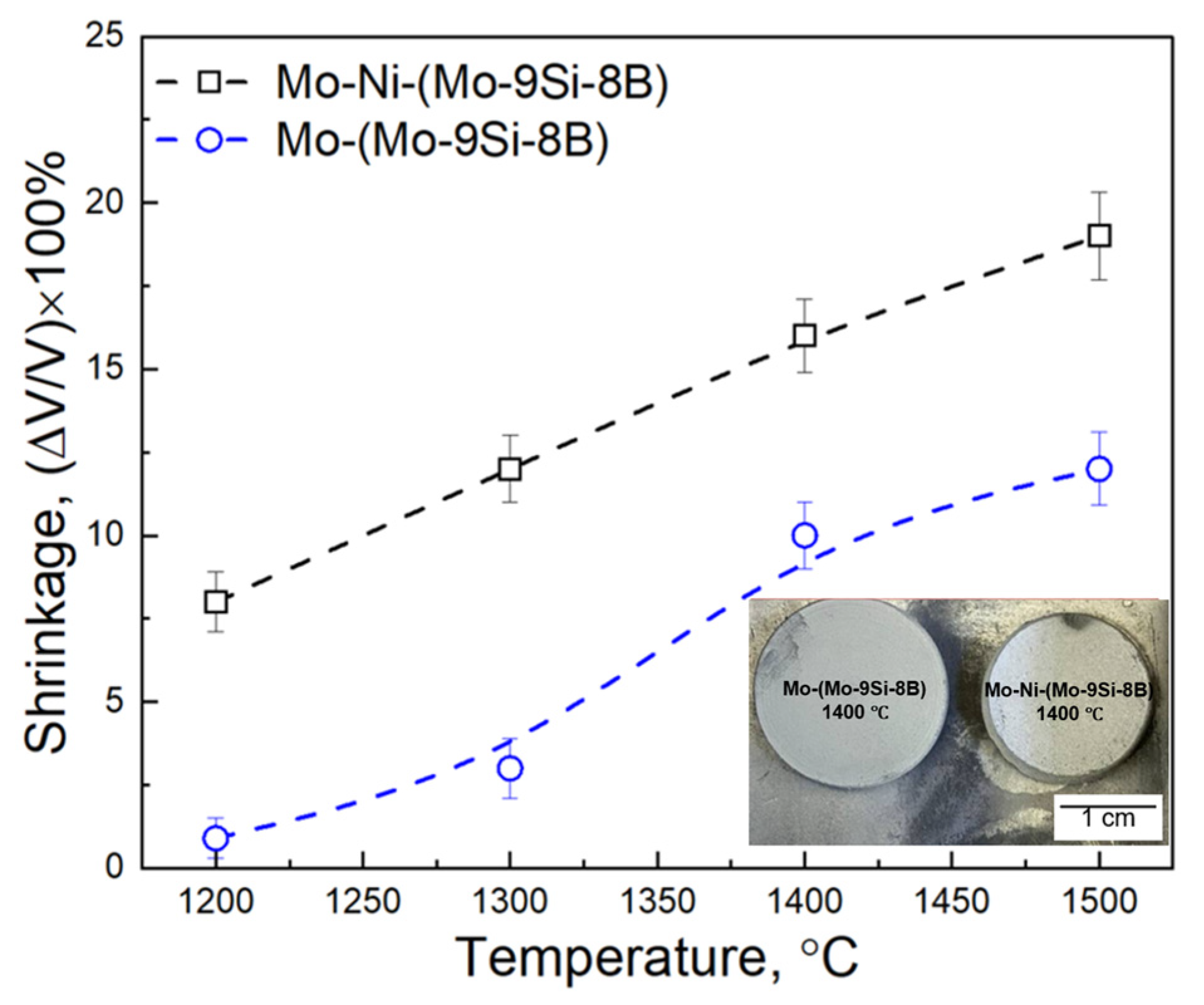
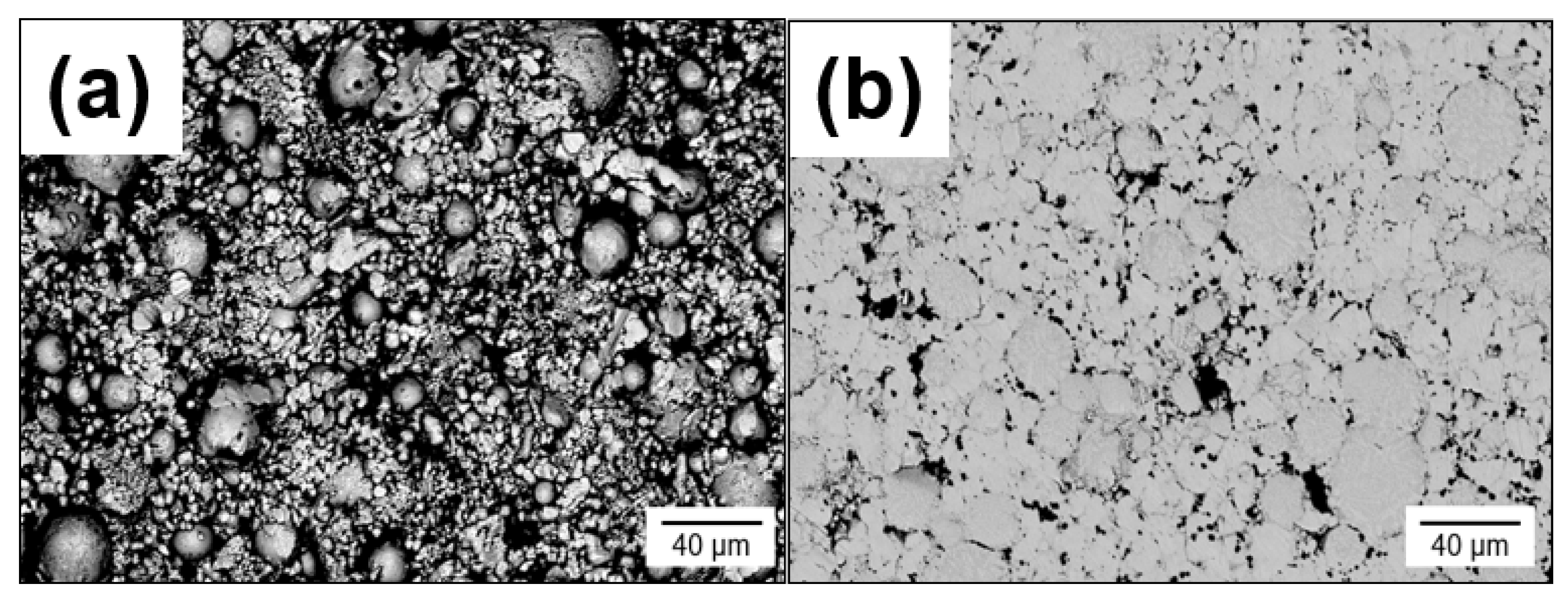
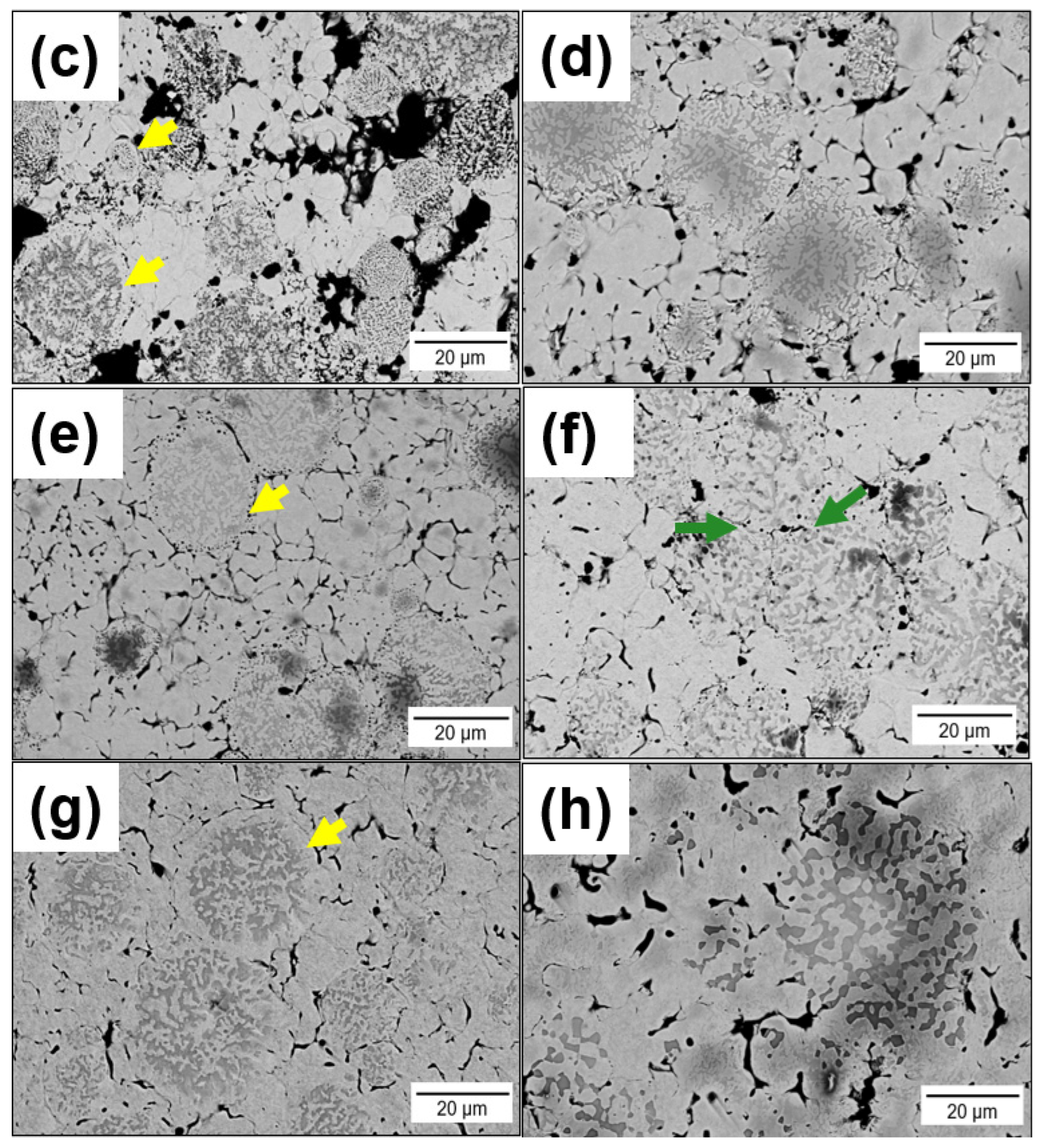
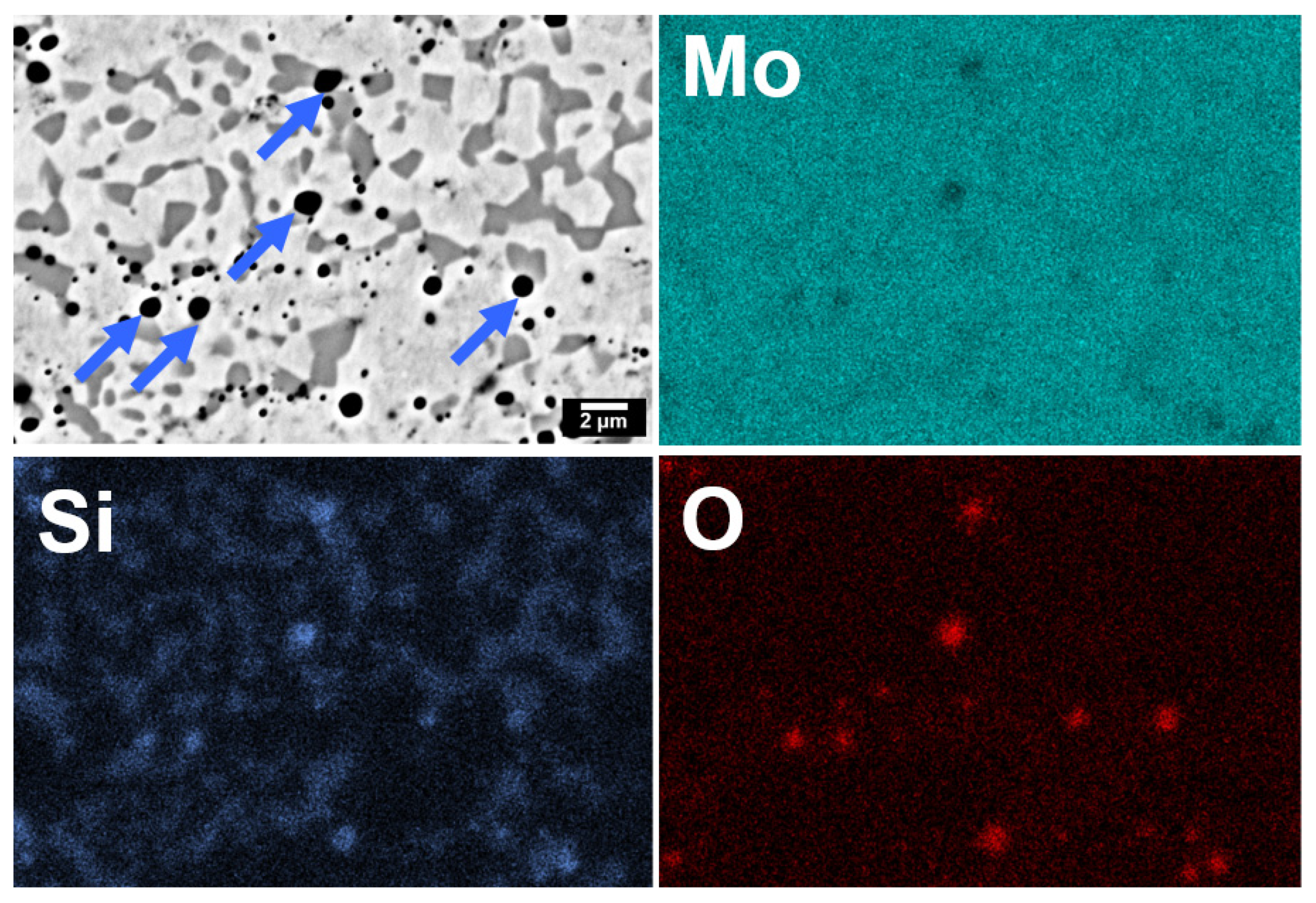
3.1. Pressure-Less Sintering of Mo Matrix Composites
3.2. Spark Plasma Sintering of Mo Matrix Composites
4. Conclusions
- (1)
- The addition of 1 wt.% of Ni to the mixture of 60 wt.% Mo—40 wt.% (Mo-9Si-8B) powder increases the kinetics of the PLS process in the temperature range between 1200 °C and 1500 °C of the composite: the shrinkage changes from 8 to 19%, while without the activator the shrinkage is in the range of 0.9–12%.
- (2)
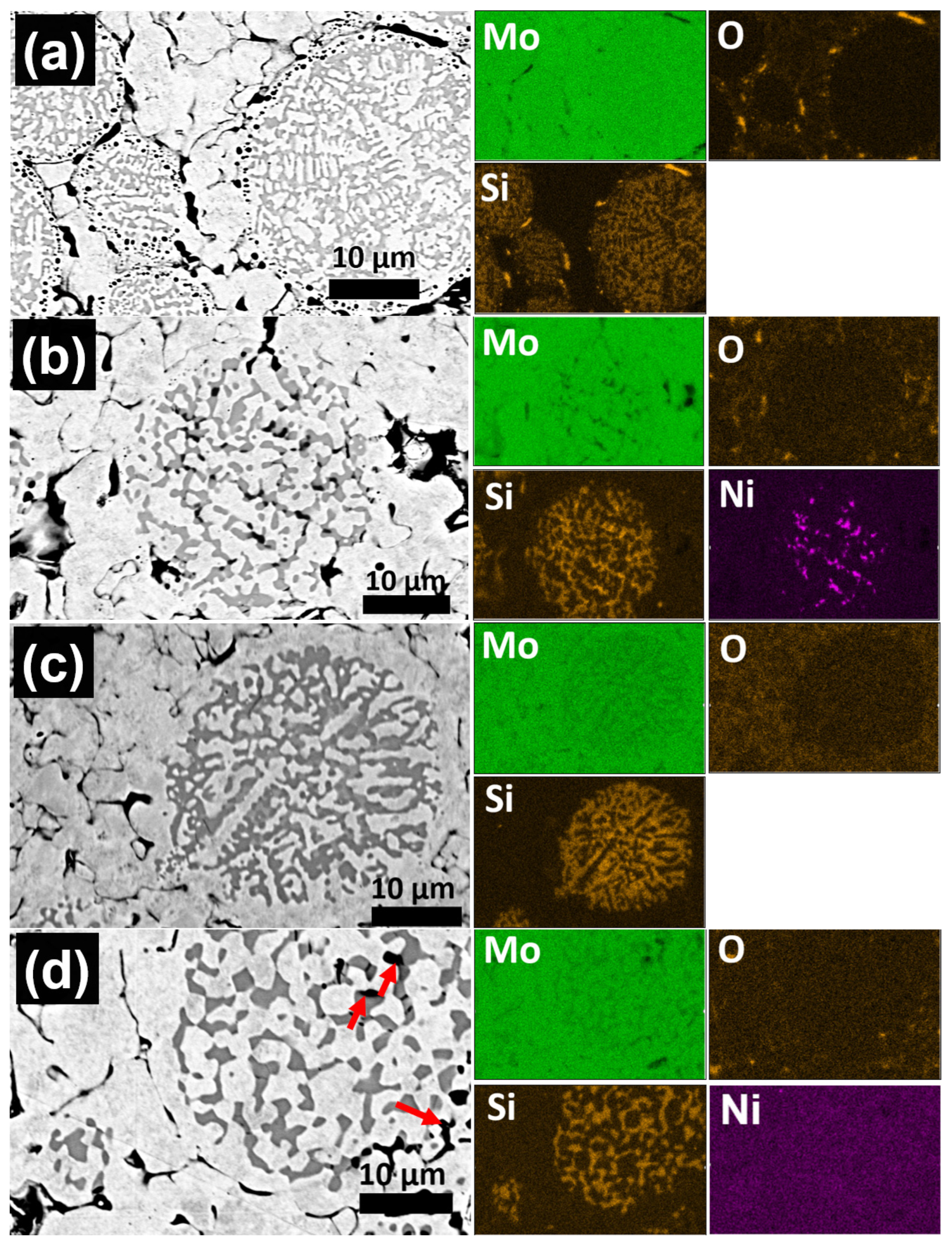
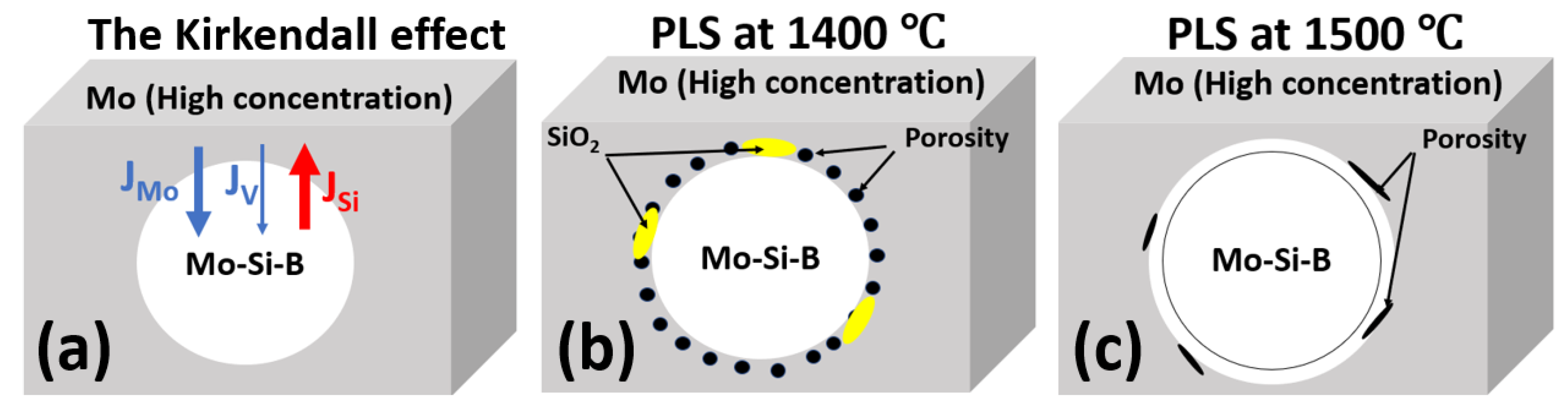
- During PLS at temperatures between 1200–1400 °C, a significant Kirkendall effect occurs in the composite Mo-(Mo-9Si-8B), which leads to the formation of porosity at the interface between the Mo matrix and Mo-9Si-8B particles.
- (3)
- The addition of 1 wt.% Ni increases the diffusion rate of Mo both in the matrix and in the Mo-9Si-8B particles, which reduces the influence of the Kirkendall effect at a sintering temperature of 1400 °C. Moreover, there is no diffusion of Si from the volume of Mo-9Si-8B particles to their surface with the subsequent formation of silicon oxide.
- (4)
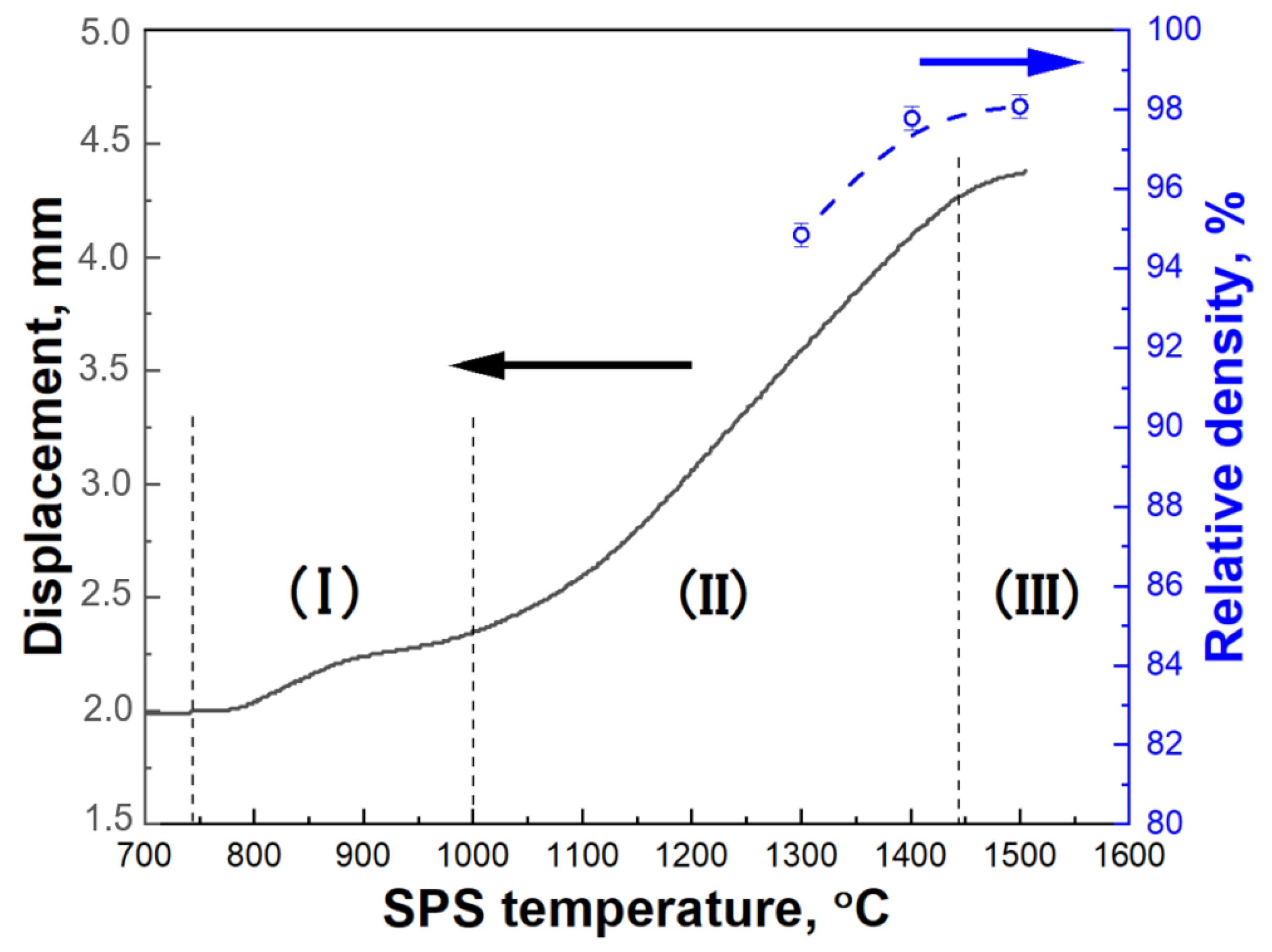
- It is possible to fabricate the high-density Mo matrix composites by PLS by increasing the sintering temperature and/or holding time.
- (5)
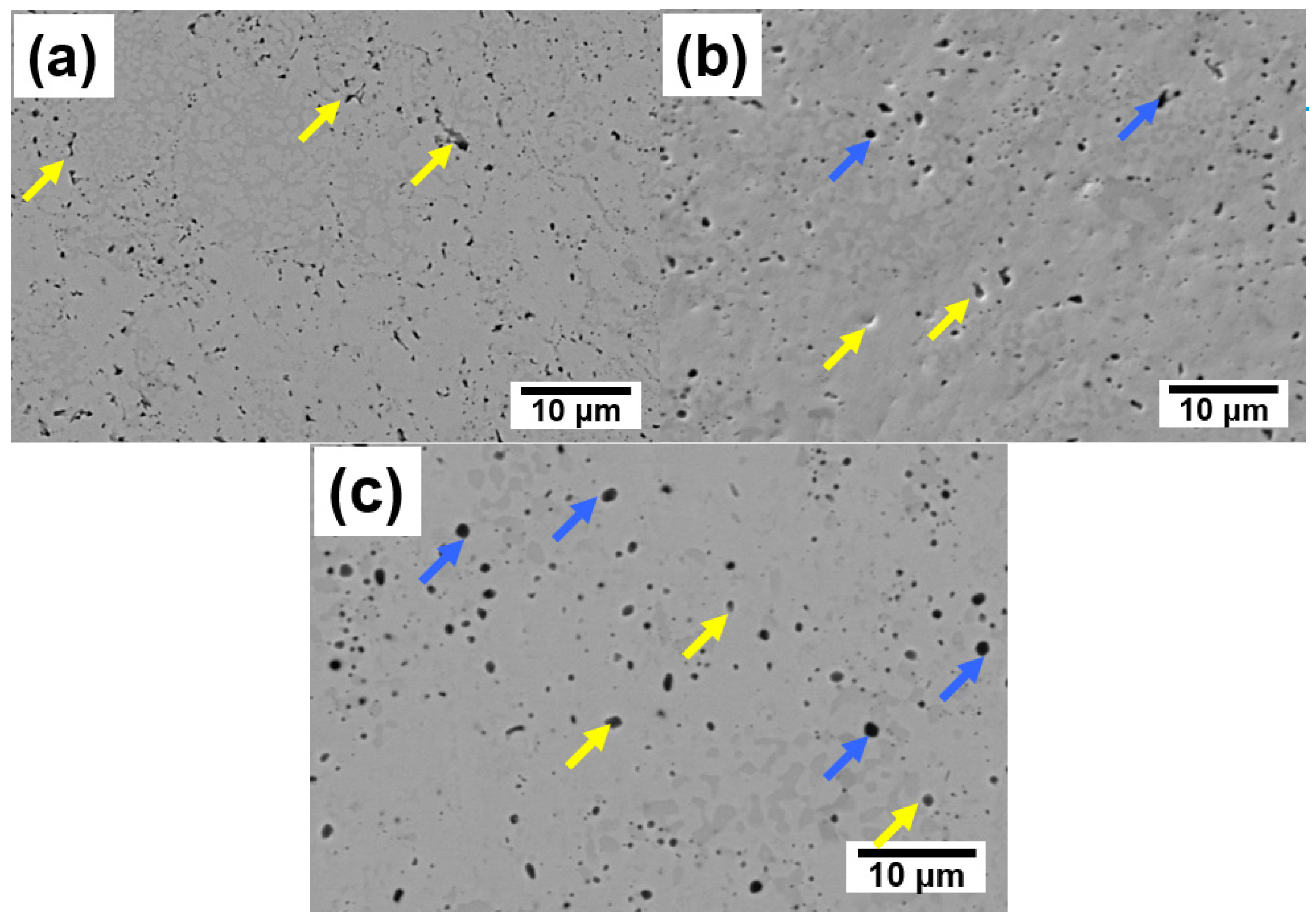
- The use of SPS at temperatures of 1300–1500 °C allows to obtain high-density composites with a relative density of 95–98%. At the same time, no changes in the phase composition and formation of carbides are observed. A small amount of fine silica is formed at sintering temperatures of 1400 °C and 1500 °C.
- (6)
- Comparison of the relative density of the sintered composites with data from the literature indicates a positive effect of activated Ni sintering, since the relative density is several percent higher, while the sintering temperature was significantly lower [30].
- (7)
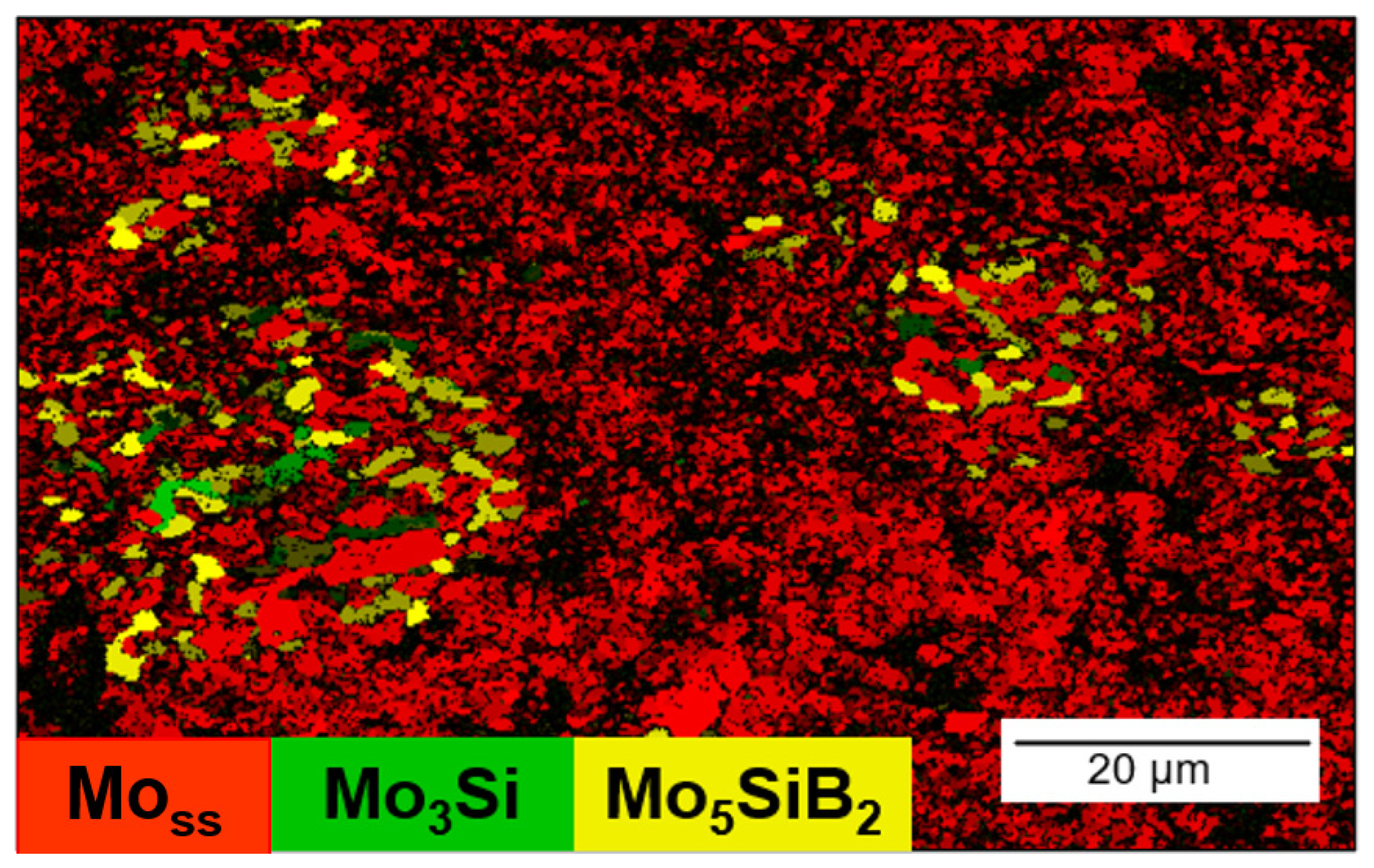
- The result of Ni-activated PLS of MMC indicates the prospects of this approach for solving two problems: (1) obtaining samples with lower residual porosity and (2) obtaining a continuous Mo matrix with Mo3Si and Mo5SiB2 inclusions. Moreover, the Ni-activated SPS of MMC allowed us to obtain a high-density and homogeneous structure. Therefore, further work could be aimed at obtaining high-density samples by PLS and SPS methods for further measurement of mechanical characteristics and oxidation resistance of MMC.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; Zheng, B.; Wu, C.; Li, P.; Xue, T.; Wu, J.; Han, F.; Chen, Y. A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials. Processes 2022, 10, 1772. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar]
- Seif, E.; Rösler, J. Reassessment of the Matrix Composition of Co-Re-Cr-Based Alloys for Particle Strengthening in High-Temperature Applications and Investigation of Suitable MC-Carbides. Materials 2023, 16, 4443. [Google Scholar] [CrossRef] [PubMed]
- Malcharcziková, J.; Skotnicová, K.; Kawulok, P.; Kawulok, R.; Szurman, I.; Růžička, J. Preparation and Properties of Directionally Solidified Ni-Al Based Alloys Modified by Molybdenum. Crystals 2022, 12, 215. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Meng, F.; Yu, H.; Sun, D. Quantitative study on Ru local atomic structure in Ni-Al-Ru ternary alloys. J. Alloys Compd. 2022, 909, 164766. [Google Scholar] [CrossRef]
- Krüger, M.; Franz, S.; Saage, H.; Heilmaier, M.; Schneibel, J.H.; Jéhanno, P.; Böning, M.; Kestler, H. Mechanically alloyed Mo-Si-B alloys with a continuous alpha-Mo matrix and improved mechanical properties. Intermetallics 2008, 16, 933–941. [Google Scholar] [CrossRef]
- Pan, K.; Yang, Y.; Wei, S.; Wu, H.; Dong, Z.; Wu, Y.; Wang, S.; Zhang, L.; Lin, J.; Mao, X. Oxidation behavior of Mo-Si-B alloys at medium-to-high temperatures. J. Mater. Sci. Technol. 2021, 60, 113–127. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Mendiratta, M.G.; Dimiduk, D.M. Oxidation mechanisms in Mo-reinforced Mo5SiB2(T-2)-Mo3Si alloys. Acta Mater. 2002, 50, 1857–1868. [Google Scholar] [CrossRef]
- Lemberg, J.A.; Ritchie, R.O. Mo-Si-B alloys for ultrahigh-temperature structural applications. Adv. Mater. 2012, 24, 3445–3480. [Google Scholar] [CrossRef]
- Perepezko, J.H. The Hotter the Engine, the Better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef]
- Krüger, M.; Jain, P.; Kumar, K.S.; Heilmaier, M. Correlation between microstructure and properties of fine grained Mo–Mo3Si–Mo5SiB2 alloys. Intermetallics 2014, 48, 10–18. [Google Scholar] [CrossRef]
- Majumdar, S.; Dönges, B.; Gorr, B.; Christ, H.-J.; Schliephake, D.; Heilmaier, M. Mechanisms of Oxide Scale Formation on Yttrium-Alloyed Mo–Si–B Containing Fine-Grained Microstructure. Corros. Sci. 2015, 90, 76–88. [Google Scholar] [CrossRef]
- Yakang, K.; Wang, C.; Chen, X.; Qu, Y.; Yu, J.; Ju, H.; Yilei, X. Review of Research Progress on Mo–Si–B Alloys. Materials 2023, 16, 5495. [Google Scholar] [PubMed]
- Schneibel, J.H. High temperature strength of Mo-Mo3Si-Mo5SiB2 molybdenum silicides. Intermetallics 2003, 11, 625–632. [Google Scholar] [CrossRef]
- Schneibel, J.; Kramer, M.; Easton, D. A Mo–Si–B intermetallic alloy with a continuous α-Mo matrix. Scr. Mater. 2002, 46, 217–221. [Google Scholar] [CrossRef]
- Becker, J.; Breuer, D.; Bogomol, I.; Krüger, M. Enhanced Fracture Toughness and High-Temperature Strength of Directionally Solidified Mo-XC Alloys. Crystals 2022, 12, 1534. [Google Scholar] [CrossRef]
- Xing, H.; Hu, P.; He, C.; Zhang, X.; Han, J.; Yang, F.; Bai, R.; Zhang, W.; Wang, K.; Volinsky, A.A. Design of high-performance molybdenum alloys via doping metal oxide and carbide strengthening: A review. J. Mater. Sci. Technol. 2023, 160, 161–180. [Google Scholar]
- Baroch, E.F.; Ostermann, M.; Patrick, G. Applications of powder metallurgy molybdenum in the 1990s. Adv. Powder Metall. 1991, 5, 321–331. [Google Scholar]
- Deschamps, I.S.; dos Santos Avila, D.; Vanzuita Piazera, E.; Dudley Cruz, R.C.; Aguilar, C.; Klein, A.N. Design of In Situ Metal Matrix Composites Produced by Powder Metallurgy—A Critical Review. Metals 2022, 12, 2073. [Google Scholar]
- Panichkina, V.V.; Skorohod, V.V.; Khrienko, A.F. Activated sintering of tungsten and molybdenum powders. Sov. Powder Metall. Met. Ceram. 1967, 6, 558–560. [Google Scholar] [CrossRef]
- Smith, J.T. Diffusion Mechanism for the Nickel-Activated Sintering of Molybdenum. J. Appl. Phys. 1965, 36, 595–598. [Google Scholar] [CrossRef]
- Mouawad, B.; Soueidan, M.; Fabrègue, D.; Buttay, C.; Bley, V.; Allard, B.; Morel, H. Full Densification of Molybdenum Powders Using Spark Plasma Sintering. Metall Mater Trans A. 2012, 43, 3402–3409. [Google Scholar] [CrossRef]
- Flem, M.L.; Allemand, A.; Urvoy, S.; Cédat, D.; Rey, C. Microstructure and thermal conductivity of Mo–TiC cermets processed by hot isostatic pressing. J. Nucl. Mater. 2008, 380, 85–92. [Google Scholar] [CrossRef]
- German, R.M.; Munir, Z.A. Heterodiffusion model for the activated sintering of molybdenum. J. Less-Common. Met. 1878, 58, 61–74. [Google Scholar] [CrossRef]
- Hwang, K.S.; Huang, H.S. Identification of the segregation layer and its effect on the activated sintering and ductility of Ni-doped molybdenum. Acta Mater. 2003, 51, 3915–3926. [Google Scholar] [CrossRef]
- Smigelskas, A.D.; Kirkendall, E.O. Zinc Diffusion in Alpha Brass, Trans. AIME 1947, 17, 130–142. [Google Scholar]
- Askill, J. Tracer Diffusion Data for Metals, Alloys, and Simple Oxides; Plenum Press: New York, NY, USA, 1970. [Google Scholar]
- Lee, G.; Manière, C.; McKittrick, J.; Gattuso, A.; Back, C.; Olevsky, E.A. Oxidation effects on spark plasma sintering of molybdenum nanopowders. Am. Ceram. Soc. 2019, 102, 801–812. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Hao, Z.; Shu, Y.; He, J. Study on reduction of MoO2 by carbon diffusion to prepare molybdenum powder. J. Today Commun. 2023, 35, 105643. [Google Scholar] [CrossRef]
- Ohser-Wiedemann, R.; Martin, U.; Seifert, H.J.; Müller, A. Densification behaviour of pure molybdenum powder by spark plasma sintering. Int. J. Refract. Hard Met. 2010, 28, 550–557. [Google Scholar] [CrossRef]
- Wen, S.H.; Zhou, C.G.; Sha, J.B. Improvement of oxidation resistance of a Mo-62Si-5B (at.%) alloy at 1250 °C and 1350 °C via an in situ pre-formed SiO2 fabricated by spark plasma sintering. Corros. Sci. 2017, 127, 175–185. [Google Scholar] [CrossRef]


| TSPS, °C | Relative Density (%) | |
|---|---|---|
| Mo-(Mo-9Si-8B) | Mo-Ni-(Mo-9Si-8B) | |
| 1200 | 66 ± 2.1 | 76 ± 1.5 |
| 1300 | 71 ± 1.9 | 81 ± 1.6 |
| 1400 | 77 ± 1.7 | 86 ± 1.8 |
| 1500 | 82 ± 1.8 | 89 ± 1.8 |
| TSPS, °C | HV20 |
|---|---|
| 1300 | 385 ± 8 |
| 1400 | 435 ± 9 |
| 1500 | 482 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solodkyi, I.; Petrusha, V.; Grigoroscuta, M.A.; Schmelzer, J.; Hasemann, G.; Betke, U.; Badica, P.; Krüger, M. Efficient Sintering of Mo Matrix Composites—A Study of Temperature Dependences and the Use of the Sinter Additive Ni. Metals 2023, 13, 1715. https://doi.org/10.3390/met13101715
Solodkyi I, Petrusha V, Grigoroscuta MA, Schmelzer J, Hasemann G, Betke U, Badica P, Krüger M. Efficient Sintering of Mo Matrix Composites—A Study of Temperature Dependences and the Use of the Sinter Additive Ni. Metals. 2023; 13(10):1715. https://doi.org/10.3390/met13101715
Chicago/Turabian StyleSolodkyi, Ievgen, Vadym Petrusha, Mihai Alexandru Grigoroscuta, Janett Schmelzer, Georg Hasemann, Ulf Betke, Petre Badica, and Manja Krüger. 2023. "Efficient Sintering of Mo Matrix Composites—A Study of Temperature Dependences and the Use of the Sinter Additive Ni" Metals 13, no. 10: 1715. https://doi.org/10.3390/met13101715
APA StyleSolodkyi, I., Petrusha, V., Grigoroscuta, M. A., Schmelzer, J., Hasemann, G., Betke, U., Badica, P., & Krüger, M. (2023). Efficient Sintering of Mo Matrix Composites—A Study of Temperature Dependences and the Use of the Sinter Additive Ni. Metals, 13(10), 1715. https://doi.org/10.3390/met13101715






