Abstract
The blast furnace and basic oxygen furnace (BF-BOF) is still the main process used for the production of iron and steel in China. With the approach of the “dual carbon” target, the iron and steel industry needs to transform and upgrade to “green” and “low-carbon” practices. At present, the low-carbon hydrogen metallurgy technology route based on hydrogen instead of carbon is mainly adopted at home and abroad, and the domestic route is mainly based on oxygen-rich BFs and hydrogen-based shaft furnaces (SFs). It promotes the transformation of the traditional BF to hydrogen-rich, oxygen-rich, and carbon-recycled (Hy-O-CR) technology. A new ironmaking system and method for a reduction smelting furnace (RSF) with Hy-O-CR is presented in this paper. The ironmaking system includes nine sets of equipment, such as an RSF, gas dust collector, dryer, CO2 separator, electrolytic water device, blower, heat exchanger, storage tank of reduction gas, and chimney. From top to bottom, the RSF includes an indirect reduction zone, a soft melting dripping zone, and a coke combustion zone. The ironmaking methods include coke and ore mixed charging, injection of the mixed reduction gas composed of electrolytic green hydrogen and circulating gas from the furnace gas into the indirect reduction zone, injection of oxygen into the coke combustion zone, CO2 recovery of the furnace top gas, and slag and iron treatment. By redesigning the size of the furnace type and optimizing the parameters, the metallization rate of the indirect reduction zone can be as high as 85–95%, and the carbon consumption per ton of hot metal can be greatly reduced. By using oxygen to recycle the reduction gas produced by its reactor, the process achieves the goal of reducing CO2 emissions by more than 50%, thus realizing green and low-carbon metallurgy.
1. Introduction
Climate change is one of the major global challenges to achieving green development in the future. China proposed a “dual carbon” strategy during the general debate of the 75th United Nations General Assembly (China aims to have carbon dioxide (CO2) emissions peak before 2030 and achieve carbon neutrality before 2060). The “dual carbon” strategy is the inevitable choice and solemn commitment to build a community with a shared future for humanity [1]. At the same time, the “dual carbon” strategy will inevitably promote China's carbon emission reduction to usher in a historic turning point, bringing about an upgrade and a transformation of energy and related industries so as to achieve the long-term, healthy, and sustainable development of the national economy.
Edwin Batson, Director General of the World Steel Association, addressed the Global Low-carbon Metallurgical Innovation Forum and the Eighth Baosteel Annual Academic Conference and pointed out that steel is a recyclable green material and that the steel industry is one of the most important basic industries for the country in terms of supporting the rapid development of the modern world [2]. A total of 75% of the carbon emissions in the iron and steel industries of China come directly or indirectly from fossil fuels, accounting for 15% to 16% of the total national carbon emissions and ranking first outside the power industry [3]. China's iron and steel industry must rapidly transform in the direction of green and low-carbon practices. At this stage, the steel production capacity of the long process (blast furnace and basic oxygen furnace (BF-BOF)) accounts for about 90%, of which the CO2 emissions of BFs account for more than 70% of the total emissions of the steel process. Compared with the short process, BF ironmaking has greater advantages in terms of production costs and production efficiency, so it is estimated that BF ironmaking under the long process should remain the prominent ironmaking method in China for the short term.
If the long-process ironmaking technology of China does not undergo major technological reforms in the short term, it will lose its cost-competitiveness in the international market in the future. Therefore, in order to achieve a smooth transition in the long process, it is urgent to greatly reduce CO2 emissions in the BF. In recent years, with the decreasing cost of hydrogen production, the application of hydrogen has tended to replace fossil fuels. Its advantages, such as zero pollution, high energy, and rich sources, position it as a new strategic energy for the iron and steel industry and also provide a new direction for development in the industry [4]. Metallurgical workers believe that if hydrogen can be used as a reducing agent in the BF ironmaking process, the carbon emissions will be reduced from the root, which will greatly alleviate environmental problems and realize the transition to green, low-carbon metallurgy [5].
First of all, this paper describes the mechanism of hydrogen metallurgy. Secondly, the characteristics of typical low-carbon process routes at home and abroad are compared and analyzed, and the problems existing in the hydrogen metallurgy technology route at present are clarified. Finally, starting with the key parameters of limited carbon emission reduction for the traditional BF process, an ironmaking system using the new hydrogen-rich, oxygen-rich, and carbon-recycled (Hy-O-CR) technology and an ironmaking method are proposed. Technical upgrading of the furnace type, reduction gas injection from shaft tuyeres, mixed burden charging, and controlling the ratio of upper and lower gas distribution overcome the defect of the traditional BF, which prevents the upper metallization rate from increasing to more than 70%, and achieves a circulation technology of process gas by combining CO2 removal in top gas and hydrogen-rich gas heating, significantly reducing the use of fossil fuels in ironmaking production. This technology significantly reduces the amount of carbon used and CO2 emissions, intending to provide a reference for the future of the steel industry about “dual carbon” development strategies and technology choices.
2. Mechanisms of Hydrogen Metallurgy
For a long time, metallurgical workers have been paying attention to the development prospects of hydrogen metallurgy. Hydrogen metallurgy technology uses hydrogen instead of fossil fuels (coal or coke) as the reducing agent in the BF to reduce or even completely eliminate CO2 emissions in the process of iron and steel production from the root. At present, the consensus is to reduce carbon from carbon metallurgy by transitioning to hydrogen metallurgy, and two typical carbon reduction paths have been formed, which are the short-process route of green hydrogen direct reduction with an electric arc furnace (EAF) and the long-process route of conventional BF hydrogen-rich reconstitution [6].
As an efficient and clean energy source, hydrogen is considered a future alternative to fossil fuels because of its wider source, high thermal efficiency, and better diffusivity. As a reductant, hydrogen has proven its unique advantages in kinetics and thermodynamics, and its reduction efficiency and reduction rate are higher than those of carbon reductants at high temperatures; thus, hydrogen metallurgy is a green and advanced low-carbon process [7]. As the inevitable product of fossil fuels, CO2 cannot be avoided in the traditional BF ironmaking process; however, the green hydrogen metallurgy process has attracted more attention because of its environmental protection qualities and zero carbon emissions. The chemical reaction equations involved in carbon metallurgy and hydrogen metallurgy under iron oxides are listed in Table 1. From the point of view of the reactants and products of the chemical reaction, the carbon metallurgical process based on fossil fuels takes C or CO as the reducing agent where the product is CO or a mixture of CO and CO2, and carbon emissions cannot be avoided from the production process. The green hydrogen metallurgy process uses H2 as a reducing agent to achieve a final product of H2O, which produces zero pollution and secondary utilization and belongs to the zero carbon emission green process. According to the Gibbs Free Energy of chemical reactions, the direct reduction of C in the lower high-temperature zone of the reactor for the carbon metallurgical process is an endothermic process, and the indirect reduction of CO in the upper zone of the reactor is an exothermic process, except for the Fe3O4→FeO step, and the whole indirect reduction process belongs to a micro exothermic process. In the hydrogen metallurgy process, except for the Fe2O3→Fe3O4 step, the whole process of indirect reduction is an endothermic process.

Table 1.
Chemical equations of carbon metallurgy and hydrogen metallurgy processes.
To sum up, if green hydrogen is used to replace fossil fuels partially or completely as a BF reducing agent, the problem of reducing or completely eliminating carbon emissions in the process of iron and steel production can be solved, thus promoting a transformation of the iron and steel industry to become green and low-carbon [8]. Promoting the development of hydrogen metallurgy technology cannot only reduce costs, but also be adapted to national development policy, which is the best choice for the future development of the iron and steel industry [6]. Although the substitution of hydrogen for carbon inevitably brings an increase in energy consumption for reduction, there are two ways to solve this problem: the first is to establish a technique for supplying heat to the reactor from outside or injecting high-temperature hydrogen, and the second is to establish an oxygen-enriched combustion technology to steadily supply a large amount of hydrogen-based gas into the reactor and consider the endothermic characteristics of hydrogen-rich fuel injection pyrolysis. The above two technologies need to optimize the process parameters in order to maximize the matching of energy balance and reduction potential.
3. Low-Carbon Routes of the Long Process
At present, the means of achieving a low-carbon transformation in the iron and steel industries are different across different countries. Europe, the United States, and other developed countries are developing a new process of hydrogen reduction metallurgy that is mainly based on the short-process of green hydrogen direct reduction. However, there are a large number of BFs in China, and so the development of low-carbon metallurgical technology requires transformation. It is expected that over the next 20 years, the BF process will become the main process for iron and steel production in China. Iron and steel enterprises all over the world have formulated a low-carbon technology route of hydrogen metallurgy, which has accelerated the process of research and development, demonstrated the application of hydrogen metallurgy, and helped the world achieve the goal of carbon neutralization [1]. Large iron and steel enterprises in China, such as Baowu Steel Group, Angang Steel Group, and HBIS Group, have issued low-carbon metallurgical routes [4] through joint research with domestic universities and other industries in the development of hydrogen-rich BF processes and gas contained by H2 reduction shaft furnace processes. Zhu [9] reviewed the development history and technological status of green hydrogen direct reduction [10,11], molten oxide electrolysis [12,13], and the electrodeposition of iron [14,15,16]. This paper focuses on domestic and international process innovation technologies for long processes, with iron as the main product.
3.1. The Low-Carbon Process, at Home and Abroad
In 2008, Japan launched a new process (COURSE50) aimed at carbon neutralization. This method is an environmentally friendly ironmaking process that reduces CO2 emissions by increasing the proportion of iron ore reduced by hydrogen in the BF [17]. The proportion of hydrogen is increased by redesigning the size of the BF using hydrogen reduction and reforming coke oven gas to achieve the goal of a 30% reduction in CO2 emissions [18,19]. The test involves a common reduction of carbon and hydrogen, which combines various processes and is a model process for the transformation of the BF ironmaking process into an efficient, clean, and low-carbon process. The project is divided into three phases. At present, the first phase of the test has been completed, and it has proven that the emission reduction of CO2 can be reduced by nearly 10%. The second phase of the Super-COURSE50 project is currently in development. The goal of the third stage is to popularize the application of the hydrogen reduction BF process before 2050 [20]. A reduction in the carbon rate of approximately 10% was achieved, as predicted by the mathematical BF model [21]. Additionally, the reduction rate of CO2 emissions was affected by the amount of hydrogen injected. About 16% of CO2 emissions were reduced with a 359 Nm3/tHM of H2 injection. [22].
The main objective of the European ULCOS project is to develop feasible, low-carbon steel production technologies that will reduce CO2 emissions by at least 50% [23]. The three new ironmaking processes in the project are the top gas circulating oxygen blast furnace (OBF) process, the new smelting reduction process (HIsarna), and the iron ore electrolysis process (ULCOWIN) [24].
The China Baowu Steel Group has upgraded the BF at its core, and the green raw materials produced by microwave sintering and new burden technology are added to the hydrogen-rich carbon cycle BF. CO2 is removed from the top gas of the upgraded BF to form a long, green process with BF-BOF. Experiments on hydrogen-rich carbon cycle oxygen blast furnaces (named HyCORFTM) were carried out at Bayi Steel Plant (XINJIANG, China) [25,26,27]. The project finally realized the condition of 100% oxygen and injected decarbonization gas. At the same time, the industrial experiments of 1473 K high-temperature gas circulation injection and hydrogen enrichment were carried out, and the goals of reducing solid fuel consumption by 30% and carbon emissions by more than 20% were achieved.
3.2. Summary
Compared with the low-carbon technologies of the long process at home and abroad, the majority of iron and steel enterprises across the world reduce carbon emissions by increasing the proportion of hydrogen reduction in the furnace, such as by increasing the proportion of natural gas injection, using biomass injection, or even directly using hydrogen for injection. Two key problems need to be solved to increase the proportion of hydrogen reduction: The first is to solve the preparation of hydrogen-based gas, and if it requires injecting hydrogen, it is necessary to upgrade renewable energy to produce “green hydrogen” or optimize other hydrogen production technologies to reduce the cost of hydrogen production. The second is to solve the impact of increasing the reduction ratio of hydrogen on the BF process. Many metallurgical workers have conducted a lot of research on the reduction process of high-proportion hydrogen injections in the BF. The results show that other problems will occur in the BF production process when the hydrogen injection ratio is too high. The effects of different levels of high proportions of hydrogen under different process conditions are summarized in Table 2.

Table 2.
The effects of hydrogen enrichment.
The above pieces of literature are all high-temperature experiments carried out under laboratory conditions. The results show that there are two main problems in the current production of high-proportion hydrogen injection: The first is that the reduction process of hydrogen is an endothermic reaction, and so the additional heat increases energy consumption. The second is that although the reduction product, H2O, is clean energy, it will aggravate the Boudouard reaction of coke in the lower high-temperature zone, resulting in a decrease in the high-temperature strength of coke, which may lead to unfavorable furnace conditions. However, for the BF of vanadium and titanium ore, the start temperature of the melt and drip increases at 10% hydrogen enrichment. So, the melting temperature range becomes narrower, the cohesive zone becomes the worst place for gas permeability, the thickness of which becomes thinner, the position moves down, and the gas permeability increases, which promotes smooth and high production levels.
To sum up, the BF process has been developed for hundreds of years and is characterized by high thermal efficiency, a large capacity scale, and perfect technology. From the practice of operations at home and abroad, the heat utilization coefficient of the traditional BF is generally 80–85%, while that of some advanced BFs can be as high as 90%. However, the carbon utilization coefficient in the BF is generally 50% to 60%, and the coefficient can continue to improve. From the point of view of gas utilization efficiency combined with production, the BF has raised the gas utilization rate to a very high level by combining the thermodynamics and kinetics of the reduction reaction, which makes it difficult to further improve it in a short time. The recycling of top gas in the BF is another way to reduce fossil fuels; that is, the CO2 in the top gas is removed and then recycled. However, the problem of N2 accumulation will occur if the N2 of the top gas is not removed during the cycle; therefore, the top gas cycle can only be realized in the OBF. If the OBF is used, the blast kinetic energy brought by pure oxygen is very small and cannot be injected into the central area, so the designed OBF is very difficult to produce on a large scale, and it will lose the advantage of high output, which is a key problem to be solved. Through the above analysis, it is known that hydrogen enrichment in the BF is the best scheme to reduce CO2 emissions. After hydrogen enrichment, it is necessary to meet not only the requirements of thermal energy and reduction potential, but also the needs of coke as a skeleton support. A new solution needs to be put forward based on these two requirements. This provides a new theoretical basis and process plan for realizing the limited emission reduction in ironmaking.
4. Proposal of a New Process for RSF with Hy-O-CR
4.1. The Basis for the Development of a New Process
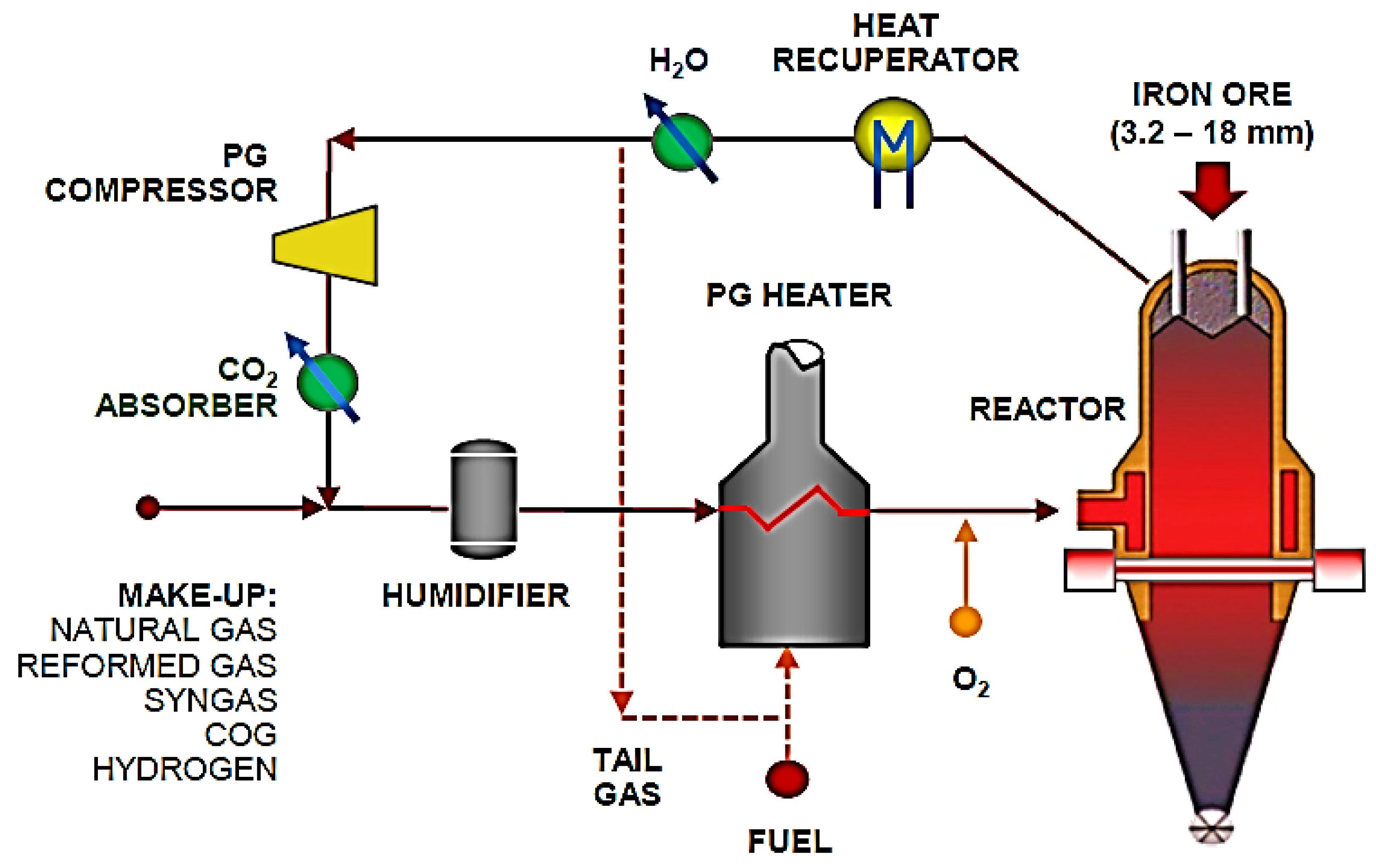
The HBIS Group, using the most advanced technology, built the first hydrogen metallurgy demonstration project in the world with a scale of 1.2 million tons of direct reducing iron (DRI) [35]. The technological process is shown in Figure 1. The project focuses on the low cost of hydrogen production and purification, the reformation of the coke oven gas (COG), and CO2 capture and utilization storage (CCUS) through innovative research and development of the whole process in order to explore the development path of low-carbon or even free-carbon emissions in the process of iron and steel production. The project aimed to adjust the structure of energy consumption, solve the problems of environmental pollution and carbon emissions in the whole process of iron and steel production, promote the reform of traditional processes, and contribute new plans to the national “dual carbon” strategic goal.
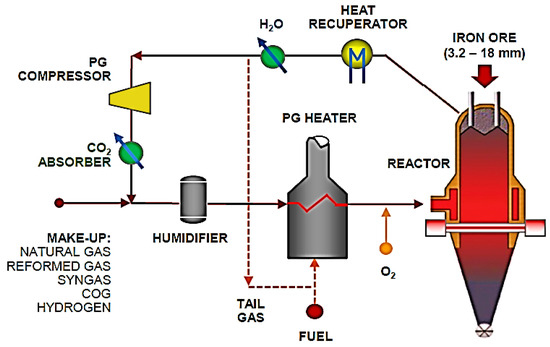
Figure 1.
Process flow chart of Energiron direct reduction production.
The hydrogen metallurgy demonstration project of the HBIS Group is the first in the world to use COG reforming to produce hydrogen and high-quality direct reducing iron, which has gradually changed to hydrogen metallurgy technology. COG is used as a reducing gas in the first stage of the demonstration project. By combining the advantages of the local resources in Zhangjiakou, China, the second stage will develop a clean energy power-generation technology to realize the process of producing “green hydrogen” using “green electricity”. In the near future, the second stage of the project will use hydrogen by electrolyzing water, reducing or eliminating the use of fossil fuels, and leading the development and reform of low-carbon green metallurgy across the world.
Based on the technology of the hydrogen reduction shaft furnace introduced by the HBIS Group, new equipment and new technological processes are being developed. Combined with the unique advantages of the abundance of renewable energy in Zhangjiakou, the HBIS Group and Northeastern University independently developed a new reduction smelting furnace (RSF) process with Hy-O-CR [36]. The production of the hydrogen reduction shaft furnace is solid DRI, which also needs to be used by an electric arc furnace, while the RSF product of the new process is liquid molten iron (hot metal). The new process is more efficient, more resistant to fluctuations, and better matched with subsequent traditional processes, and it can make full use of the existing equipment to significantly reduce investment costs.
4.2. Development Ideas for the New RSF Process
Combined with the basic Chinese domestic conditions, it is necessary to carry out technical innovation of ironmaking processes with high carbon emissions. At present, the BF process is still the main ironmaking process in China, using reducing agents such as coke and pulverized coal. Under the constraint of the “dual carbon” goal, the traditional BF needs to solve the following four problems to adapt to the inevitable low-carbon period. First, according to many years of research experience into traditional BF, the best direct reduction degree is 21.5%, under the condition that both reduction carbon consumption and burning carbon heating are satisfied. In the reduction process of traditional BF, the mode generally combines direct reduction (30–40%) and indirect reduction (60–70%), which greatly limits the carbon emission reduction of the BF. From another point of view, the energy consumption of BF ironmaking technology is close to the limits of thermodynamic theory, which means it will be difficult to continue to reduce emissions. Second, it is impossible to carry out a top gas cycle to improve carbon utilization. The traditional BF uses hot air for injecting, resulting in a large amount of N2 in the top gas, which will lead to the problem of N2 accumulation if recycled. Third, use of the OBF will also face a lot of problems, such as the existence of a lower limit of direct reduction, heat in the lower zone with cold in the upper zone, and insufficient central gas supply due to the kinetic energy of oxygen becoming smaller [37]. Fourth, the hydrogen metallurgical BF is used to replace carbon, but there is an upper limit of the ratio of hydrogen. The study shows that it is feasible to use the hydrogen-rich fuel of BFs. However, its product, H2O, will affect the high-temperature performance of coke and lead to unfavorable furnace conditions. Thus, it is not feasible for the traditional BF to achieve a significant reduction in carbon emissions by enriching hydrogen in high proportions.
Combined with the previous literature, the authors collate the iron ore reduction process in high-proportion hydrogen-rich atmospheres from aspects of engineering practice [35], thermodynamic theory, kinetic simulations [38], and related physical experimental data [39]. Wang X.D. [35] introduced a hydrogen metallurgy process with rich hydrogen using COG as the gas source. The reduction gas temperature was 1223 K, the volume fractions of H2 and CO were 60.5% and 7.3%, respectively, and the metallization rate of the product DRI was 92%. Xu H. [38] found that, according to the mass and energy balance equations of the gas phase and the solid phase, the variation curves of concentration and temperature of each substance were obtained using a single-interface unreacted core model. In this model, the indirect reduction section length of the reactor was 9.75 m, the diameter of the furnace was 4.26 m, the pellet radius was 5.5 mm, the reduction gas temperature was 1223 K, and the flow rate of the gas was 5.39 × 104 Nm3/h, of which H2 occupied 52.6% and CO accounted for 30.0%. The simulation results show that in the indirect reduction section, iron-containing raw materials can be completely transformed into Fe3O4 at a distance of 0.5 m and into FeO at a distance of 2.0 m. When the indirect reduction height is 9.75 m, the metallization rate can reach more than 90%. Liu X.C. [39] studied the reduction process of pellets by high-temperature reduction experiments. The effects of different H2/CO ratios, temperatures, and atmospheres on direct reduction were obtained. The suitable process parameters for the production of pellets with different metallization rates were obtained through high-temperature experiments. The results show that the metallization rate of the product is more than 92% when the temperature is 1323 K; 30 min is needed in a pure hydrogen atmosphere, and 50 min is needed in a hydrogen-rich atmosphere (H2 = 74%). The experimental results show that the metallization rate of the product increases gradually with the increase in the reduction time, reduction temperature, gas flow rate, H2 ratio, and other parameters.
From the above engineering practice and simulation experiments, it can be seen that the reduction process of iron ore is closely related to the pellet diameter, reduction section height, temperature, atmosphere and gas flow rate, reduction time, and so on. By reasonably adjusting the above parameters, the metallization rate can reach 85% to 95%, in theory.
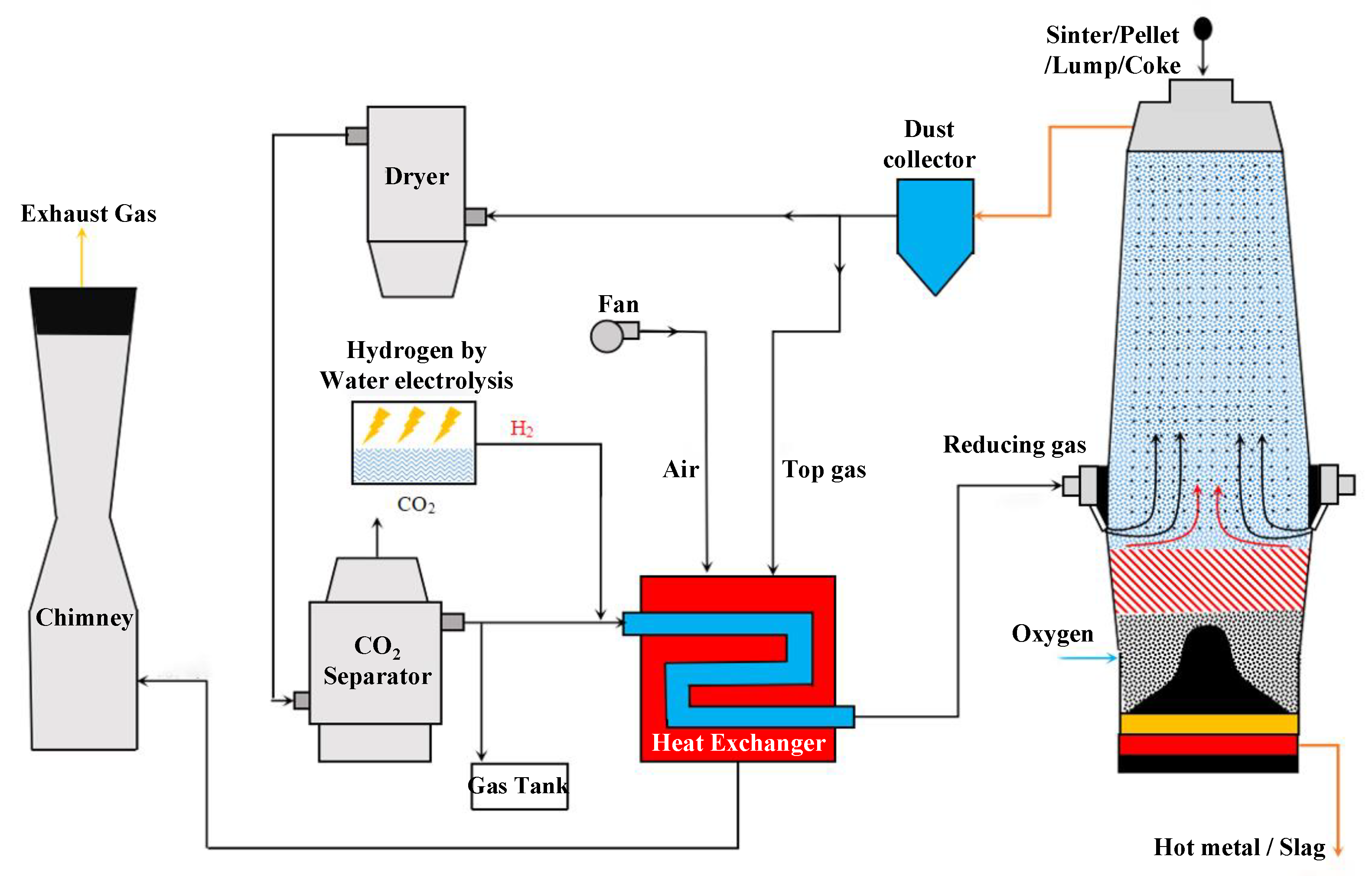
Following the above discussion, the authors think that, in order to improve the proportion of carbon emission reduction, it is necessary to upgrade the size of the existing traditional BF. Four kinds of problems existing in the traditional BF are solved by upgrading the furnace size and the cycle of top gas so as to break through the limits of the proportion of direct reduction and indirect reduction in the traditional BF. Therefore, a new RSF process with Hy-O-CR is proposed in this paper. A new furnace type is designed to change the matching ratio of direct reduction and indirect reduction in the reduction process, reduce the carbon consumption of hot metal per ton, and improve the utilization coefficient of the new furnace through the reuse of top gas to achieve carbon recycling, and finally achieve the goal of reducing CO2 emissions by at least 40%. Based on this, a new ironmaking system and method of RSF with Hy-O-CR are presented in this paper, as shown in Figure 2. In this system, the two-stage RSF with shaft tuyeres and hearth tuyeres is the core reactor of the ironmaking system, which can replace the BF reactor in the long process.
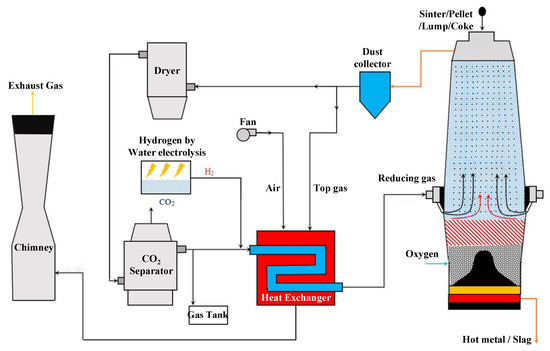
Figure 2.
The ironmaking system and process flow chart of RSF with Hy-O-CR.
The new process evenly mixes ore and coke and then is charged into the RSF from the top charging devices, and oxygen is injected into the furnace from the lower hearth tuyeres. The combustion of coke in the furnace forms the bosh gas dominated by CO, which meets the high-temperature hydrogen-rich reducing gas injected from the shaft tuyeres in the middle, and the two kinds of gas work together to preheat and reduce the downward materials. The metallization rate at the bottom of the indirect reduction zone reached 85–95% due to the enhanced reduction of the high proportion of hydrogen. The top gas produced is treated by dedusting, drying, and CCUS and forms a high reducing potential gas with CO and H2, which can be recycled. The recycled gas is mixed with the green hydrogen produced by the electrolytic water equipment and heated by the heat exchanger to form a high-temperature reducing gas of 1123–1273 K, which is then injected into the RSF from the shaft tuyeres, and the remaining reducing gas is stored and reserved. The heat source of the heat exchanger comes from the gas combustion separated from the RSF top gas, and the produced CO2 is either discharged directly or decarbonized.
The function of the upper zone of the core equipment in the new process is indirect reduction, and the function of the lower zone is to burn coke to provide direct reduction heat and the necessary smelting heat for the formation of liquid slag and hot metal. Therefore, the core equipment is named the reduction smelting furnace (RSF). The key to the technical characteristics is hydrogen-rich, oxygen-rich, and carbon-recycled (Hy-O-CR) technology. The oxygen injection can get rid of the recycling of N2 into the top gas, which is more conducive to carbon cycle and CO2 removal. The high reducing potential CO obtained from the cycle is used for the reduction of iron ore, which can fully realize the extreme use of the chemical energy of carbon elements. At the same time, circulating gas is beneficial to realizing the recycling of hydrogen in the top gas, solving the problem of decreasing hydrogen utilization caused by a high proportion of rich hydrogen, and providing a prerequisite for subsequent increases in the hydrogen ratio. As a result, the traditional BF ironmaking process gradually gets rid of its dependence on fossil fuels.
5. Comparative Analysis of Typical Low-Carbon Process Indicators
German scholar Wenzel [40] first put forward the OBF technology in 1975. After years of in-depth research, ironmaking scholars found that there are two problems of “upper colder” and “lower hotter” in the actual production of OBFs. On this basis, metallurgical experts at home and abroad have proposed to improve the process [41]. The Fink process [42], full oxygen BF (FOBF) process [43], oxygen-coal-Flux (OCF) process [44], NKK process [45] and so on use different technologies to solve the above two problems of OBFs. In addition, Xia Z.X. [46] from the University of Science and Technology Beijing conducted a CO2 reduction potential analysis for four typical working conditions based on the OBF process, such as traditional BF, OBF, CCU-OBF (oxygen blast furnace with carbon capture and utilization), CCU-OBFh-ec (oxygen blast furnace with carbon capture, utilization, and hydrogen-rich injecting). The study shows that the CO2 emission of conventional BFs is 1572 kg/tHM and the CO2 emission of OBFs is reduced by 10.7% per ton of hot metal, and the CO2 emission of the CCU-OBF process is 822 kg/tHM, which is reduced by 47.7%. The article calculates and analyzes the reduction of CO2 emissions in the range of 38% to 44% for the CCU-OBFh-ec process under different hydrogen enrichment conditions, and the process reduction is attributed to an increase in H2 reduction in the furnace, the reduction of coke usage, and the use of CO2 storage technology. In summary, it can be seen that OBFs have a greater potential to reduce emissions under hydrogen-rich fuel injection conditions.
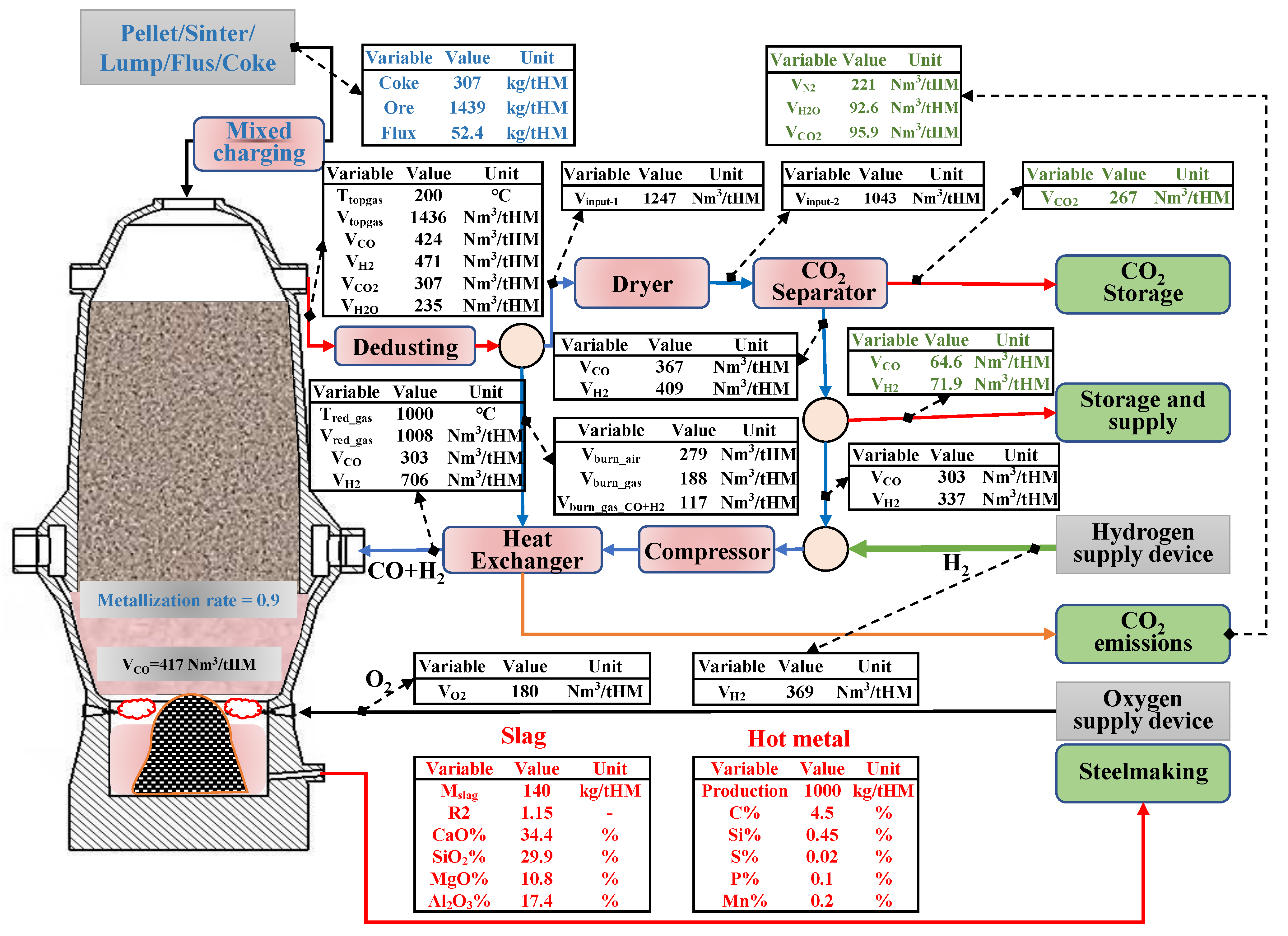
According to the principles of mass balance and heat balance, the authors have developed a mass and energy distribution model of RSFs with the Hy-O-CR ironmaking process, as shown in Figure 3.
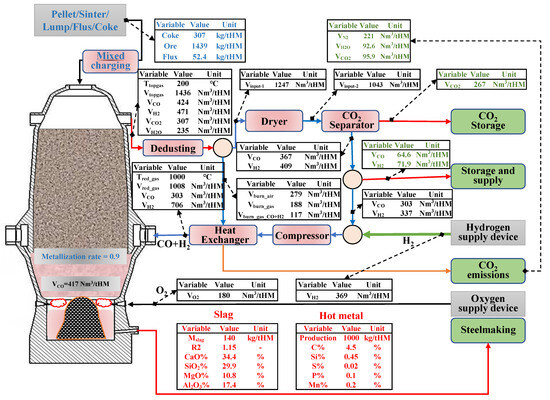
Figure 3.
Material distribution map of new low-carbon ironmaking process.
The process model adopts the method of combining a regional balance with an overall furnace balance, the indirect reduction reaction of step-by-step reduction of iron oxides using CO and H2 (Fe2O3→Fe3O4→FeO→Fe), and the water–gas reaction in the upper reduction area. The chemical reactions considered in the lower smelting zone include direct carbon reduction reactions of elements, such as Fe/Si/Mn/P/S in hot metal, coke combustion reactions, slag formation reactions, and carbonate decomposition reactions. The model obtains the burden distribution information of the new low-carbon ironmaking process, which is based on producing 1 ton of hot metal at 1000 °C with CO:H2 = 3:7 injection of shaft tuyere, the upper metallization rate of 90% as the boundary conditions, and the lowest top gas reduction potential (1.22) as the constraints.
The volume of bosh gas needs 1.40 × 103 Nm3/tHM in the traditional BF process. In the new process, the volume of the reducing gas injected into the furnace shaft is approximately 1.0 × 103 Nm3/tHM, and the bottom hearth tuyeres produce approximately 415 Nm3/tHM. This gas distribution method not only promotes an increase in the proportion of indirect reduction in the furnace shaft, but it also reduces the heat consumption of direct reduction in the lower zone. The heat balance calculation is carried out from the regional (high-temperature zone) balance with the whole furnace balance, respectively, and the feasibility of the process is confirmed. The calculated results are shown in Table 3 and Table 4.

Table 3.
Heat balance data of the whole furnace (energy, MJ/tHM).

Table 4.
Heat balance data in high-temperature area (energy, MJ/tHM).
It can be seen that the heat balance of the whole furnace and the high-temperature zone can meet process requirements when the metallization rate in the lower zone reaches 90% from Table 3 and Table 4. From the point of view of the heat balance of the whole furnace, the heat of carbon oxidation, the heat of reducing gas, and the heat of hydrogen oxidation account for 58.4%, 14.9%, and 26.3% of the total heat income of the whole furnace, respectively. The heat surplus (sensible heat and heat loss of the top gas) of the whole furnace is 1035 MJ/tHM, accounting for 10.6%. Compared with the heat surplus of the traditional BF, 7% to 10%, the actual operation of the new process has a certain feasibility. It can be seen from Table 4 that the heat consumption of the direct reduction in high-temperature zones is 390 MJ/tHM. Compared with the lower direct reduction heat consumption of the traditional BF, 904 MJ/tHM (when the direct reduction degree is 0.40), the reduction is as high as 57.8%, which is the main reason for saving fuels in this process and is closely related to the design idea of reducing the lower direct reduction heat consumption. Compared with the heat surplus of 35% to 40% in the high-temperature zone of the traditional BF, the heat surplus (gas output and heat loss) of the high-temperature RSF is 844 MJ/tHM, which accounts for 30.6%. The heat surplus still meets the heat needs for the smelting of slag and hot metal and direct reduction heat consumption. Although the heat generated by the bosh gas in this process is less than that of the traditional BF, the heat produced by injecting high-temperature gas into the furnace shaft makes up for the lack of heat. It can be seen that the actual operation is feasible.
In order to more intuitively reflect the difference between the existing two-stage oxygen-injection BF process and the top gas cycle, the authors make a comparative analysis with the relevant process flow distribution diagram [46]. By comparison, it can be found that it heats the reducing gas, removing CO2 to the temperature of 1173 K in the OBF process of reference [46], and injects it from the shaft tuyeres and hearth tuyeres, respectively. The gas injection volumes for producing 1 ton of hot metal are 360 Nm3 (shaft tuyeres) and 261 Nm3 (hearth tuyeres), respectively, and the mass of fuel includes 306 kg/tHM of coke, 160 kg/tHM of pulverized coal (PC), and 50 Nm3/tHM of natural gas (NG). The proportion of H2 in the furnace shaft is 17.3%. It is calculated that the metallization rate at the furnace shaft is between 70% and 80%. Compared with the new low-carbon process proposed in this paper, 307 kg/tHM of coke and 1008 Nm3/tHM of reducing gas with 70% H2 are injected from the furnace shaft tuyere, and the proportion of H2 after injection into the furnace at the furnace shaft is 50.5% (much higher than 17.3%) when it is mixed with the lower CO gas. The metallization rate of the ore in the furnace shaft is increased to 90%, which reduces the heat required for direct reduction in the high-temperature zone. The heat in the high-temperature zone only needs to meet the smelting heat of slag and hot metal iron and the heat consumed by direct reduction, which is 10% lower than that of the OBF.
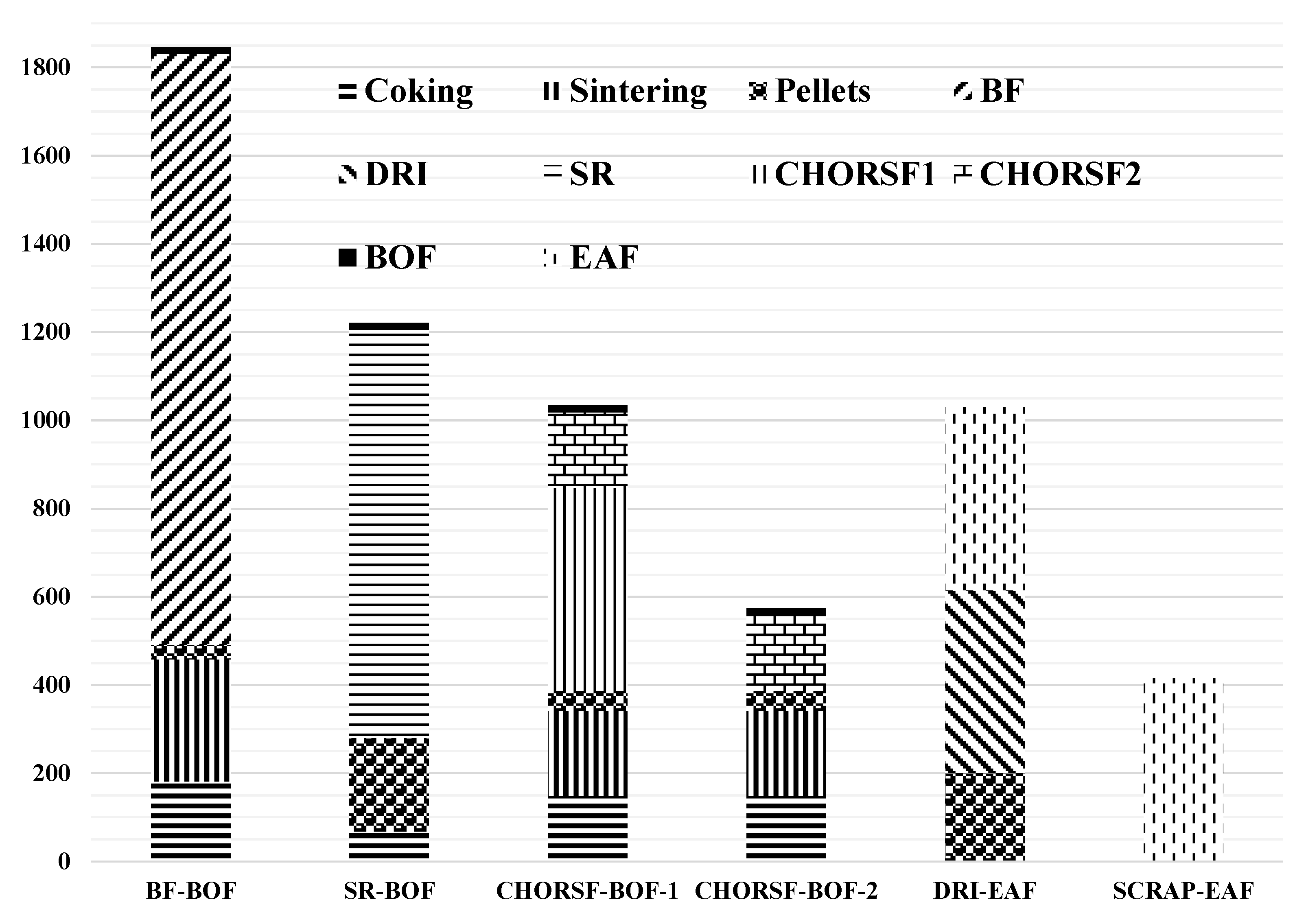
The results of this model were used to compare and analyze the CO2 emissions per ton of steel for the whole process under six typical processes, as shown in Figure 4, and the CO2 emissions per ton of steel of the sub-processes corresponding to six typical processes are shown in Table 5. The six processes are as follows: BF-BOF, SR (smelting reduction)-BOF, CHORSF-BOF-1, CHORSF-BOF-2 (CCS technology has been added), DRI-EAF, and the short-process of SCRAP-EAF.
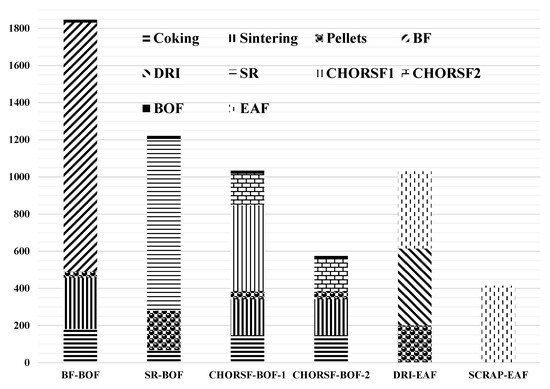
Figure 4.
CO2 emission analysis of six typical processes.

Table 5.
CO2 emission values of sub-processes under six typical processes (kg/ts).
From Figure 4 and Table 5, it can be seen that the mass values of CO2 emissions are 1846 kg/ts, 1221 kg/ts, 1034 kg/ts, 574 kg/ts, 1030 kg/ts, and 415 kg/ts for the six processes. From the above data, it can be seen that the CO2 emissions per ton of steel of the new low-carbon process of RSFs are comparable to the short process of DRI-EAF because both processes indirectly reduce ore to more than 90%, breaking the bottleneck of the upper limit of indirect reduction in the traditional BF. If the CO2 removed by the new process is reused as a raw material for chemical products, an integrated steel and chemical process will be formed, and the CO2 emissions per ton of steel corresponding to the process will be reduced to 574 kg/ts, or even lower. Compared to the long process, the CO2 emissions per ton of steel of the new process correspond to a reduction of between 44% and 69%.
6. Conclusions
Under the “dual carbon” strategy, green and low-carbon development has become the core proposal for the transformation and development of the iron and steel industries. The long process of iron and steel production in China is still dominated by BF-BOF. The authors systematically combed the existing low-carbon metallurgical routes, at home and abroad, and learned that hydrogen metallurgy has become the mainstream low-carbon ironmaking technology. The route of replacing carbon with hydrogen is studied systematically, and the following conclusions are obtained:
- The authors compare the difference between hydrogen metallurgy and carbon metallurgy in terms of thermodynamics and point out that replacing carbon with hydrogen will inevitably increase reduction energy consumption and protect the environment. Two technologies should be used to optimize the matching of thermal energy and reduction potential. These are heating the reactor from the outside (injecting high-temperature hydrogen) and steadily supplying a large amount of hydrogen gas into the reactor.
- The BF-BOF long process is the main process of steel production in China. The authors think that the BF process should be mainly used until 2050 before reaching “carbon neutrality”. The authors evaluated the hydrogen-rich BF process in Japan, Europe, and China and think that promoting a transformation from the traditional BF to a hydrogen-rich BF is the most feasible method at present. However, the existing lower-zone injection process has certain restrictions on the proportion of hydrogen-rich processes, and the emission reduction of CO2 per ton of hot metal is less than 30%. If we want to achieve substantial emission reduction, we still need to change our concept and develop disruptive, innovative technologies.
- Starting with the key parameters and the limits of traditional BF emission reduction, the authors put forward an ironmaking system and an ironmaking method of RSF with Hy-O-CR. Technical upgrades such as furnace size redesign, furnace shaft gas injection, mixed charging methods, and reasonable control of the gas distribution ratio between the upper and lower zones broke the defect that the upper metallization rate of the traditional BF cannot be raised to more than 70%. Circulation technology for self-gas is realized by combining the top gas removal of CO2 with the hydrogen-rich gas of heating. Compared with the long process, the target of a 44–69% reduction in CO2 emissions per ton steel can be achieved, which provides a new means of realizing green low-carbon metallurgy.
The idea of low-carbon ironmaking involved in this study is based on a high proportion of rich hydrogen. The new process will inevitably bring new challenges, such as the evolution of the material bed and structure, the melting and dripping behavior of iron ore under high metallization rates, and the matching restriction of physical energy and chemical energy in each functional area of the new RSF reactor, which is essentially different from the traditional BF. At the same time, it is also faced with the mutual matching between the hydrogen-rich devices outside the furnace and the gas circulation devices on the top gas in the RSF. These problems still need to be deeply analyzed from the perspectives of the unit phenomenon, reactor parameter optimization, and process equipment capacity matching in the later stage so as to realize the application of industrialization.
Author Contributions
Conceptualization, H.L.; Data curation, J.C.; Funding acquisition, H.L.; Investigation, H.L.; Methodology, H.L.; Project administration, H.L.; Resources, H.L.; Software, H.L.; Supervision, H.L.; Writing—original draft, J.C.; Writing—review and editing, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the National Key R&D Program of China (No. 2022YFE0208100) and the Fundamental Research Funds for the Central Universities (N2225022).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Q.; Shen, J.; Xu, L.S. Carbon peak and low-carbon transition path of China’s iron and steel industry. Iron Steel 2021, 56, 152–163. (In Chinese) [Google Scholar]
- Zhang, P. Results of the world’s first 400 m3 industrial grade hydrogen-rich carbon circulating oxygen blast furnace released. China Metallurgical News, 23 November 2022. 172(A01). (In Chinese) [Google Scholar]
- Li, H.F.; Xu, W.R.; Zhang, X.H.; Zou, Z.S. Theoretical analysis and practice of CO2 emission capability under hydrogen-rich fuel injection in blast furnace. In Proceedings of the 13th China Iron and Steel Annual Meeting—Ironmaking and Raw Fuel, Beijing, China, 23–24 November 2022; The Chinese Society for Metals: Beijing, China, 2022. (In Chinese). [Google Scholar]
- Lin, S.H. Research on the application and development trend of hydrogen energy in iron and steel metallurgy. China Coal 2022, 48, 95–102. (In Chinese) [Google Scholar]
- Gao, J.J.; Qi, Y.H.; Yan, D.L.; Wang, F.; Xu, H.C. Development path and key technical problems of low carbon ironmaking in China. China Metall. 2021, 31, 64–72. (In Chinese) [Google Scholar]
- Shangguan, F.Q.; Zhou, J.C.; Wang, H.F.; Li, X.P. Climate change and decarbonization development of steel industry. Iron Steel 2021, 56, 1–6. (In Chinese) [Google Scholar]
- Xiao, W.X.; Wang, G.; Li, M.M.; Zhou, Z.P.; Zhao, Y.J. Analysis of carbon reduction strategy based on hydrogen-rich/carbon-containing gas used for smelting reductant. China Metall. 2023, 33, 121–127. (In Chinese) [Google Scholar]
- Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials 2021, 14, 7540. [Google Scholar] [CrossRef]
- Zhu, Q.S. Development pathway analyses for various ironmaking routes with ultra-low CO2 emission. Chem. Ind. Eng. Prog. 2022, 41, 1391–1398. (In Chinese) [Google Scholar]
- Chufarov, G.; Tatijevskaja, E. The reaction zones in the reduction of magnetite and hematite with hydrogen. Acta Physicochim. URSS 1935, 3, 957–974. [Google Scholar]
- Schenk, J.L. Recent status of fluidized bed technologies for producing iron input materials for steelmaking. Particuology 2011, 9, 14–23. [Google Scholar] [CrossRef]
- Wiencke, J.; Lavelaine, H.; Panteix, P.J.; Petitjean, C.; Rapin, C. Electrolysis of iron in a molten oxide electrolyte. J. Appl. Electrochem. 2018, 48, 115–126. [Google Scholar] [CrossRef]
- Allanore, A. Features and challenges of molten oxide electrolytes for metal extraction. J. Electrochem. Soc. 2014, 162, E13–E22. [Google Scholar] [CrossRef]
- Picard, G.; Oster, D.; Tremillon, B. Electrochemical reduction of iron oxides in suspension in water-sodium hydroxide mixtures between 25 °C and 140 °C, Part II. Experimental study. J. Chem. Res. 1980, 8, 252–253. [Google Scholar]
- Yuan, B.Y.; Kongstein, O.E.; Haarberg, G.M. Electrowinning of iron in aqueous alkaline solution using a rotating cathode. J. Electrochem. Soc. 2009, 156, D64. [Google Scholar] [CrossRef]
- Monteiro, J.F.; Ivanova, Y.A.; Kovalevsky, A.V.; Ivanou, D.K.; Frade, J.R. Reduction of magnetite to metallic iron in strong alkaline medium. Electrochim. Acta 2016, 193, 284–292. [Google Scholar] [CrossRef]
- Zhang, F.M.; Liu, Q.M. Development and understanding on low carbon technology based on BF-BOF steel manufacturing processes. China Metall. 2023, 33, 1–17. (In Chinese) [Google Scholar]
- Nishioka, K.; Ujisawa, Y.; Tonomura, S.; Ishiwata, N.; Sikstrom, P. Sustainable aspects of CO2 ultimate reduction in the steelmaking process (COURSE50 Project), part 1: Hydrogen reduction in the blast furnace. J. Sustain. Metall. 2016, 2, 200–208. [Google Scholar] [CrossRef]
- Watakabe, S.; Miyagawa, K.; Matsuzaki, S.; Inada, T.; Tomita, Y.; Saito, K.; Wikstrom, J.O. Operation trial of hydrogenous gas injection of COURSE50 project at an experimental blast furnace. ISIJ Int. 2013, 53, 2065–2071. [Google Scholar] [CrossRef]
- Kaoru, N.; Yutaka, U. Experimental blast furnace operation for CO2 ultimate reduction. In Proceedings of the 1st International Conference on Science and Technology for an Internet of Things (ICSTI 2018), Yogyakarta, Indonesia, 19–20 October 2018; Austrian Society of Metallurgy and Materials: Vienna, Austria, 2018. [Google Scholar]
- Kaoru, N.; Hiroshi, S.; Yutaka, U.; Kazumoto, K.; Koki, N.; Kohei, S.; Yoshinori, M.; Hirokazu, Y. Development of Low Carbon Blast Furnace Operation Technology by using Experimental Blast Furnace. ISIJ Int. 2022, 62, 2424–2432. [Google Scholar]
- Chikashi, K.; Yoshinori, M.; Hirokazu, Y.; Kohei, S.; Kazumoto, K.; Hiroshi, S.; Kaoru, N.; Yutaka, U.; Koki, N. Influence of Large Amount of Hydrogen Containing Gaseous Reductant Injection on Carbon Consumption and Operation Conditions of Blast Furnace-Development of Low Carbon Blast Furnace Operation Technology by using Experimental Blast Furnace: Part II. ISIJ Int. 2022, 62, 2433–2441. [Google Scholar]
- Hirsch, A.; Van, D.; Sert, D. ULCOS top gas recycling blast furnace. In Proceedings’ Abstracts of Asia Steel International Conference 2012; The Chinese Society for Metals: Beijing, China, 2012; p. 35. [Google Scholar]
- Quader, M.A.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Tian, B.S.; Zhang, J.S. Industrial Test of High Oxygen Enriched Smelting in Oxygen Blast Furnace of Bayi Steel. Xinjiang Iron Steel. 2020, 4, 1–3. (In Chinese) [Google Scholar]
- Tian, B.S. Research and practice on low carbon smelting technology of Hydrogen-rich carbon cycle blast furnace at Bayi Steel. Xinjiang Iron Steel 2021, 4, 1–4. (In Chinese) [Google Scholar]
- Yuan, W.N.; Li, T.; Liu, Z.X. Thoughts and Practice of Low Carbon Ironmaking Technology of Bayi Steel. Xinjiang Iron Steel 2022, 1, 1–3. (In Chinese) [Google Scholar]
- Dou, M.H.; Sun, Y.; Han, J.W.; Sun, Z.; Liang, Y.H. Solution loss characteristics of cokes in H2O+CO2 atmosphere. Iron Steel 2022, 57, 26–33. (In Chinese) [Google Scholar]
- Lan, C.C.; Liu, R.; Zhang, S.H.; Lv, Q. Effect of H2 volume percent on gasification reaction of coke in blast furnace. Iron Steel 2020, 55, 100–106. (In Chinese) [Google Scholar]
- Zhao, Z.; Tang, J.; Chu, M.; Wang, X.; Zheng, A.; Wang, X.; Li, Y. Direct reduction swelling behavior of pellets in hydrogen-based shaft furnaces under typical atmospheres. Int. J. Miner. Metall. Mater. 2022, 29, 1891–1900. [Google Scholar] [CrossRef]
- Mao, X.; Garg, P.; Hu, X.; Li, Y.; Nag, S.; Kundu, S.; Zhang, J. Kinetic analysis of iron ore powder reaction with hydrogen—Carbon monoxide. Int. J. Miner. Metall. Mater. 2022, 29, 1882–1890. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of iron oxides with hydrogen—A review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Li, J.; Kuang, S.; Jiao, L.; Liu, L.; Zou, R.; Yu, A. Numerical modeling and analysis of hydrogen blast furnace ironmaking process. Fuel 2022, 323, 124368. [Google Scholar] [CrossRef]
- Qie, Y.N.; Jin, Y.T.; Kang, Y.; Li, L.F.; Wang, Y.F.; Wang, X.D. Influence of hydrogen-rich on the softening and melting property of blast furnace burden with vanadium and titanium. Iron Steel 2023, 33, 31–38. (In Chinese) [Google Scholar]
- Wang, X.D.; Zhao, Z.L.; Li, C.M.; Yang, Y.Q. Hydrogen metallurgy engineering technologies based on zero reforming of hydrogen enriched coke oven gas. Iron Steel 2023, 38, 11–19. (In Chinese) [Google Scholar]
- Yu, Y.; Wang, X.D.; Luo, Z.G. Low-Carbon Ironmaking System and Method in Reduction and Smelting Furnace with Pure Oxygen and Hydrogen-Rich. China Patent ZL 200210084753.X, 31 January 2023. (In Chinese). [Google Scholar]
- Liu, J.Z. Fundamental Research on Shaft Gas and Cycle Gas Behaviors in Oxygen Blast Furnace. Ph.D. thesis, University of Science and Technology Beijing, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Xu, H.; Zou, Z.S.; Yu, A.B. A Preliminary Numerical Simulation of Midrex Reduction Shaft Furnace. Iron Steel 2008, 43, 12–17, 60. (In Chinese) [Google Scholar]
- Liu, X.C.; Shi, N.Z.; Zhao, Z.C.; Xu, C.J.; Tang, Y.; Chu, M.S. Research on preparation of pellets with different metallization rates under hydrogen-based shaft furnace condition. Sinter. Pelletiz. 2022, 47, 1–8. (In Chinese) [Google Scholar]
- Wenzel, W.; Gudenau, H.W.; Fukushima, T. Blast furnace operating methods. U.S. Patent 3,884,677, 20 May 1975. [Google Scholar]
- Xue, Q.G.; Yang, F.; Zhang, X.X.; Wang, J.S.; Zuo, H.B.; Jiang, Z.Y.; Yu, X.F.; Wang, G. Development of an oxygen blast furnace and its research progress in University of Science and Technology Beijing. Chin. J. Eng. 2021, 43, 1579–1591. (In Chinese) [Google Scholar]
- Fink, F. Suspension smelting reduction–a new method of hot iron production. Steel Times 1996, 224, 398. [Google Scholar]
- Qin, M.S.; Xie, Y.K.; Yang, Y.Y.; Yang, N.F.; Gu, F.; Ren, D.N. Feasibility study of a new blast furnace process of injecting coal at unusual high rate and partially recycling of top gas. Iron Steel 1985, 20, 13. (In Chinese) [Google Scholar]
- Gao, Z.K.; Zhang, J.L.; Kong, L.T. New technology for blast furnace ironmaking with oxygen-coal. Iron Steel 1997, 32, 372. (In Chinese) [Google Scholar]
- Ohno, Y.; Hotta, H.; Matsuura, M.; Mitsufuji, H.; Satito, H. Development of oxygen blast furnace process with preheating gas injection into upper shaft. Tetsu-Hagane 1989, 75, 1278. [Google Scholar] [CrossRef]
- Xia, Z.; Jiang, Z.; Zhang, X.; Li, Z.; Lu, Y.; He, Y.; Chen, J. The CO2 reduction potential for the oxygen blast furnace with CO2 capture and storage under hydrogen-enriched conditions. Int. J. Greenh. Gas Control 2022, 121, 103793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).