Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-ray Diffraction Analyses
Abstract
:1. Introduction
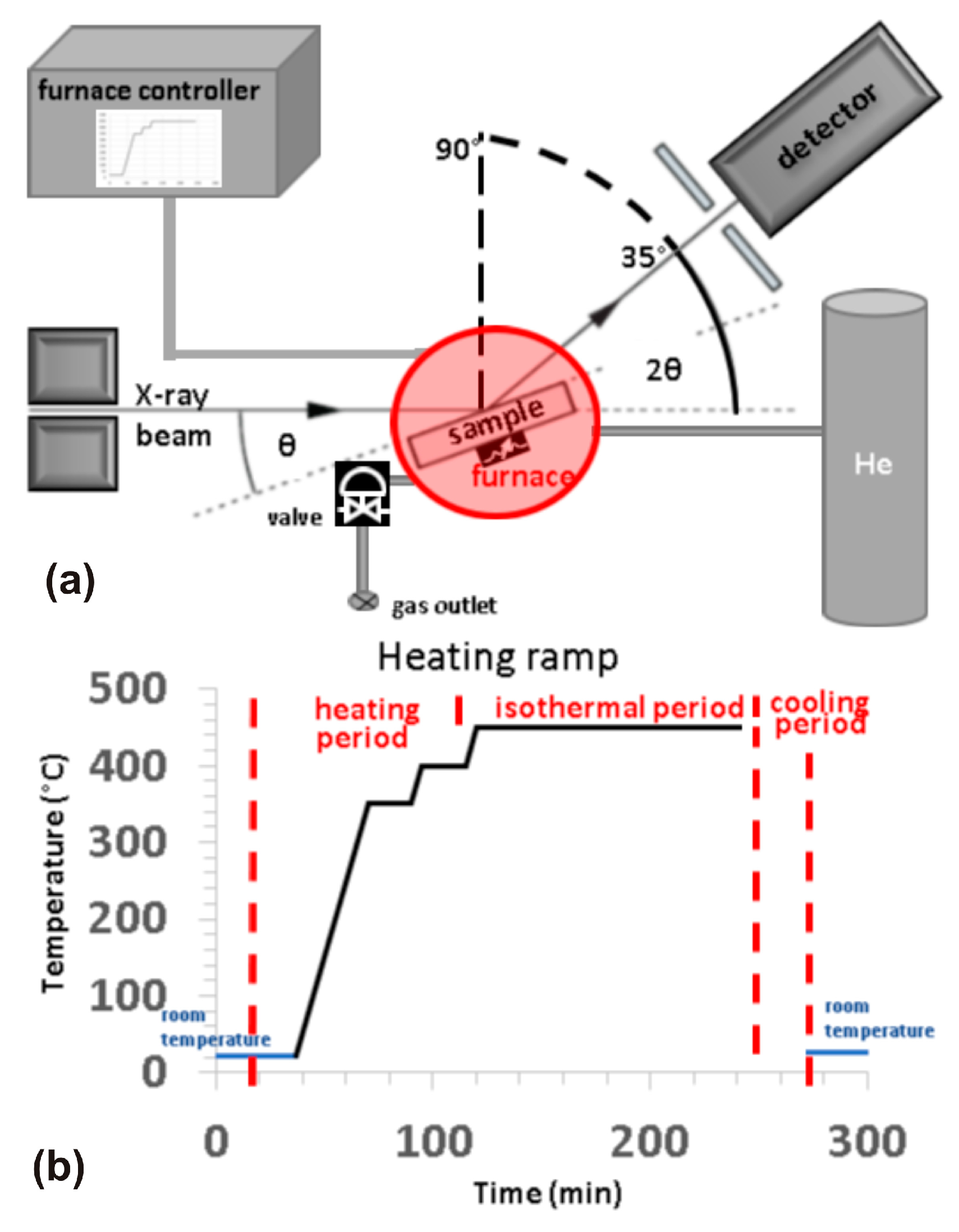
2. Materials and Methods
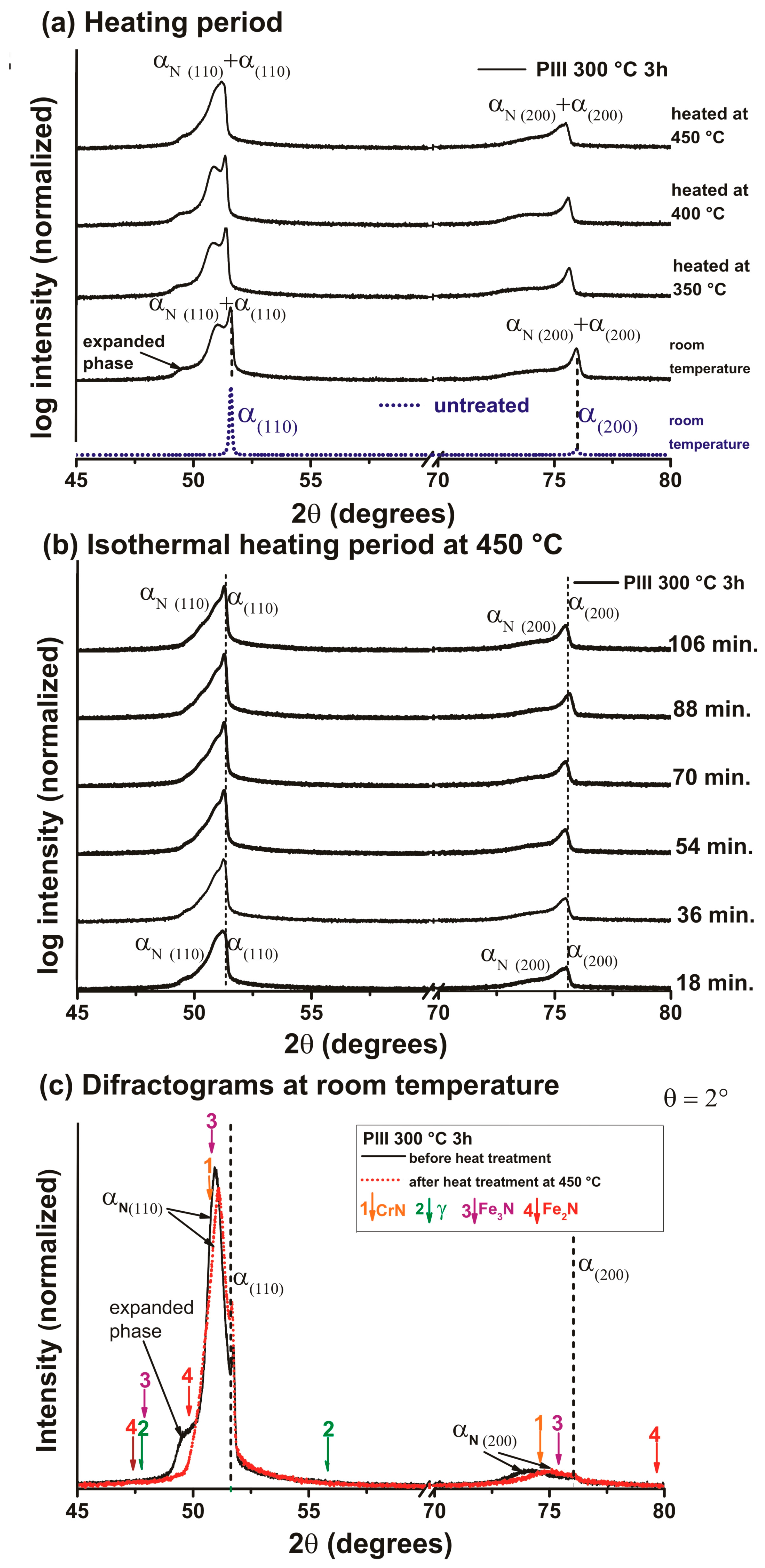
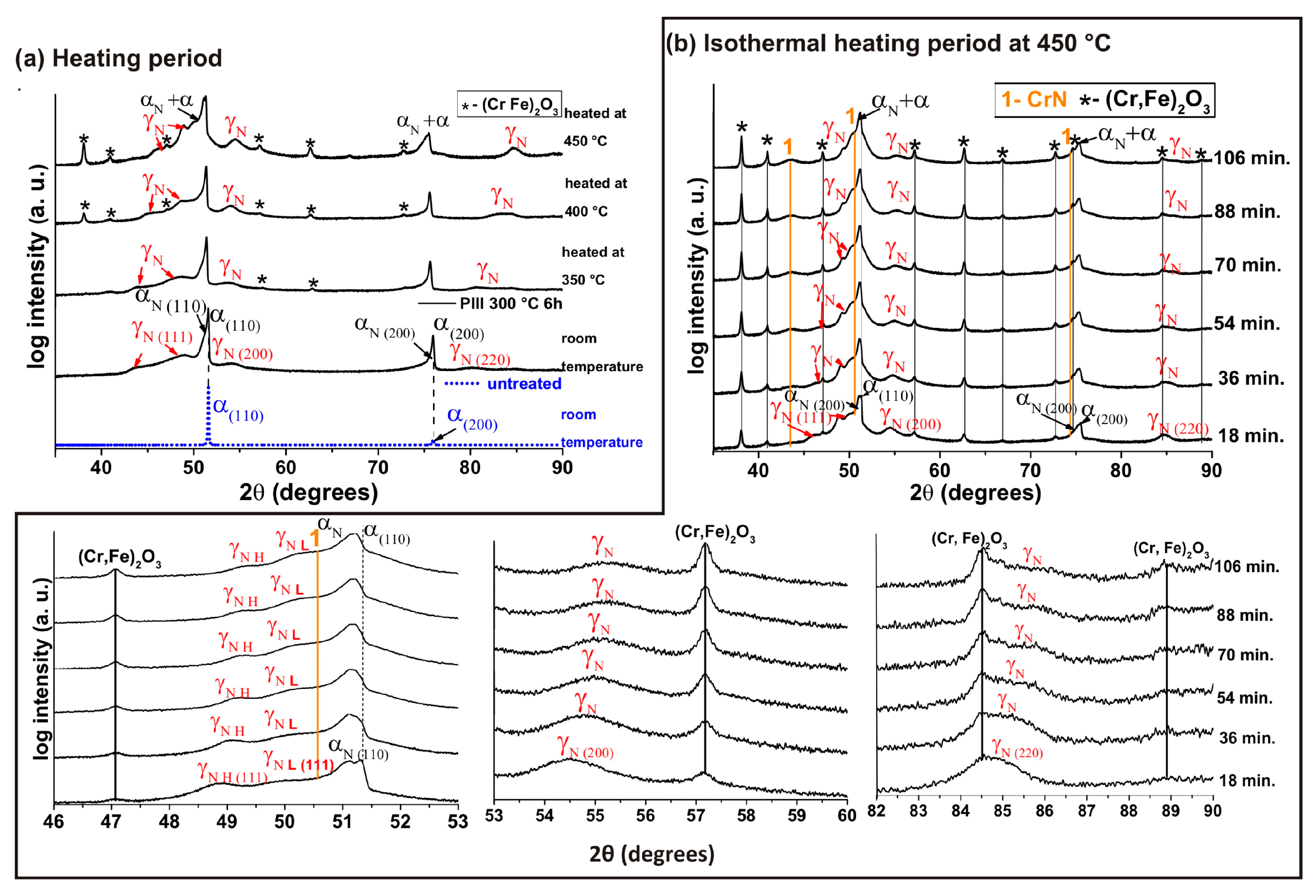
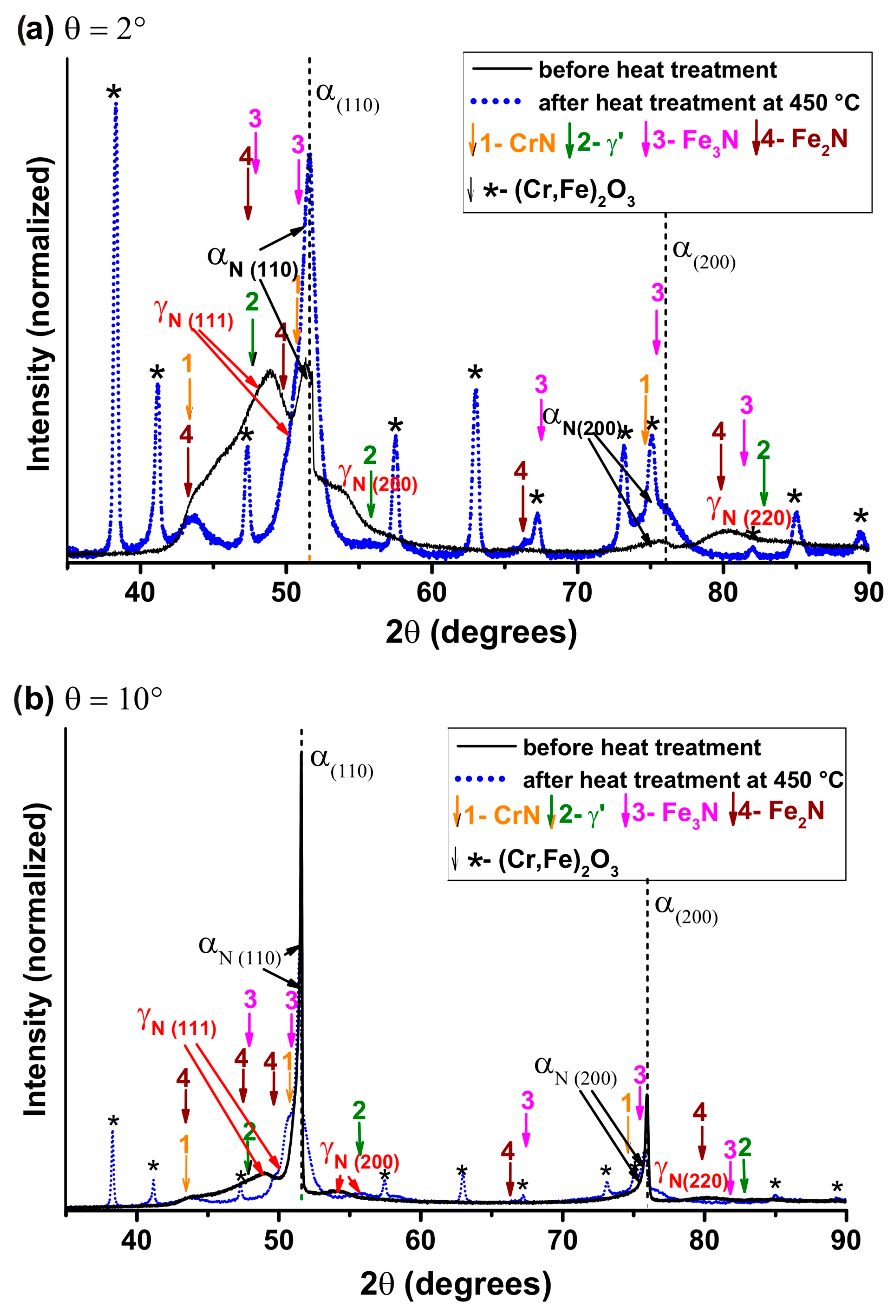
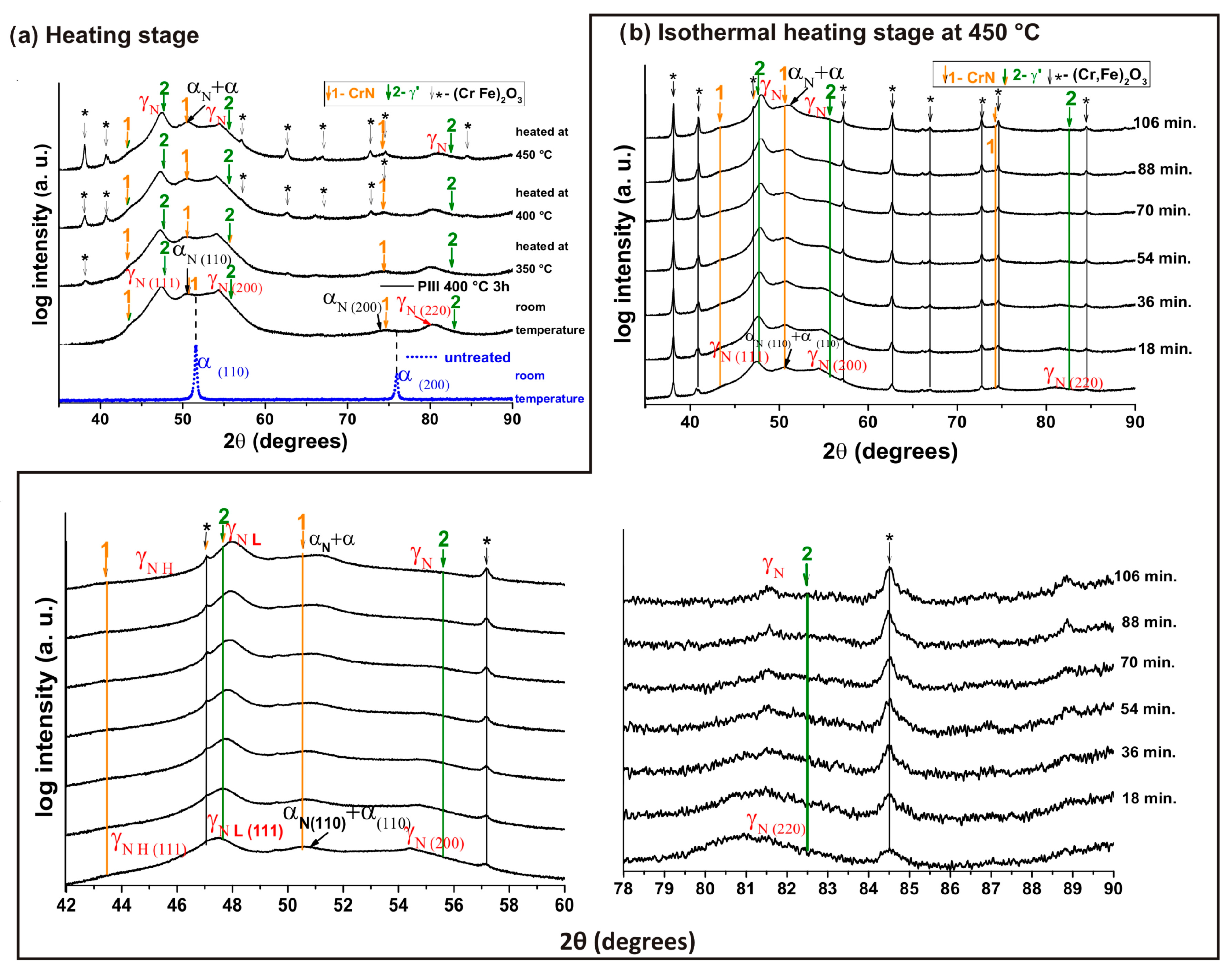
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alphonsa, J.; Mukherjee, S.; Raja, V.S. Study of Plasma Nitriding and Nitrocarburising of AISI 430F Stainless Steel for High Hardness and Corrosion Resistance. Corros. Eng. Sci. Technol. 2018, 53, 51–58. [Google Scholar] [CrossRef]
- De Araújo, E.; Bandeira, R.M.; Manfrinato, M.D.; Moreto, J.A.; Borges, R.; Vales, S.D.S.; Suzuki, P.A.; Rossino, L.S. Effect of Ionic Plasma Nitriding Process on the Corrosion and Micro-Abrasive Wear Behavior of AISI 316L Austenitic and AISI 470 Super-Ferritic Stainless Steels. J. Mater. Res. Technol. 2019, 8, 2180–2191. [Google Scholar] [CrossRef]
- Gontijo, L.C.; Machado, R.; Casteletti, L.C.; Kuri, S.E.; Nascente, P.A.P. X-Ray Diffraction Characterisation of Expanded Austenite and Ferrite in Plasma Nitrided Stainless Steels. Surf. Eng. 2010, 26, 265–270. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Liu, Q.; Wu, Z.; Meng, X.; Fang, Y. Effects of Initial Microstructure on the Low-Temperature Plasma Nitriding of Ferritic Stainless Steel. Coatings 2022, 12, 1404. [Google Scholar] [CrossRef]
- Tadepalli, L.D.; Gosala, A.M.; Kondamuru, L.; Bairi, S.C.; Subbiah, R.; Singh, S.K. A Review on Effects of Nitriding of AISI409 Ferritic Stainless Steel. Mater. Today Proc. 2020, 26, 1014–1020. [Google Scholar] [CrossRef]
- Jimenez, L.B.V.; Umemura, M.T.; Calderón-Hernández, J.W.; Magnabosco, R.; Pinedo, C.E.; Tschiptschin, A.P. Plasma Nitriding of 410S Ferritic/Martensitic Stainless Steel: Microstructure, Wear and Corrosion Properties. Tecnol. Metal. Mater. Mineração 2023, 20, e2809. [Google Scholar] [CrossRef]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Blawert, C.; Mordike, B.L.; Rensch, U.; Oettel, H. The Effect of HV in the Nitriding of Ferritic Steels by Plasma Immersion Ion Implantation. Surf. Coatings Technol. 2001, 142–144, 376–383. [Google Scholar] [CrossRef]
- Schreiber, G.; Rensch, U.; Oettel, H.; Blawert, C.; Mordike, B.L. Thermal Stability of PI 3 Nitrided Surface Layers on Ferritic Steels. Surf. Coat. Technol. 2003, 170, 447–451. [Google Scholar] [CrossRef]
- Manova, D.; Eichentopf, I.-M.; Hirsch, D.; Mandl, S.; Neumann, H.; Rauschenbach, B. Influence of Microstructure on Nitriding Properties of Stainless Steel. IEEE Trans. Plasma Sci. 2006, 34, 1136–1140. [Google Scholar] [CrossRef]
- Schibicheski Kurelo, B.C.E.; de Souza, G.B.; Serbena, F.C.; Lepienski, C.M.; Borges, P.C. Mechanical Properties and Corrosion Resistance of AN-Rich Layers Produced by PIII on a Super Ferritic Stainless Steel. Surf. Coatings Technol. 2020, 403, 126388. [Google Scholar] [CrossRef]
- Luiz, L.A.; Kurelo, B.C.E.S.; de Souza, G.B.; de Andrade, J.; Marino, C.E.B. Effect of Nitrogen Plasma Immersion Ion Implantation on the Corrosion Protection Mechanisms of Different Stainless Steels. Mater. Today Commun. 2021, 201, 8131–8135. [Google Scholar] [CrossRef]
- Schibicheski Kurelo, B.C.E.; de Souza, G.B.; Serbena, F.C.; Lepienski, C.M.; Chuproski, R.F.; Borges, P.C. Improved Saline Corrosion and Hydrogen Embrittlement Resistances of Superaustenitic Stainless Steel by PIII Nitriding. J. Mater. Res. Technol. 2022, 18, 1717–1731. [Google Scholar] [CrossRef]
- Borgioli, F. From Austenitic Stainless Steel to Expanded Austenite-s Phase: Formation, Characteristics and Properties of an Elusive Metastable Phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Blawert, C.; Mordike, B.L.; Jirásková, Y.; Schneeweiss, O. Structure and Composition of Expanded Austenite Produced by Nitrogen Plasma Immersion Ion Implantation of Stainless Steels X6CrNiTi1810 and X2CrNiMoN2253. Surf. Coatings Technol. 1999, 116, 189–198. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Casteletti, L.C.; Gallego, J. Microstructure of Nitrided and Nitrocarburized Layers Produced on a Superaustenitic Stainless Steel. J. Mater. Res. Technol. 2013, 2, 158–164. [Google Scholar] [CrossRef]
- Stróz, D.; Psoda, M. TEM Studies of Plasma Nitrided Austenitic Stainless Steel. J. Microsc. 2010, 237, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Che, H.L.; Lei, M.K. Microstructure of Perfect Nitrogen-Expanded Austenite Formed by Unconstrained Nitriding. Scr. Mater. 2021, 194, 113705. [Google Scholar] [CrossRef]
- Blawert, C.; Weisheit, A.; Mordike, B.L.; Knoop, R.M. Plasma Immersion Ion Implantation of Stainless Steel: Austenitic Stainless Steel in Comparison to Austenitic-Ferritic Stainless Steel. Surf. Coatings Technol. 1996, 85, 15–27. [Google Scholar] [CrossRef]
- Alphonsa, J.; Raja, V.S.; Mukherjee, S. Study of Plasma Nitriding and Nitrocarburizing for Higher Corrosion Resistance and Hardness of 2205 Duplex Stainless Steel. Corros. Sci. 2015, 100, 121–132. [Google Scholar] [CrossRef]
- Núñez de la Rosa, Y.E.; Palma Calabokis, O.; Borges, P.C.; Ballesteros Ballesteros, V. Effect of Low-Temperature Plasma Nitriding on Corrosion and Surface Properties of Duplex Stainless Steel UNS S32205. J. Mater. Eng. Perform. 2020, 29, 2612–2622. [Google Scholar] [CrossRef]
- Jirásková, Y.; Blawert, C.; Schneeweiss, O. Thermal Stability of Stainless Steel Surfaces Nitrided by Plasma Immersion Ion Implantation. Phys. Stat. Sol. (a) 1999, 175, 537–548. [Google Scholar] [CrossRef]
- Piekoszewski, J.; Sartowska, B.; Waliś, L.; Werner, Z.; Kopcewicz, M.; Prokert, F.; Stanisławski, J.; Kalinowska, J.; Szymczyk, W. Interaction of Nitrogen Atoms in Expanded Austenite Formed in Pure Iron by Intense Nitrogen Plasma Pulses. Nukleonika 2004, 49, 57–60. [Google Scholar]
- Manne, V.; Singh, S.K.; Sateesh, N.; Ram, S. A Review on Influence of Nitriding on AISI430 Ferritic Stainless Steel. Mater. Today Proc. 2020, 26, 1010–1013. [Google Scholar] [CrossRef]
- Sasidhar, K.N.; Meka, S.R. Thermodynamic Reasoning for Colossal N Supersaturation in Austenitic and Ferritic Stainless Steels during Low-Temperature Nitridation. Sci. Rep. 2019, 9, 7996. [Google Scholar] [CrossRef]
- Wang, D.; Kahn, H.; Ernst, F.; Heuer, A.H. “Colossal” Interstitial Supersaturation in Delta Ferrite in Stainless Steels: (II) Low-Temperature Nitridation of the 17-7 PH Alloy. Acta Mater. 2017, 124, 237–246. [Google Scholar] [CrossRef]
- Manova, D.; Mändl, S.; Neumann, H.; Rauschenbach, B. Formation of Metastable Diffusion Layers in Cr-Containing Iron, Cobalt and Nickel Alloys after Nitrogen Insertion. Surf. Coatings Technol. 2017, 312, 81–90. [Google Scholar] [CrossRef]
- Manova, D.; Lutz, J.; Gerlach, J.W.; Neumann, H.; Mändl, S. Relation between Lattice Expansion and Nitrogen Content in Expanded Phase in Austenitic Stainless Steel and CoCr Alloys. Surf. Coatings Technol. 2011, 205, S290–S293. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Nishikawa, A.S.; Varela, L.B.; Pinedo, C.E. Thermal Stability of Expanded Austenite Formed on a DC Plasma Nitrided 316L Austenitic Stainless Steel. Thin Solid Films 2017, 644, 156–165. [Google Scholar] [CrossRef]
- Borgioli, F. The Corrosion Behavior in Different Environments of Austenitic Stainless Steels Subjected to Thermochemical Surface Treatments at Low Temperatures: An Overview. Metals 2023, 13, 776. [Google Scholar] [CrossRef]
- Brink, B.; Ståhl, K.; Christiansen, T.L.; Somers, M.A.J. Thermal Expansion and Phase Transformations of Nitrogen-Expanded Austenite Studied with in Situ Synchrotron X-Ray Diffraction. J. Appl. Crystallogr. 2014, 47, 819–826. [Google Scholar] [CrossRef]
- Somers, M.A.J.; van der Pers, N.M.; Schalkoord, D.; Mittemeijer, E.J. Dependence of the Lattice Parameter of γ′ Iron Nitride, Fe4N, on Nitrogen Content; Accuracy of the Nitrogen Absorption Data. Metall. Trans. A 1989, 20, 1533–1539. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Tischler, G.; Mitterer, C. Microstructure and Mechanical/Thermal Properties of Cr-N Coatings Deposited by Reactive Unbalanced Magnetron Sputtering. Surf. Coatings Technol. 2001, 142–144, 78–84. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tsai, C.J.; Lu, F.H. The Young’s Modulus of Chromium Nitride Films. Surf. Coatings Technol. 2004, 184, 69–73. [Google Scholar] [CrossRef]
- Bian, L.; Chen, Z.; Wang, L.; Li, F.; Chou, K. Oxidation Resistance, Thermal Expansion and Area Specific Resistance of Fe-Cr Alloy Interconnector for Solid Oxide Fuel Cell. J. Iron Steel Res. Int. 2017, 24, 77–83. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- de Oliveira, W.R.; Chuproski, R.F.; Valadão, G.M.; Cintho, O.M.; de Souza, E.C.F.; Serbena, F.C.; de Souza, G.B. Symmetry between the Anisotropic N Behavior in the Lattice under High Pressures and the Formation of Expanded Austenite. J. Alloys Compd. 2021, 871, 159509. [Google Scholar] [CrossRef]
- Fernandes, B.B.; Mändl, S.; Oliveira, R.M.; Ueda, M. Mechanical Properties of Nitrogen-Rich Surface Layers on SS304 Treated by Plasma Immersion Ion Implantation. Appl. Surf. Sci. 2014, 310, 278–283. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Decomposition Kinetics of Expanded Austenite with High Nitrogen Contents. Int. J. Mater. Res. 2006, 97, 79–88. [Google Scholar] [CrossRef]
- Galdikas, A. The Anisotropic Stress-Induced Di Ff Usion and Trapping of Nitrogen in Austenitic Stainless Steel during Nitriding. Metals 2020, 10, 1319. [Google Scholar] [CrossRef]
- Moskalioviene, T.; Galdikas, A. Crystallographic Orientation Dependence of Nitrogen Mass Transport in Austenitic Stainless Steel. Metals 2020, 10, 615. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. On the Crystallographic Structure of S-Phase. Scr. Mater. 2004, 50, 35–37. [Google Scholar] [CrossRef]
- Ye, S.; Cao, Y. Structure of Iron Nitrides under Different Nitridation Temperatures. In Proceedings of the AMITP 2016, Guilin, China, 24–25 September 2016; Zheng, Z., Zhuo, X., Eds.; Atlantis Press: Guilin, China, 2016; pp. 6–9. [Google Scholar]
- Rohith, K.V.; Saravanan, P.; Sakar, M.; Balakumar, S. Insights into the Nitridation of Zero-Valent Iron Nanoparticles for the Facile Synthesis of Iron Nitride Nanoparticles. RSC Adv. 2016, 6, 45850–45857. [Google Scholar] [CrossRef]
- Cao, Y.; Norell, M. Role of Nitrogen Uptake during the Oxidation of 304L and 904L Austenitic Stainless Steels. Oxid. Met. 2013, 80, 479–491. [Google Scholar] [CrossRef]
- Manova, D.; Lotnyk, A.; Mändl, S.; Neumann, H.; Rauschenbach, B. CrN Precipitation and Elemental Segregation during the Decay of Expanded Austenite. Mater. Res. Express 2016, 3, 066502. [Google Scholar] [CrossRef]


| Sample | Nitriding Parameters | ||||
|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Frequency—f (Hz) | Current Density—j (mA/cm2) | Ion Fluence—Γ (ions/cm2) | |
| Untreated | --- | --- | --- | --- | --- |
| PIII 300 °C 3 h | 300 °C | 3 | 728 | 7.8 ± 0.2 | 2.5 × 1018 |
| PIII 300 °C 6 h | 300 °C | 6 | 728 | 7.7 ± 0.2 | 5.0 × 1018 |
| PIII 400 °C 3 h | 400 °C | 3 | 981 | 13.1 ± 0.1 | 5.7 × 1018 |
| Sample | Thickness (µm) |
|---|---|
| PIII 300 °C 3 h | 1.1 ± 0.1 |
| PIII 300 °C 3 h HT | 2.1 ± 0.3 |
| PIII 300 °C 6 h | 1.3 ± 0.2 |
| PIII 300 °C 6 h HT | 3.2 ± 0.2 |
| PIII 400 °C 3 h | 9.3 ± 1.3 |
| PIII 400 °C 3 h HT | 13.3 ± 1.4 |
| Sample | Average Lattice Parameter (Å) | |
|---|---|---|
| αN | γN | |
| PIII 300 3 h | 2.91 ± 0.01 | --- |
| PIII 300 3 h HT | 2.90 ± 0.01 | --- |
| PIII 300 6 h | 2.91 ±0.01 | 3.85 ± 0.03 |
| PIII 300 6 h HT | 2.91 ± 0.01 | 3.71 ± 0.06 |
| PIII 400 3 h | 2.90 ± 0.03 | 3.86 ± 0.04 |
| PIII 400 3 h HT | 2.90 ± 0.02 | 3.81± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schibicheski Kurelo, B.C.E.; Lepienski, C.M.; de Oliveira, W.R.; de Souza, G.B.; Serbena, F.C.; Cardoso, R.P.; das Neves, J.C.K.; Borges, P.C. Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-ray Diffraction Analyses. Metals 2023, 13, 1744. https://doi.org/10.3390/met13101744
Schibicheski Kurelo BCE, Lepienski CM, de Oliveira WR, de Souza GB, Serbena FC, Cardoso RP, das Neves JCK, Borges PC. Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-ray Diffraction Analyses. Metals. 2023; 13(10):1744. https://doi.org/10.3390/met13101744
Chicago/Turabian StyleSchibicheski Kurelo, Bruna C. E., Carlos M. Lepienski, Willian R. de Oliveira, Gelson B. de Souza, Francisco C. Serbena, Rodrigo P. Cardoso, Julio C. K. das Neves, and Paulo C. Borges. 2023. "Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-ray Diffraction Analyses" Metals 13, no. 10: 1744. https://doi.org/10.3390/met13101744
APA StyleSchibicheski Kurelo, B. C. E., Lepienski, C. M., de Oliveira, W. R., de Souza, G. B., Serbena, F. C., Cardoso, R. P., das Neves, J. C. K., & Borges, P. C. (2023). Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-ray Diffraction Analyses. Metals, 13(10), 1744. https://doi.org/10.3390/met13101744








