Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum
Abstract
:1. Introduction
2. Materials and Methods
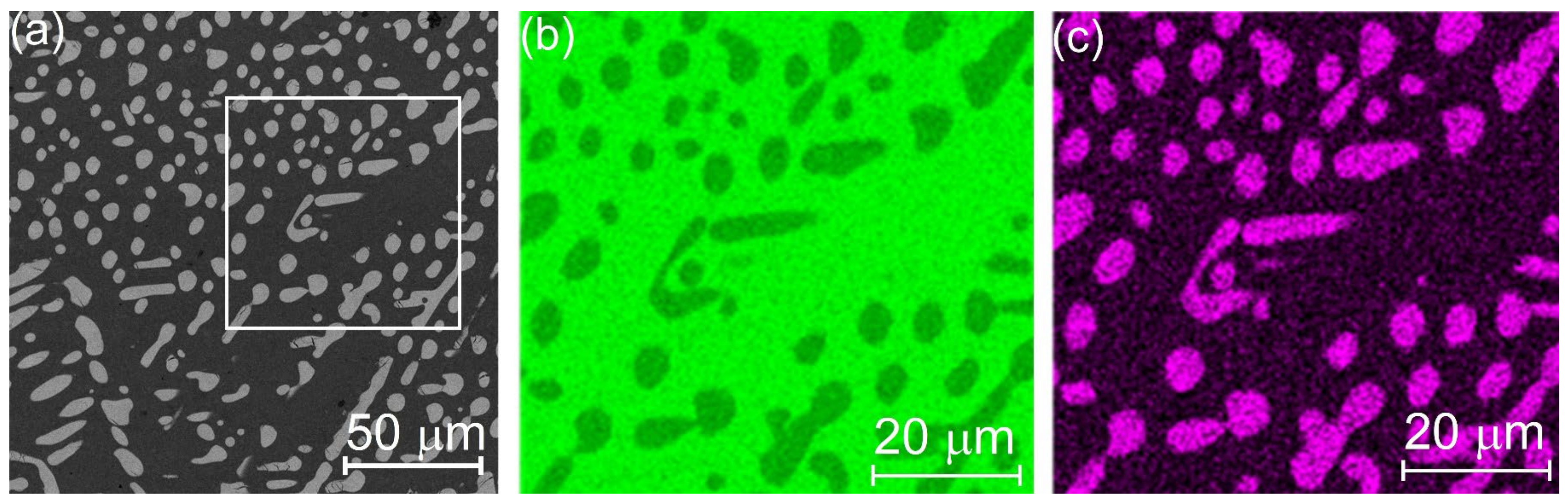
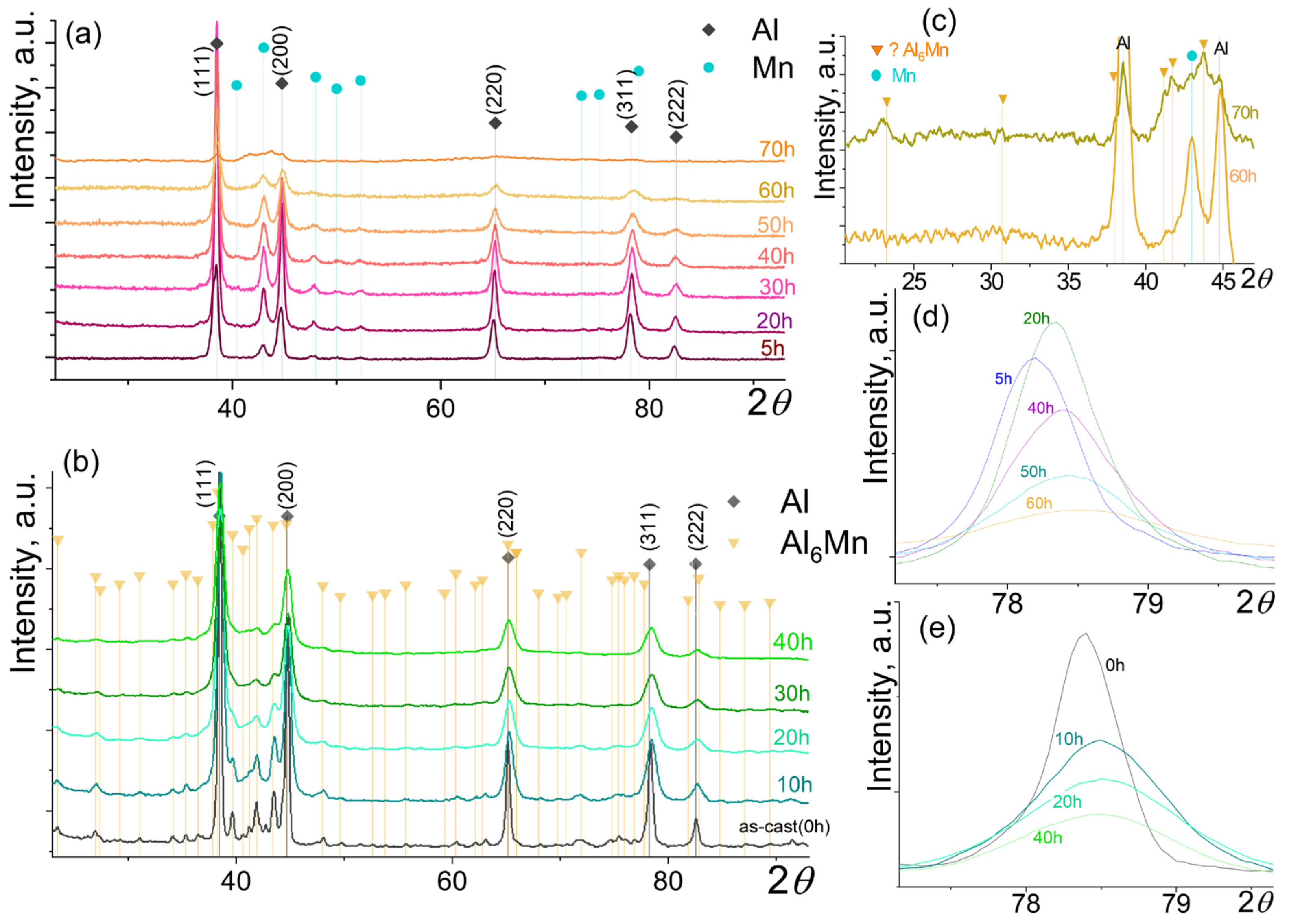
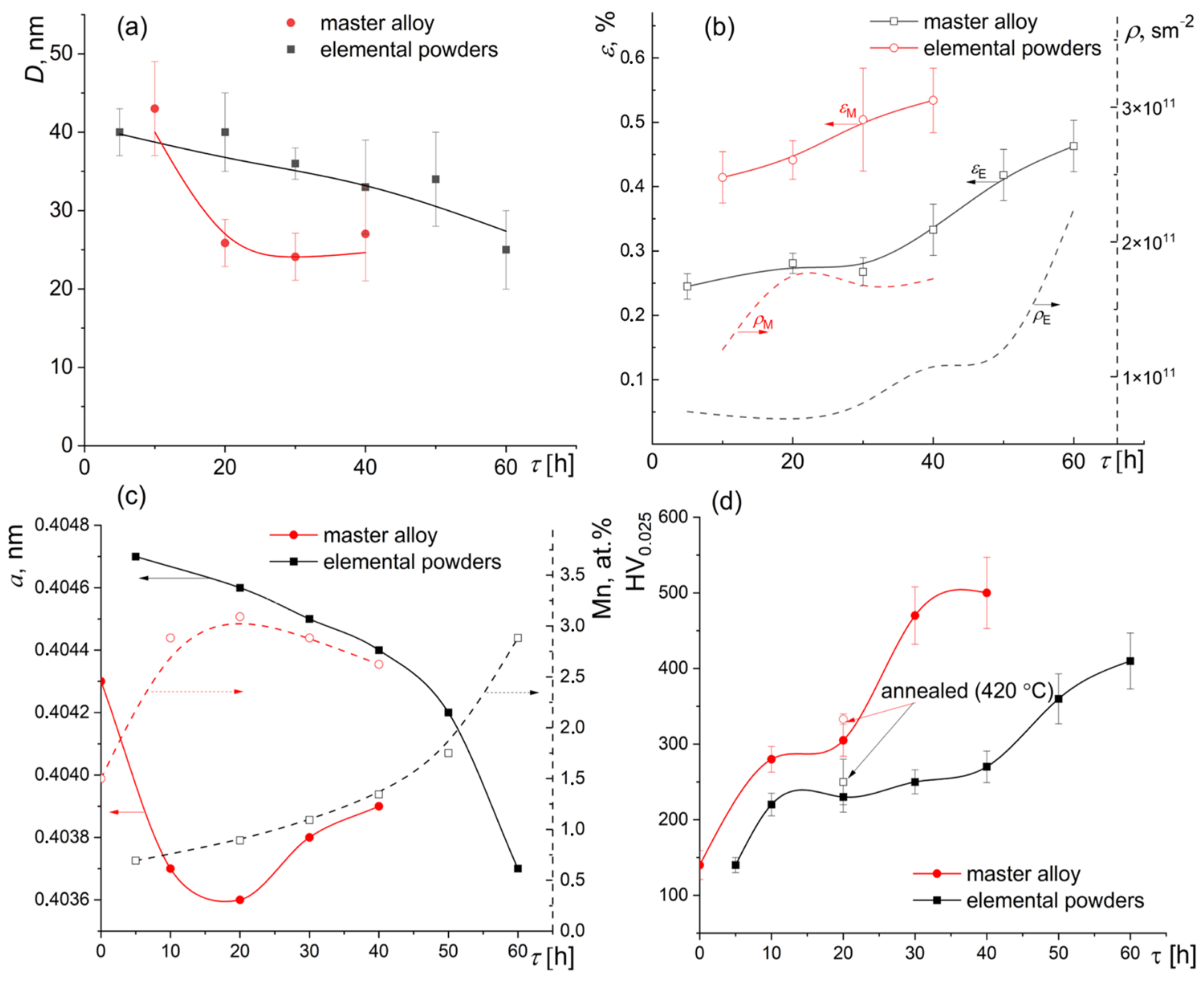
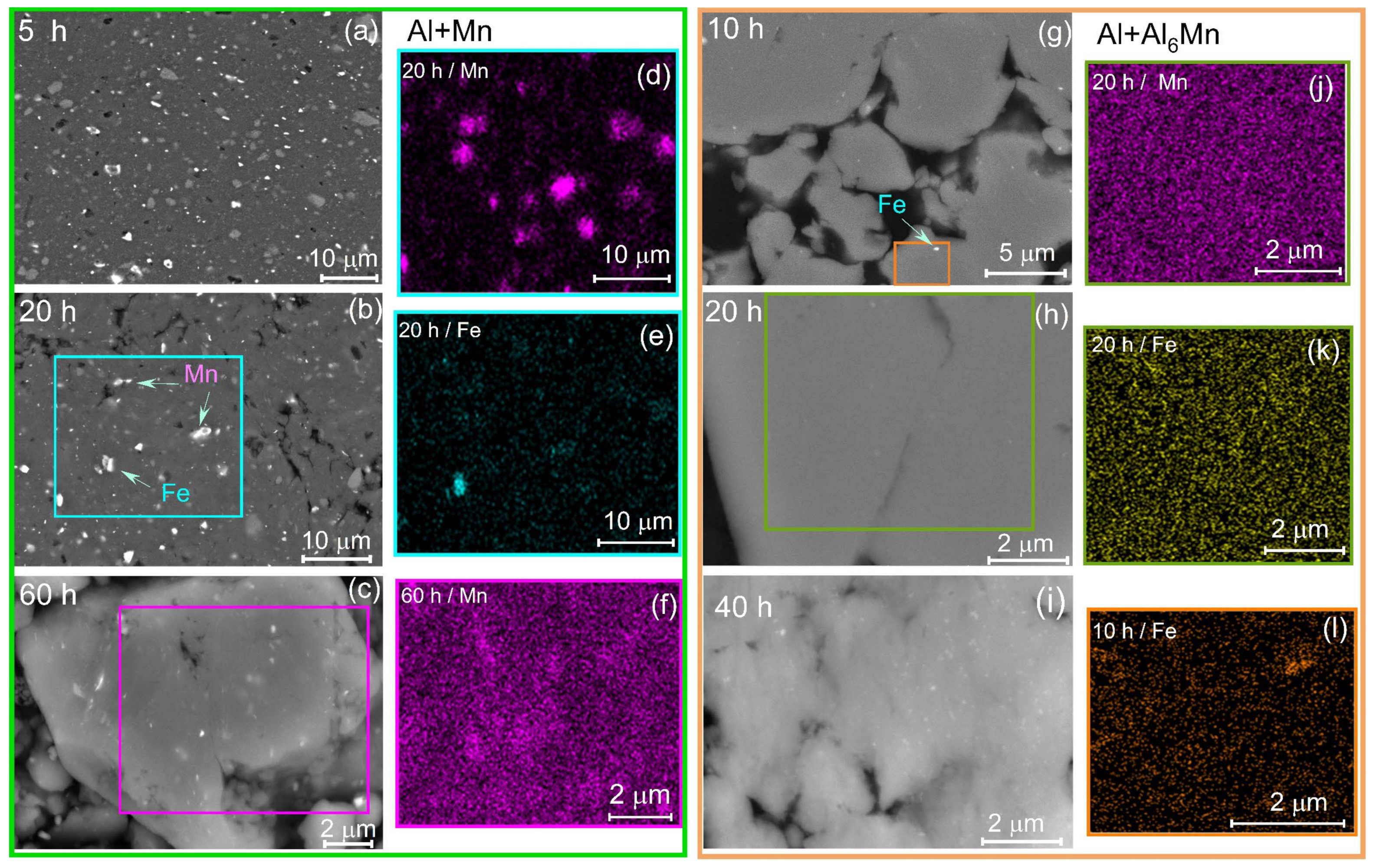
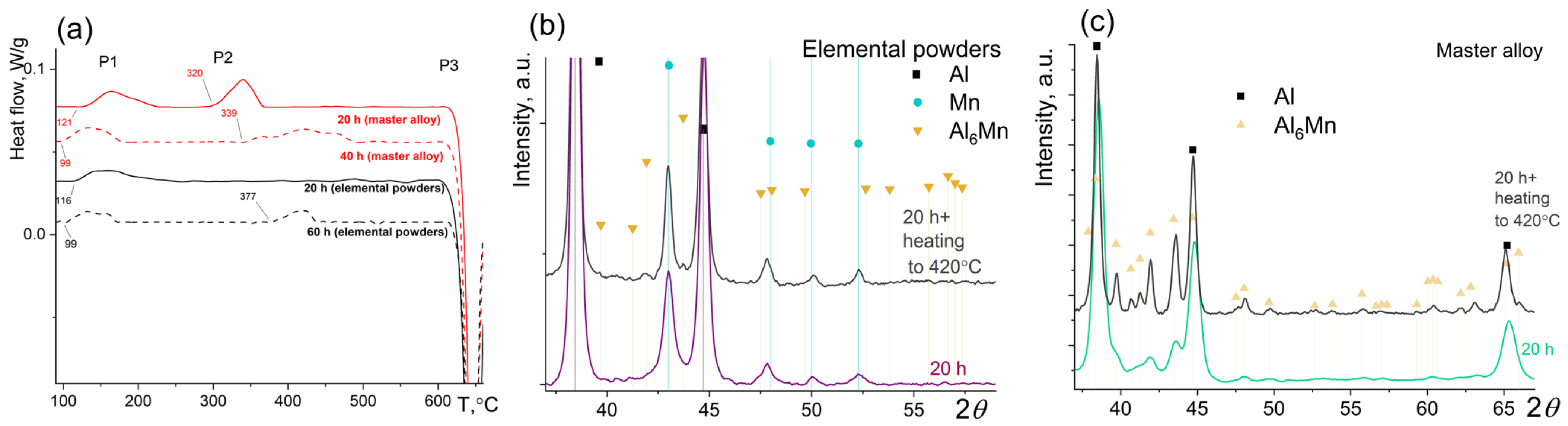
3. Results
4. Discussion
4.1. Dissolution and Precipitation Effects
4.2. The Strengthening Mechanisms
4.2.1. The Strengthening Mechanisms for the Elemental Powders
4.2.2. The Strengthening Mechanisms for the Master Alloy
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueiredo, R.B.; Kawasaki, M.; Langdon, T.G. Seventy Years of Hall-Petch, Ninety Years of Superplasticity and a Generalized Approach to the Effect of Grain Size on Flow Stress. Prog. Mater. Sci. 2023, 137, 101131. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Peng, S.; Qi, F.; Wang, G.; Min, A.; Yang, W.; Wang, W. Mechanical Alloying of Immiscible Metallic Systems: Process, Microstructure, and Mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Senkov, O.N.; Froes, F.H.; Stolyarov, V.V.; Valiev, R.Z.; Liu, J. Non-Equilibrium Structures in Aluminum-Iron Alloys Subjected to Severe Plastic Deformation. Proc. Adv. Part. Mater. Process. 1997, 38, 95–102. [Google Scholar]
- Sauvage, X.; Ganeev, A.; Ivanisenko, Y.; Enikeev, N.; Murashkin, M.; Valiev, R. Grain Boundary Segregation in UFG Alloys Processed by Severe Plastic Deformation. Adv. Eng. Mater. 2012, 14, 968–974. [Google Scholar] [CrossRef]
- Bachmaier, A.; Kerber, M.; Setman, D.; Pippan, R. The Formation of Supersaturated Solid Solutions in Fe–Cu Alloys Deformed by High-Pressure Torsion. Acta Mater. 2012, 60, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Bachmaier, A.; Rathmayr, G.B.; Bartosik, M.; Apel, D.; Zhang, Z.; Pippan, R. New Insights on the Formation of Supersaturated Solid Solutions in the Cu–Cr System Deformed by High-Pressure Torsion. Acta Mater. 2014, 69, 301–313. [Google Scholar] [CrossRef]
- Manchón-Gordón, A.F.; Ipus, J.J.; Blázquez, J.S.; Conde, C.F.; Conde, A. Evolution of Fe Environments and Phase Composition during Mechanical Amorphization of Fe70Zr30 and Fe70Nb30 Alloys. J. Non. Cryst. Solids 2018, 494, 78–85. [Google Scholar] [CrossRef]
- Yakin, A.; Avar, B.; Simsek, T.; Chattopadhyay, A.K. Synthesis of Boron-Based Alloys and Compounds by Mechanical Alloying: A Review. Mater. Today Commun. 2023, 37, 106980. [Google Scholar] [CrossRef]
- Manchón-Gordón, A.F.; Ipus, J.J.; Blázquez, J.S.; Conde, C.F.; Conde, A. Influence of Milling Time on the Homogeneity and Magnetism of a Fe70Zr30 Partially Amorphous Alloy: Distribution of Curie Temperatures. Materials 2020, 13, 490. [Google Scholar] [CrossRef]
- Ipus, J.J.; Blázquez, J.S.; Franco, V.; Millán, M.; Conde, A.; Oleszak, D.; Kulik, T. An Equivalent Time Approach for Scaling the Mechanical Alloying Processes. Intermetallics 2008, 16, 470–478. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [PubMed]
- Calka, A.; Kaczmarek, W.; Williams, J.S. Extended Solid Solubility in Ball-Milled Al-Mg Alloys. J. Mater. Sci. 1993, 28, 15–18. [Google Scholar] [CrossRef]
- Esquivel, J.; Murdoch, H.A.; Darling, K.A.; Gupta, R.K. Excellent Corrosion Resistance and Hardness in Al Alloys by Extended Solid Solubility and Nanocrystalline Structure. Mater. Res. Lett. 2018, 6, 79–83. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.; Shcherbachev, K.D.; Tabachkova, N.Y. Investigation of Nanostructured Al-10wt.% Zr Material Prepared by Ball Milling for High Temperature Applications. Mater. Charact. 2017, 123, 173–177. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Microstructural Evolution and Grain Subdivision Mechanisms during Severe Plastic Deformation of Aluminum Particles by Ball Milling. Philos. Mag. 2015, 95, 1425–1447. [Google Scholar] [CrossRef]
- Mohamed, F.A. A Dislocation Model for the Minimum Grain Size Obtainable by Milling. Acta Mater. 2003, 51, 4107–4119. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S. Novel Materials Synthesis by Mechanical Alloying/Milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Fujiwara, H.; Oda, E.; Ameyama, K. Mechanical Milling Process as Severe Plastic Deformation Method. Tetsu-Hagane 2008, 94, 608–615. [Google Scholar] [CrossRef]
- Wei, L.; Abd Rahim, S.; Al Bakri Abdullah, M.; Yin, A.; Ghazali, M.; Omar, M.; Nemeș, O.; Sandu, A.; Vizureanu, P.; Abdellah, A. Producing Metal Powder from Machining Chips Using Ball Milling Process: A Review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef]
- Gupta, R.K.; Fabijanic, D.; Dorin, T.; Qiu, Y.; Wang, J.T.; Birbilis, N. Simultaneous Improvement in the Strength and Corrosion Resistance of Al via High-Energy Ball Milling and Cr Alloying. Mater. Des. 2015, 84, 270–276. [Google Scholar] [CrossRef]
- Muthaiah, V.M.S.; Mula, S. Effect of Zirconium on Thermal Stability of Nanocrystalline Aluminium Alloy Prepared by Mechanical Alloying. J. Alloys Compd. 2016, 688, 571–580. [Google Scholar] [CrossRef]
- Zhou, F.; Lee, Z.; Lavernia, E.J.; Nutt, S.R. The Influence of Sc on Thermal Stability of a Nanocrystalline Al-Mg Alloy Processed by Cryogenic Ball Milling. Metall. Mater. Trans. 2005, 36, 1587–1594. [Google Scholar] [CrossRef]
- Xiao, X.; Gao, C.; Hou, J.S.; Guo, J.T. Superplastic Extensibility Deformation of Al-3%Mn Alloy with Submicrometer Grain Size. Trans. Nonferrous Met. Soc. China 2012, 22, 1035–1040. [Google Scholar] [CrossRef]
- Glazoff, M.V.; Khvan, A.; Zolotorevsky, V.S.; Belov, N.A.; Dinsdale, A.; Glazoff, M.V. Casting Aluminum Alloys; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128118054. [Google Scholar]
- Suzuki, Y.; Hashimoto, T.; Muramatsu, T. Refinement of Recrystallized Grain Structure in Al-Mn-Mg Alloy Sheets via Strip Casting. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2022; Volume 396–402, pp. 113–118. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hashimoto, T.; Muramatsu, T. Fine Grain Structure of High-Mn Aluminum Alloy Sheets. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 331–337, pp. 811–816. [Google Scholar]
- Belov, N.A.; Akopyan, T.K.; Shurkin, P.K.; Korotkova, N.O. Comparative Analysis of Structure Evolution and Thermal Stability of Commercial AA2219 and Model Al-2wt%Mn-2wt%Cu Cold Rolled Alloys. J. Alloys Compd. 2021, 864, 158823. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Belov, N.A.; Cherkasov, S.O.; Sundeev, R.V. Joint Effect of Electromagnetic Casting and High-Pressure Torsion on the Structure and Hardening of Al3.3Cu2.5Mn0.5Zr (wt%) Alloy. Mater. Lett. 2022, 324, 132776. [Google Scholar] [CrossRef]
- Shechtman, D.; Schaefer, R.J.; Biancaniello, F.S. Precipitation in Rapidly Solidified Al-Mn Alloys. Metall. Trans. A 1984, 15, 1987–1997. [Google Scholar] [CrossRef]
- Ruan, S.; Schuh, C.A. Electrodeposited Al-Mn Alloys with Microcrystalline, Nanocrystalline, Amorphous and Nano-Quasicrystalline Structures. Acta Mater. 2009, 57, 3810–3822. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Armstrong, L.; Kapoor, D.; Tschopp, M.A.; Kecskes, L.J.; Mathaudhu, S.N. Influence of Mn Solute Content on Grain Size Reduction and Improved Strength in Mechanically Alloyed Al–Mn Alloys. Mater. Sci. Eng. A 2014, 589, 57–65. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Bazlov, A.I.; Prosviryakov, A.S.; Emelina, N.B.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The Influence of the Al2O3 Particles on the Microstructure of the Mechanically Alloyed Al-Mn-Cu Alloy. J. Alloys Compd. 2023, 930, 167452. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Emelina, N.B.; Mochugovskiy, A.G.; Tabachkova, N.Y.; Prosviryakov, A.S.; Mikhaylovskaya, A.V. Influence of Pre-Milling on the Mn Solid Solubility in the Al-Mn-Cu Alloy during Mechanical Alloying. Metals 2023, 13, 756. [Google Scholar] [CrossRef]
- Zhou, E.; Suryanarayana, C.; Froes, F.H. (Sam) Effect of Premilling Elemental Powders on Solid Solubility Extension of Magnesium in Titanium by Mechanical Alloying. Mater. Lett. 1995, 23, 27–31. [Google Scholar] [CrossRef]
- Yang, P.; Engler, O.; Klaar, H.-J. Orientation Relationship between Al6 Mn Precipitates and the Al Matrix during Continuous Recrystallization in Al–1.3%Mn. J. Appl. Crystallogr. 1999, 32, 1105–1118. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, W.Z.; Marthinsen, K. Precipitation Crystallography of Plate-Shaped Al6 (Mn,Fe) Dispersoids in AA5182 Alloy. Acta Mater. 2012, 60, 5963–5974. [Google Scholar] [CrossRef]
- Torre, F.; Pia, G.; Carta, M.; Takacs, L.; Delogu, F. Grain Size Reduction in Cu Powders Subjected to Ball Milling and Ball Drop Experiments. Mater. Lett. 2018, 232, 33–35. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Prosviryakov, A.S.; Cheverikin, V.V.; Zanaeva, E.N.; Mikhaylovskaya, A.V. Influence of High-Energy Ball Milling on the Microstructure, Phase Composition, and Microhardness of the Al–Mn–Cu Alloy. Russ. J. Non-Ferrous Met. 2022, 63, 426–433. [Google Scholar] [CrossRef]
- Zolotorevsky, N.Y.; Solonin, A.N.; Churyumov, A.Y.; Zolotorevsky, V.S. Study of Work Hardening of Quenched and Naturally Aged Al-Mg and Al-Cu Alloys. Mater. Sci. Eng. A 2009, 502, 111–117. [Google Scholar] [CrossRef]
- Darling, K.A.; Roberts, A.J.; Catalano, J.E.; Tschopp, M.A.; Kecskes, L.J. Effect of Processing Parameters on the Microstructure of Mechanically Alloyed Nanostructured Al-Mn Alloys. In Advanced Composites for Aerospace, Marine, and Land Applications II; Springer: Cham, Switzerland, 2016; pp. 3–11. [Google Scholar] [CrossRef]
- Zhou, F.; Lee, Z.; Lavernia, E.J.; Nutt, S.R. Bimodal Microstructures in Nanocrystalline Al and Al-Mg Alloy Powders Prepared by Cryogenic Ball Milling. Mater. Res. Soc. Symp. Proc. 2004, 821, 83–88. [Google Scholar] [CrossRef]
- Chuev, I.I.; Kovalev, D.Y. Effects of Titanium High Energy Ball Milling on the Solid-Phase Reaction Ti + C. Mater. Mater. Chem. Phys. 2022, 283, 126025. [Google Scholar] [CrossRef]
- Sallez, N.; Boulnat, X.; Borbély, A.; Béchade, J.L.; Fabrègue, D.; Perez, M.; De Carlan, Y.; Hennet, L.; Mocuta, C.; Thiaudière, D.; et al. In Situ Characterization of Microstructural Instabilities: Recovery, Recrystallization and Abnormal Growth in Nanoreinforced Steel Powder. Acta Mater. 2015, 87, 377–389. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Dunand, D.C.; Seidman, D.N. Enhanced Age-Hardening Response and Creep Resistance of an Al-0.5Mn-0.3Si (at.%) Alloy by Sn Inoculation. Acta Mater. 2022, 240, 118344. [Google Scholar] [CrossRef] [PubMed]
- Rozman, N.; Medved, J.; Zupanič, F. Microstructural Evolution in Al − Mn − Cu − (Be) Alloys. Philos. Mag. 2011, 91, 4230–4246. [Google Scholar] [CrossRef]
- Belov, N.A.; Akopyan, T.K.; Korotkova, N.O.; Cherkasov, S.O.; Yakovleva, A.O. Effect of Fe and Si on the Phase Composition and Microstructure Evolution in Al-2wt.% Cu-2wt.% Mn Alloy During Solidification, Cold Rolling and Annealing. JOM 2021, 73, 3827–3837. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. Nanodispersoids of the Quasicrystalline I-Phase in Mn- and Mg-Bearing Aluminum-Based Alloys. Mater. Lett. 2021, 284, 128945. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Mukhamejanova, A.B.; Kotov, A.D.; Yakovtseva, O.A.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The Effect of Pre-Straining on the Annealing-Induced Precipitation Behavior of the Icosahedral I-Phase in an Aluminum-Based Alloy. Mater. Lett. 2022, 310, 131517. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Tabachkova, N.Y.; Ghayoumabadi, M.E.; Cheverikin, V.V.; Mikhaylovskaya, A.V. Joint Effect of Quasicrystalline Icosahedral and L12-Strucutred Phases Precipitation on the Grain Structure and Mechanical Properties of Aluminum-Based Alloys. J. Mater. Sci. Technol. 2021, 87, 196–206. [Google Scholar] [CrossRef]
- Prosviryakov, A.S.S.; Shcherbachev, K.D.D. Strengthening of Mechanically Alloyed Al-Based Alloy with High Zr Contents. Mater. Sci. Eng. A 2018, 713, 174–179. [Google Scholar] [CrossRef]
- Witharamage, C.S.; Christudasjustus, J.; Gupta, R.K. The Effect of Milling Time and Speed on Solid Solubility, Grain Size, and Hardness of Al-V Alloys. J. Mater. Eng. Perform. 2021, 30, 3144–3158. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.; Jang, J.; Lee, J.; Lee, C. Orowan Strengthening Effect on the Nanoindentation Hardness of the Ferrite Matrix in Microalloyed Steels. Mater. Sci. Eng. A 2008, 487, 552–557. [Google Scholar] [CrossRef]
- Farhat, Z.N.; Ding, Y.; Northwood, D.O.; Alpas, A.T. Effect of Grain Size on Friction and Wear of Nanocrystalline Aluminum. Mater. Sci. Eng. A 1996, 206, 302–313. [Google Scholar] [CrossRef]
- Souza, P.H.L.; de Oliveira, C.A.S.; do Vale Quaresma, J.M. Precipitation Hardening in Dilute Al–Zr Alloys. J. Mater. Res. Technol. 2018, 7, 66–72. [Google Scholar] [CrossRef]
- Ardell, A.J. Precipitation Hardening. Metall. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]
- Lefebvre, W.; Masquelier, N.; Houard, J.; Patte, R.; Zapolsky, H. Tracking the Path of Dislocations across Ordered Al3Zr Nano-Precipitates in Three Dimensions. Scr. Mater. 2014, 70, 43–46. [Google Scholar] [CrossRef]
- Zhu, A.W.; Starke, E.A. Strengthening Effect of Unshearable Particles of Finite Size: A Computer Experimental Study. Acta Mater. 1999, 47, 3263–3269. [Google Scholar] [CrossRef]
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Ekström, H.-E. Strengthening Mechanisms in Solid Solution Aluminum Alloys. Metall. Mater. Trans. A 2006, 37, 1999–2006. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Fujita, H.; Tabata, T. The Effect of Grain Size and Deformation Sub-Structure on Mechanical Properties of Polycrystalline Aluminum. Acta Metall. 1973, 21, 355–365. [Google Scholar] [CrossRef]
- Mohammadi, A.; Enikeev, N.A.; Murashkin, M.Y.; Arita, M.; Edalati, K. Examination of Inverse Hall-Petch Relation in Nanostructured Aluminum Alloys by Ultra-Severe Plastic Deformation. J. Mater. Sci. Technol. 2021, 91, 78–89. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.W.; Park, J.S.; Bae, D.H. Tensile Behavior of Bulk Nanocrystalline Aluminum Synthesized by Hot Extrusion of Ball-Milled Powders. Scr. Mater. 2008, 59, 1123–1126. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Heilmaier, M.; Murty, B.S.; Sarma, V.S. On the Hall–Petch Relationship in a Nanostructured Al–Cu Alloy. Mater. Sci. Eng. A 2010, 527, 7821–7825. [Google Scholar] [CrossRef]
- Hansen, N.; Huang, X. Microstructure and Flow Stress of Polycrystals and Single Crystals. Acta Mater. 1998, 46, 1827–1836. [Google Scholar] [CrossRef]
- Lan, X.; Li, K.; Wang, J.; Lu, Q.; Yang, T.; Xiao, Y.; Du, Y. Preparation of Ultra-Large Intermetallic Particles in Al Alloys for Accurate Determination of Their Mechanical Properties. Intermetallics 2023, 152, 107771. [Google Scholar] [CrossRef]
- Qi, Y.W.; Luo, Z.P.; Li, X.Y.; Lu, K. Transition of Deformation Mechanisms from Twinning to Dislocation Slip in Nanograined Pure Cobalt. J. Mater. Sci. Technol. 2022, 121, 124–129. [Google Scholar] [CrossRef]
- Moskalenko, V.A.; Smolianets, R.V.; Natsik, V.D.; Pohribna, Y.M. Dislocation Mechanisms of Low-Temperature Plasticity of Nanocrystalline Titanium: The Role of Impurity and Grain Boundary Strengthening. Low Temp. Phys. 2023, 49, 248. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Liao, X.Z.; Wu, X.L. Deformation Twinning in Nanocrystalline Materials. Prog. Mater. Sci. 2012, 57, 1–62. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical Properties of Nanocrystalline Materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- An, X.H.; Wu, S.D.; Wang, Z.G.; Zhang, Z.F. Significance of Stacking Fault Energy in Bulk Nanostructured Materials: Insights from Cu and Its Binary Alloys as Model Systems. Prog. Mater. Sci. 2019, 101, 1–45. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakovtseva, O.A.; Emelina, N.B.; Mochugovskiy, A.G.; Bazlov, A.I.; Prosviryakov, A.S.; Mikhaylovskaya, A.V. Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum. Metals 2023, 13, 1765. https://doi.org/10.3390/met13101765
Yakovtseva OA, Emelina NB, Mochugovskiy AG, Bazlov AI, Prosviryakov AS, Mikhaylovskaya AV. Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum. Metals. 2023; 13(10):1765. https://doi.org/10.3390/met13101765
Chicago/Turabian StyleYakovtseva, Olga A., Nadezhda B. Emelina, Andrey G. Mochugovskiy, Andrey I. Bazlov, Alexey S. Prosviryakov, and Anastasia V. Mikhaylovskaya. 2023. "Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum" Metals 13, no. 10: 1765. https://doi.org/10.3390/met13101765
APA StyleYakovtseva, O. A., Emelina, N. B., Mochugovskiy, A. G., Bazlov, A. I., Prosviryakov, A. S., & Mikhaylovskaya, A. V. (2023). Effect of Mechanical Alloying on the Dissolution of the Elemental Mn and Al-Mn Compound in Aluminum. Metals, 13(10), 1765. https://doi.org/10.3390/met13101765









