Effects of Current Density and Bath Temperature on the Morphological and Anticorrosive Properties of Zn-Ni Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Experiments
2.2. Preparation of the Electrolytic Bath
2.3. Substrate Preparation
2.4. Electrodeposition
2.5. Material Characterization
2.6. Corrosion Tests
3. Results
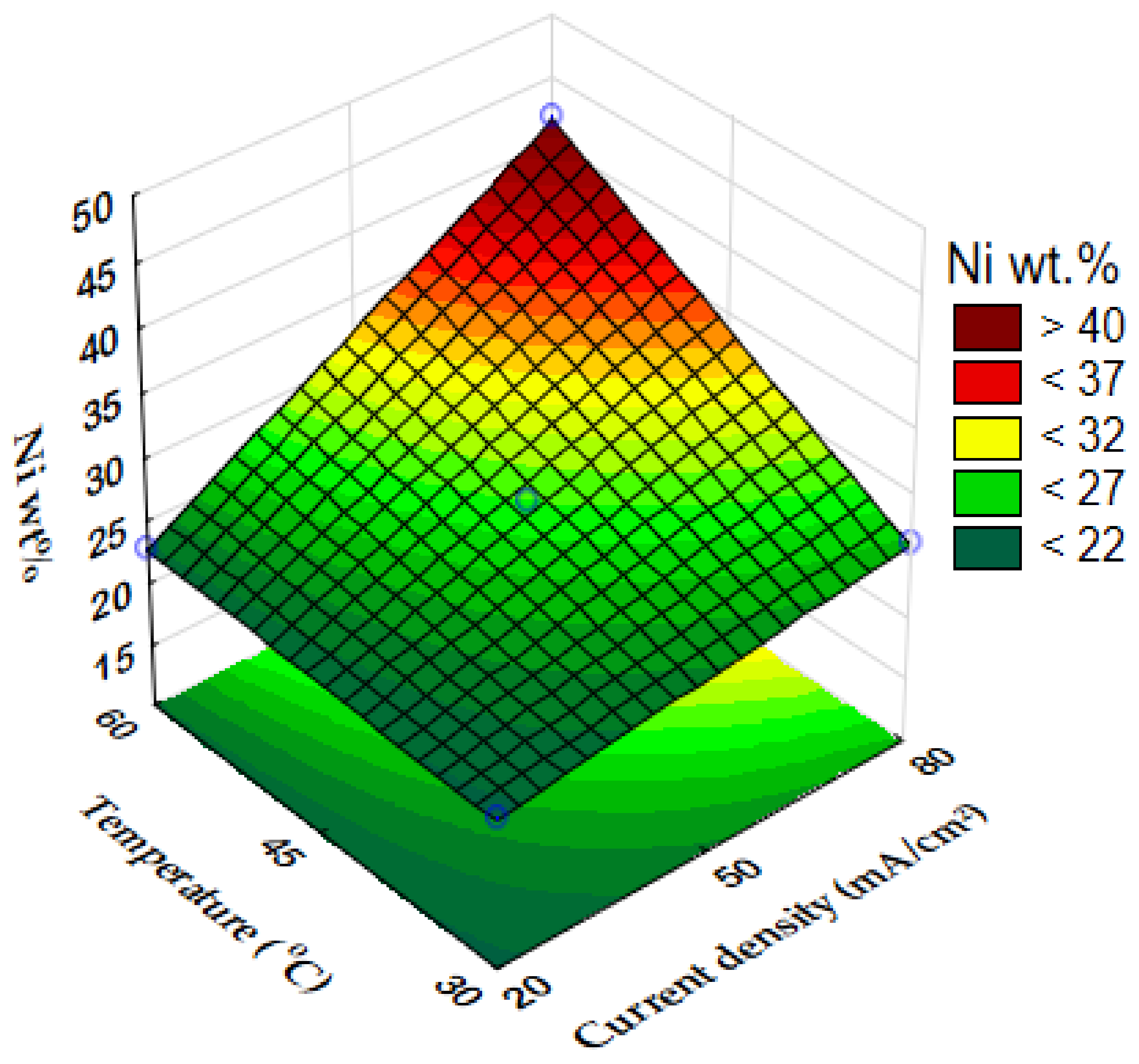
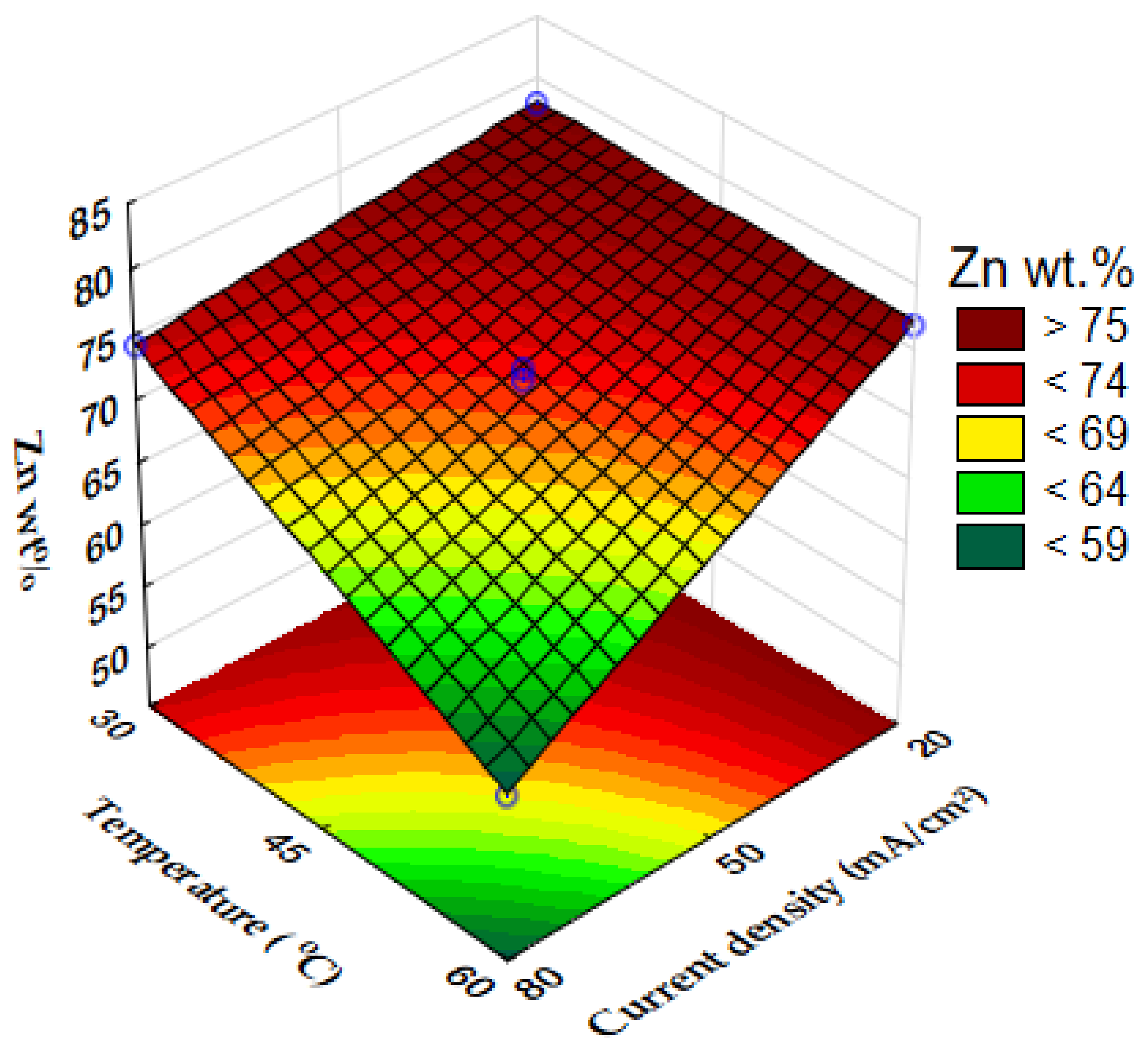
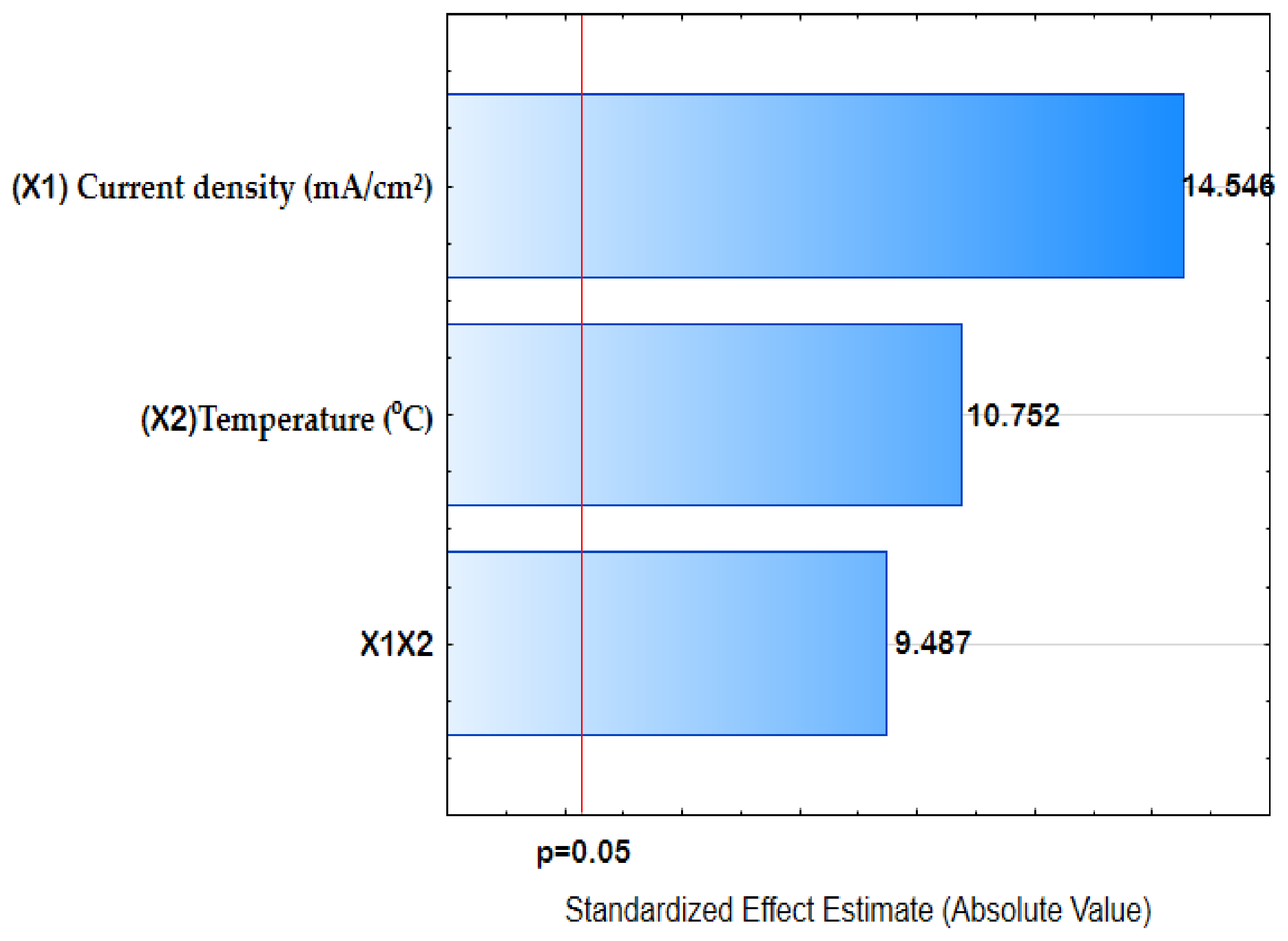
3.1. Chemical Composition
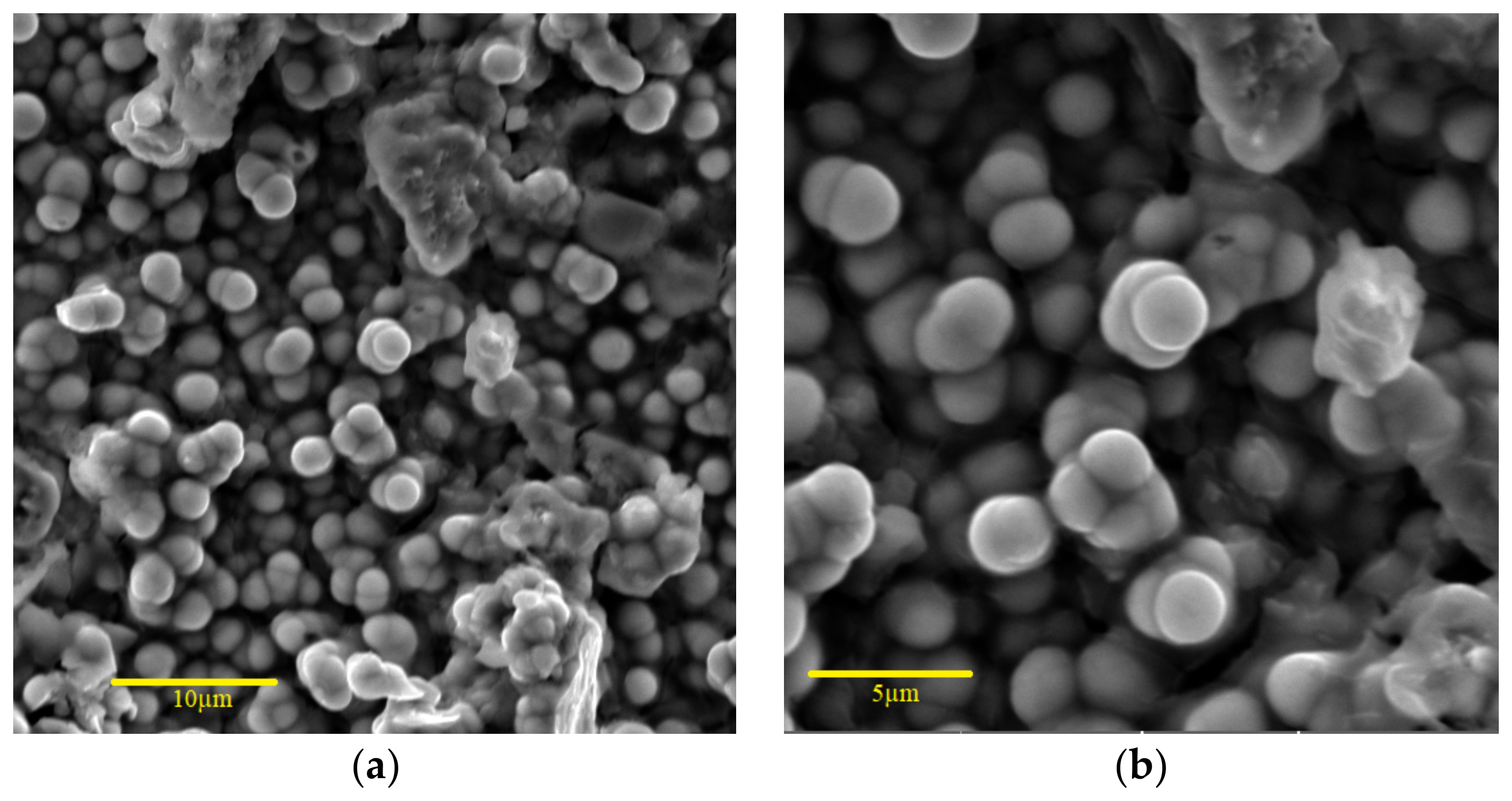
3.2. Surface Morphology
3.3. Corrosion Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe Alloys, Composites, and Nano Coatings–A Review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- He, J.; Li, D.W.; He, F.L.; Liu, Y.Y.; Liu, Y.L.; Zhang, C.Y.; Ren, F.; Ye, Y.J.; Deng, X.D.; Yin, D.C. A Study of Degradation Behaviour and Biocompatibility of Zn—Fe Alloy Prepared by Electrodeposition. Mater. Sci. Eng. C 2020, 117, 111295. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Arenas, M.A.; de Damborenea, J.J. Electrodeposition of Zn–Ni Coatings as Cd Replacement for Corrosion Protection of High Strength Steel. Corros. Sci. 2011, 53, 1489–1497. [Google Scholar] [CrossRef]
- Wang, Y.; Mandal, A.K.; Son, Y.-O.; Pratheeshkumar, P.; Wise, J.T.F.; Wang, L.; Zhang, Z.; Shi, X.; Chen, Z. Roles of ROS, Nrf2, and Autophagy in Cadmium-Carcinogenesis and Its Prevention by Sulforaphane. Toxicol. Appl. Pharmacol. 2018, 353, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C. Metal Carcinogen Exposure Induces Cancer Stem Cell-like Property through Epigenetic Reprograming: A Novel Mechanism of Metal Carcinogenesis. Semin. Cancer Biol. 2019, 57, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, C.; Ghosh, S.K.; Rajak, S.; Sahu, A.K.; Tewari, R.; Kain, V.; Dey, G.K. Effect of PH on Anomalous Co-Deposition and Current Efficiency during Electrodeposition of Ni-Zn-P Alloys. Surf. Coat. Technol. 2017, 313, 8–16. [Google Scholar] [CrossRef]
- Pagotto, S.O.; de Alvarenga Freire, C.M.; Ballester, M. Zn–Ni Alloy Deposits Obtained by Continuous and Pulsed Electrodeposition Processes. Surf. Coat. Technol. 1999, 122, 10–13. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Ghorbani, M. Ni-P/Zn-Ni Compositionally Modulated Multilayer Coatings—Part 1: Electrodeposition and Growth Mechanism, Composition, Morphology, Roughness and Structure. Appl. Surf. Sci. 2018, 442, 275–287. [Google Scholar] [CrossRef]
- Asseli, R.; Benaicha, M.; Derbal, S.; Allam, M.; Dilmi, O. Electrochemical Nucleation and Growth of Zn-Ni Alloys from Chloride Citrate-Based Electrolyte. J. Electroanal. Chem. 2019, 847, 113261. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Zhang, J.; Yang, P.; Song, H.; An, M. Electrodeposition of Nanocrystalline Zn–Ni Coatings with Single Gamma Phase from an Alkaline Bath. Surf. Coat. Technol. 2015, 270, 47–56. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Wang, X.L.; Tu, J.P. Influence of Electrodeposition Conditions on the Microstructure and Corrosion Resistance of Zn–Ni Alloy Coatings from a Deep Eutectic Solvent. Surf. Coat. Technol. 2014, 242, 34–41. [Google Scholar] [CrossRef]
- Soares, M.E.; Souza, C.A.C.; Kuri, S.E. Characteristics of a Zn–Ni Electrodeposited Alloy Obtained from Controlled Electrolyte Flux with Gelatin. Mater. Sci. Eng. A 2005, 402, 16–21. [Google Scholar] [CrossRef]
- Byk, T.V.; Gaevskaya, T.V.; Tsybulskaya, L.S. Effect of Electrodeposition Conditions on the Composition, Microstructure, and Corrosion Resistance of Zn–Ni Alloy Coatings. Surf. Coat. Technol. 2008, 202, 5817–5823. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, L.; Li, D.; Sun, Q.; Xing, P.; An, M. Electrochemical Studies of 2-Aminopyridine on Nanocrystalline Zn–Ni Alloy Electrodeposition. J. Electroanal. Chem. 2019, 835, 114–122. [Google Scholar] [CrossRef]
- Abedini, B.; Ahmadi, N.P.; Yazdani, S.; Magagnin, L. Structure and Corrosion Behavior of Zn-Ni-Mn/Zn Ni Layered Alloy Coatings Electrodeposited under Various Potential Regimes. Surf. Coat. Technol. 2019, 372, 260–267. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Bertagnolli, D.C.; da Silva, L.; Ferreira, E.A.; Paula, A.S.; da Fonseca, G.S. Effect of Fe and Co Co-Deposited Separately with Zn-Ni by Electrodeposition on ASTM A624 Steel. Appl. Surf. Sci. 2017, 420, 53–62. [Google Scholar] [CrossRef]
- Yang, Z.N.; Zhang, Z.; Zhang, J.Q. Electrodeposition of Decorative and Protective Zn–Fe Coating onto Low-Carbon Steel Substrate. Surf. Coat. Technol. 2006, 200, 4810–4815. [Google Scholar] [CrossRef]
- Swathirajan, S. Electrodeposition of Zinc + Nickel Alloy Phases and Electrochemical Stripping Studies of the Anomalous Codeposition of Zinc. J. Electroanal. Chem. Interfacial Electrochem. 1987, 221, 211–228. [Google Scholar] [CrossRef]
- Ortiz-Aparicio, J.L.; Meas, Y.; Trejo, G.; Ortega, R.; Chapman, T.W.; Chainet, E.; Ozil, P. Electrodeposition of Zinc-Cobalt Alloy from a Complexing Alkaline Glycinate Bath. Electrochim. Acta 2007, 52, 4742–4751. [Google Scholar] [CrossRef]
- Rahman, M.J.; Sen, S.R.; Moniruzzaman, M.; Shorowordi, K.M. Morphology and Properties of Electrodeposited ZN-NI Alloy Coatings on Mild Steel. J. Mech. Eng. 1970, 40, 9–14. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Sohi, M.H. Study of the Corrosion Behavior of Zinc and Zn–Co Alloy Electrodeposits Obtained from Alkaline Bath Using Direct Current. Mater. Chem. Phys. 2009, 117, 414–421. [Google Scholar] [CrossRef]
- Nayana, K.O.; Venkatesha, T.V.; Chandrappa, K.G. Influence of Additive on Nanocrystalline, Bright Zn–Fe Alloy Electrodeposition and Its Properties. Surf. Coat. Technol. 2013, 235, 461–468. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: PRINCIPLES and PRACTICE. In Electrodeposition of Alloys; Elsevier: Amsterdam, The Netherlands, 1963; Volume I, p. ii. ISBN 9781483223117. [Google Scholar]
- Roventi, G.; Cecchini, R.; Fabrizi, A.; Bellezze, T. Electrodeposition of Nickel-Zinc Alloy Coatings with High Nickel Content. Surf. Coat. Technol. 2015, 276, 1–7. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Ghorbani, M. Electrodeposition of Zn–Ni–P Compositionally Modulated Multilayer Coatings: An Attempt to Deposit Ni–P and Zn–Ni Alloys from a Single Bath. Electrochem. Commun. 2017, 81, 93–96. [Google Scholar] [CrossRef]
- Li, R.; Dong, Q.; Xia, J.; Luo, C.; Sheng, L.; Cheng, F.; Liang, J. Electrodeposition of Composition Controllable Zn Ni Coating from Water Modified Deep Eutectic Solvent. Surf. Coat. Technol. 2019, 366, 138–145. [Google Scholar] [CrossRef]
- Miranda, F.J.F.; Barcia, O.E.; Diaz, S.L.; Mattos, O.R.; Wiart, R. Electrodeposition of Zn-Ni Alloys in Sulfate Electrolytes. Electrochim. Acta 1996, 41, 1041–1049. [Google Scholar] [CrossRef]
- Hammami, O.; Dhouibi, L.; Triki, E. Influence of Zn–Ni Alloy Electrodeposition Techniques on the Coating Corrosion Behaviour in Chloride Solution. Surf. Coat. Technol. 2009, 203, 2863–2870. [Google Scholar] [CrossRef]
- Hegde, A.C.; Venkatakrishna, K.; Eliaz, N. Electrodeposition of Zn-Ni, Zn-Fe and Zn-Ni-Fe Alloys. Surf. Coat. Technol. 2010, 205, 2031–2041. [Google Scholar] [CrossRef]
- Albalat, R.; Gómez, E.; Müller, C.; Sarret, M.; Vallés, E.; Pregonas, J. Electrodeposition of Zinc-Nickel Alloy Coatings: Influence of a Phenolic Derivative. J. Appl. Electrochem. 1990, 20, 635–639. [Google Scholar] [CrossRef]
- Maciej, A.; Wadas, A.; Sowa, M.; Socha, R.; Dercz, G.; Rabe, M.; Simka, W. Improvement of Corrosion Resistance of Zn-Ni Alloy Coatings by Anodizing in Selected Alcoholic Solutions. Corros. Sci. 2019, 158, 108107. [Google Scholar] [CrossRef]
- Kwon, M.; Jo, D.h.; Cho, S.H.; Kim, H.T.; Park, J.T.; Park, J.M. Characterization of the Influence of Ni Content on the Corrosion Resistance of Electrodeposited Zn-Ni Alloy Coatings. Surf. Coat. Technol. 2016, 288, 163–170. [Google Scholar] [CrossRef]
- Pouladi, S.; Shariat, M.H.; Bahrololoom, M.E. Electrodeposition and Characterization of Ni-Zn-P and Ni-Zn-P/Nano-SiC Coatings. Surf. Coat. Technol. 2012, 213, 33–40. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Bahrololoom, M.E.; Shariat, M.H. Electrodeposition of Nanocrystalline Zn-Ni Alloy from Alkaline Glycinate Bath Containing Saccharin as Additive. Appl. Surf. Sci. 2011, 257, 8311–8316. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y. Electrochemical Analysis of an Electrodeposited Zn-Ni Alloy Films Contained EDTA Stable Baths in 3.5 Wt% NaCl Solutions. Mater. Today Proc. 2020, 28, 532–537. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. Electrodeposition of Single Gamma Phased Zn–Ni Alloy Coatings from Additive-Free Acidic Bath. Appl. Surf. Sci. 2014, 311, 635–642. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Zhang, J.L.; Huang, M.L.; Wang, X.L.; Tu, J.P. Effect of EDTA and NH4Cl Additives on Electrodeposition of Zn-Ni Films from Choline Chloride-Based Ionic Liquid. Trans. Nonferrous Met. Soc. China 2015, 25, 2054–2064. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Ghorbani, M. Ni-P/Zn-Ni Compositionally Modulated Multilayer Coatings—Part 2: Corrosion and Protection Mechanisms. Appl. Surf. Sci. 2018, 442, 313–321. [Google Scholar] [CrossRef]
- Ataie, S.A.; Zakeri, A. RSM Optimization of Pulse Electrodeposition of Zn-Ni-Al2O3 Nanocomposites under Ultrasound Irradiation. Surf. Coat. Technol. 2019, 359, 206–215. [Google Scholar] [CrossRef]
- Oliveira, J.A.M.; de Almeida, A.F.; Campos, A.R.N.; Prasad, S.; Alves, J.J.N.; de Santana, R.A.C. Effect of Current Density, Temperature and Bath PH on Properties of Ni–W–Co Alloys Obtained by Electrodeposition. J. Alloys Compd. 2021, 853, 157104. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Costa, J.D.; De Sousa, M.B.; Alves, J.J.N.; Campos, A.R.N.; Santana, R.A.C.; Prasad, S. Studies on Electrodeposition and Characterization of the Ni-W-Fe Alloys Coatings. J. Alloys Compd. 2015, 619, 697–703. [Google Scholar] [CrossRef]
- Costa, J.D.; de Sousa, M.B.; Lia Fook, N.C.M.; Alves, J.J.N.; de Araújo, C.J.; Prasad, S.; Campos, A.R.N.; de Santana, R.A.C. Obtaining and Characterization of Ni-Ti/Ti-Mo Joints Welded by TIG Process. Vacuum 2016, 133, 58–69. [Google Scholar] [CrossRef]
- Costa, J.D.; de Sousa, M.B.; Alves, J.J.N.; Evaristo, B.d.O.; Queiroga, R.A.; dos Santos, A.X.; Maciel, T.M.; Campos, A.R.N.; de Santana, R.A.C.; Prasad, S. Effect of Electrochemical Bath Composition on the Preparation of Ni-W-Fe-P Amorphous Alloy. Int. J. Electrochem. Sci. 2018, 13, 2969–2985. [Google Scholar] [CrossRef]
- Hafeez, A.; Ammar Taqvi, S.A.; Fazal, T.; Javed, F.; Khan, Z.; Amjad, U.S.; Bokhari, A.; Shehzad, N.; Rashid, N.; Rehman, S.; et al. Optimization on Cleaner Intensification of Ozone Production Using Artificial Neural Network and Response Surface Methodology: Parametric and Comparative Study. J. Clean. Prod. 2020, 252, 119833. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Soltani, M.; Noofeli, A.; Nathwani, J. An Optimization Study on Heavy Oil Upgrading in Supercritical Water through the Response Surface Methodology (RSM). Fuel 2020, 271, 117618. [Google Scholar] [CrossRef]
- Heydari, H.; Akbari, M. Investigating the Effect of Process Parameters on the Temperature Field and Mechanical Properties in Pulsed Laser Welding of Ti6Al4V Alloy Sheet Using Response Surface Methodology. Infrared Phys. Technol. 2020, 106, 103267. [Google Scholar] [CrossRef]
- Moreno Dávila, I.M.M.; Tamayo Ordoñez, M.C.; Morales Martínez, T.K.; Soria Ortiz, A.I.; Gutiérrez Rodríguez, B.; Rodríguez de la Garza, J.A.; Ríos González, L.J. Effect of Fermentation Time/Hydraulic Retention Time in a UASB Reactor for Hydrogen Production Using Surface Response Methodology. Int. J. Hydrogen Energy 2020, 45, 13702–13706. [Google Scholar] [CrossRef]
- Ali, A.N.; Huang, S.J. Ductile Fracture Behavior of ECAP Deformed AZ61 Magnesium Alloy Based on Response Surface Methodology and Finite Element Simulation. Mater. Sci. Eng. A 2019, 746, 197–210. [Google Scholar] [CrossRef]
- Abu-Sharkh, S.; Doerffel, D. Rapid Test and Non-Linear Model Characterisation of Solid-State Lithium-Ion Batteries. J. Power Sources 2004, 130, 266–274. [Google Scholar] [CrossRef]
- Yan, M.; Wang, J.; Han, E.; Ke, W. Local Environment under Simulated Disbonded Coating on Steel Pipelines in Soil Solution. Corros. Sci. 2008, 50, 1331–1339. [Google Scholar] [CrossRef]
- Santana, R.A.C.d.; Campos, A.R.N.; Prasad, S.; Leite, V.D. Otimização Do Banho Eletrolítico Da Liga Fe-WB Resistente à Corrosão. Quim. Nova 2007, 30, 360–365. [Google Scholar] [CrossRef]
- Qiao, X.; Li, H.; Zhao, W.; Li, D. Effects of Deposition Temperature on Electrodeposition of Zinc-Nickel Alloy Coatings. Electrochim. Acta 2013, 89, 771–777. [Google Scholar] [CrossRef]
- Tuaweri, T.J. Zn-Ni Electrodeposition for Enhanced Corrosion Performance. Int. J. Mater. Sci. Appl. 2013, 2, 221. [Google Scholar] [CrossRef]
- Hammami, O.; Dhouibi, L.; Berçot, P.; Rezrazi, E.M. Effect of Phosphorus Doping on Some Properties of Electroplated Zn–Ni Alloy Coatings. Surf. Coat. Technol. 2013, 219, 119–125. [Google Scholar] [CrossRef]
- Constantin, I. Microstructural Characterization and Corrosion Behavior of Electroless Ni-Zn-P Thin Films. J. Metall. 2014, 2014, 827393. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, N.; Wang, C.Q.; Li, D.Y.; Meng, F.Y.; Zhu, Y.M. Effects of CeO2 on the Microstructure and Hydrogen Evolution Property of Ni-Zn Coatings. J. Power Sources 2013, 222, 88–91. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Shariat, M.H.; Bahrololoom, M.E. Study of Corrosion Performance of Electrodeposited Nanocrystalline Zn-Ni Alloy Coatings. Corros. Sci. 2012, 59, 81–87. [Google Scholar] [CrossRef]
- Abou-Krisha, M.M.; Rageh, H.M.; Matter, E.A. Electrochemical Studies on the Electrodeposited Zn-Ni-Co Ternary Alloy in Different Media. Surf. Coat. Technol. 2008, 202, 3739–3746. [Google Scholar] [CrossRef]
- Brenner, A.; Couch, D.E.; Williams, E.K. Electrodeposition of Alloys of Phosphorus with Nickel or Cobalt. J. Res. Natl. Bur. Stand. 1950, 44, 109. [Google Scholar] [CrossRef]
- MacIej, A.; Nawrat, G.; Simka, W.; Piotrowski, J. Formation of Compositionally Modulated Zn-Ni Alloy Coatings on Steel. Mater. Chem. Phys. 2012, 132, 1095–1102. [Google Scholar] [CrossRef]
- Chang, L.M.; Chen, D.; Liu, J.H.; Zhang, R.J. Effects of Different Plating Modes on Microstructure and Corrosion Resistance of Zn–Ni Alloy Coatings. J. Alloys Compd. 2009, 479, 489–493. [Google Scholar] [CrossRef]
- Gnanamuthu, R.; Mohan, S.; Saravanan, G.; Lee, C.W. Comparative Study on Structure, Corrosion and Hardness of Zn–Ni Alloy Deposition on AISI 347 Steel Aircraft Material. J. Alloys Compd. 2012, 513, 449–454. [Google Scholar] [CrossRef]
- Kumar, C.M.; Kumar, P.; Venkatesha, T.V.; Vathsala, K.; Nayana, K.O. Electrodeposition and Corrosion Behavior of Zn-Ni and Zn-Ni-Fe 2O3 Coatings. J. Coat. Technol. Res. 2012, 9, 71–77. [Google Scholar] [CrossRef]
- Tafreshi, M.; Allahkaram, S.R.; Farhangi, H. Comparative Study on Structure, Corrosion Properties and Tribological Behavior of Pure Zn and Different Zn-Ni Alloy Coatings. Mater. Chem. Phys. 2016, 183, 263–272. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, I.H. Structural and Corrosion Protection Properties of Electrochemically Deposited Nano-Sized Zn-Ni Alloy Coatings. Appl. Surf. Sci. 2014, 318, 15–23. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Brahimi, S.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Characterization of Corrosion Resistance of Electrodeposited Zn-Ni Zn and Cd Coatings. Electrochim. Acta 2013, 105, 314–323. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Zheng, S.; Duan, S.; Cui, J.; Zhang, H. NaHCO3/Na2CO3 as an Inhibitor of Chloride-Induced Mild Steel Corrosion in Cooling Water: Electrochemical Evaluation. J. Ind. Eng. Chem. 2021, 95, 235–243. [Google Scholar] [CrossRef]
- Mishra, P.; Yavas, D.; Bastawros, A.F.; Hebert, K.R. Electrochemical Impedance Spectroscopy Analysis of Corrosion Product Layer Formation on Pipeline Steel. Electrochim. Acta 2020, 346, 136232. [Google Scholar] [CrossRef]
- De Motte, R.; Basilico, E.; Mingant, R.; Kittel, J.; Ropital, F.; Combrade, P.; Necib, S.; Deydier, V.; Crusset, D.; Marcelin, S. A Study by Electrochemical Impedance Spectroscopy and Surface Analysis of Corrosion Product Layers Formed during CO2 Corrosion of Low Alloy Steel. Corros. Sci. 2020, 172, 108666. [Google Scholar] [CrossRef]
- Lukács, Z.; Kristóf, T. A Generalized Model of the Equivalent Circuits in the Electrochemical Impedance Spectroscopy. Electrochim. Acta 2020, 363, 137199. [Google Scholar] [CrossRef]
- Costa, J.D.; Sousa, M.B.; Almeida, A.F.; Oliveira, J.A.M.; Silva, P.C.S.; Alves, J.J.N.; Campos, A.R.N.; Araújo, C.J.; Santana, R.A.C.; Delgado, J.M.P.Q.; et al. Thermal, Mechanical, and Electrochemical Characterization of Ti50Ni50−XMox Alloys Obtained by Plasma Arc Melting. Metals 2023, 13, 1637. [Google Scholar] [CrossRef]
- Silva, C.R.P.; Costa, J.D.; de Almeida, A.F.; Santana, R.A.C.; Campos, A.R.N.; Alves, J.J.N.; Abreu Santos, T.F. Chemical composition variation of the Ni–W alloy as a function of parameters used in the electrodeposition process. J. Appl. Electrochem. 2023. [Google Scholar] [CrossRef]
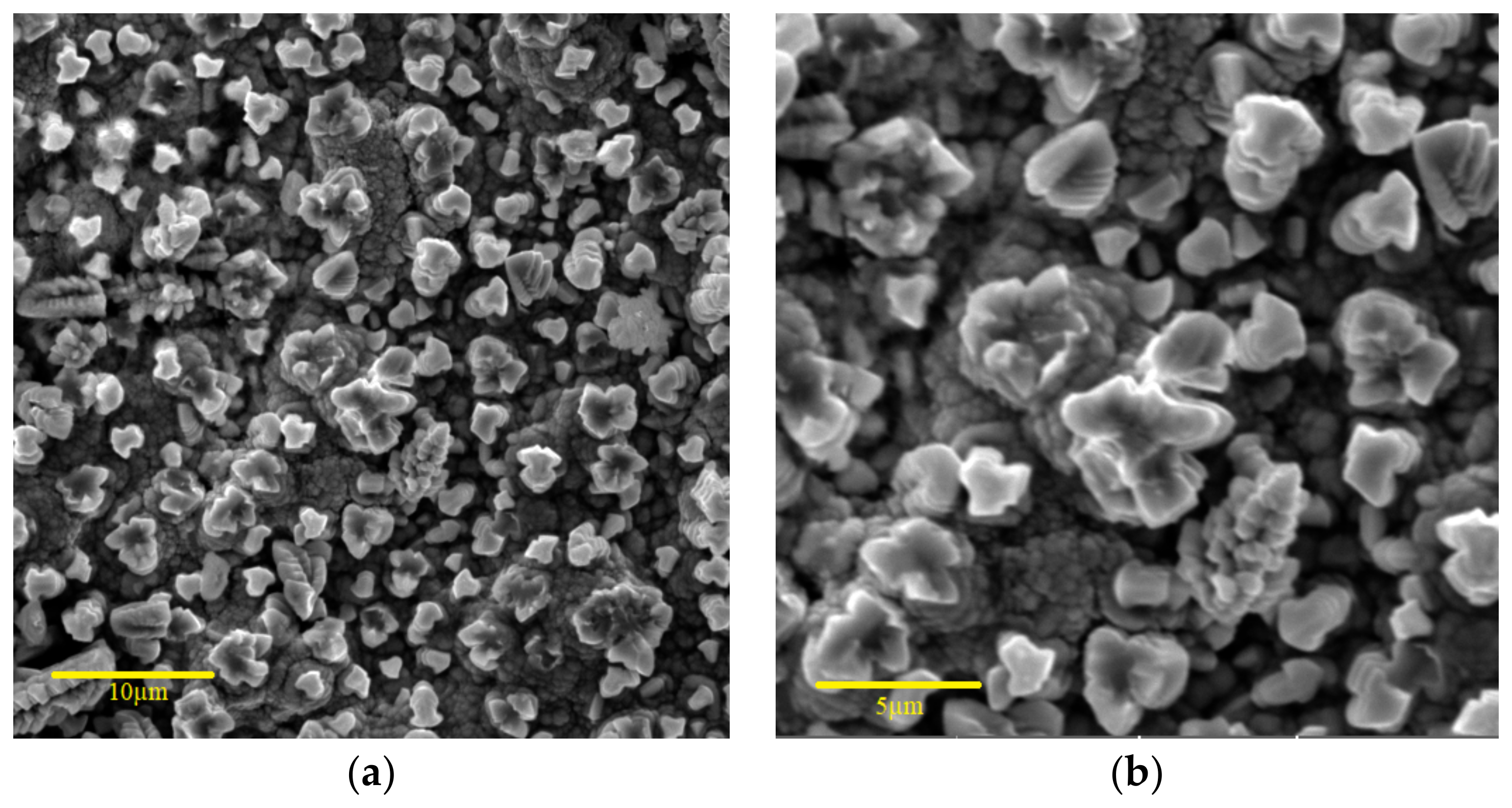
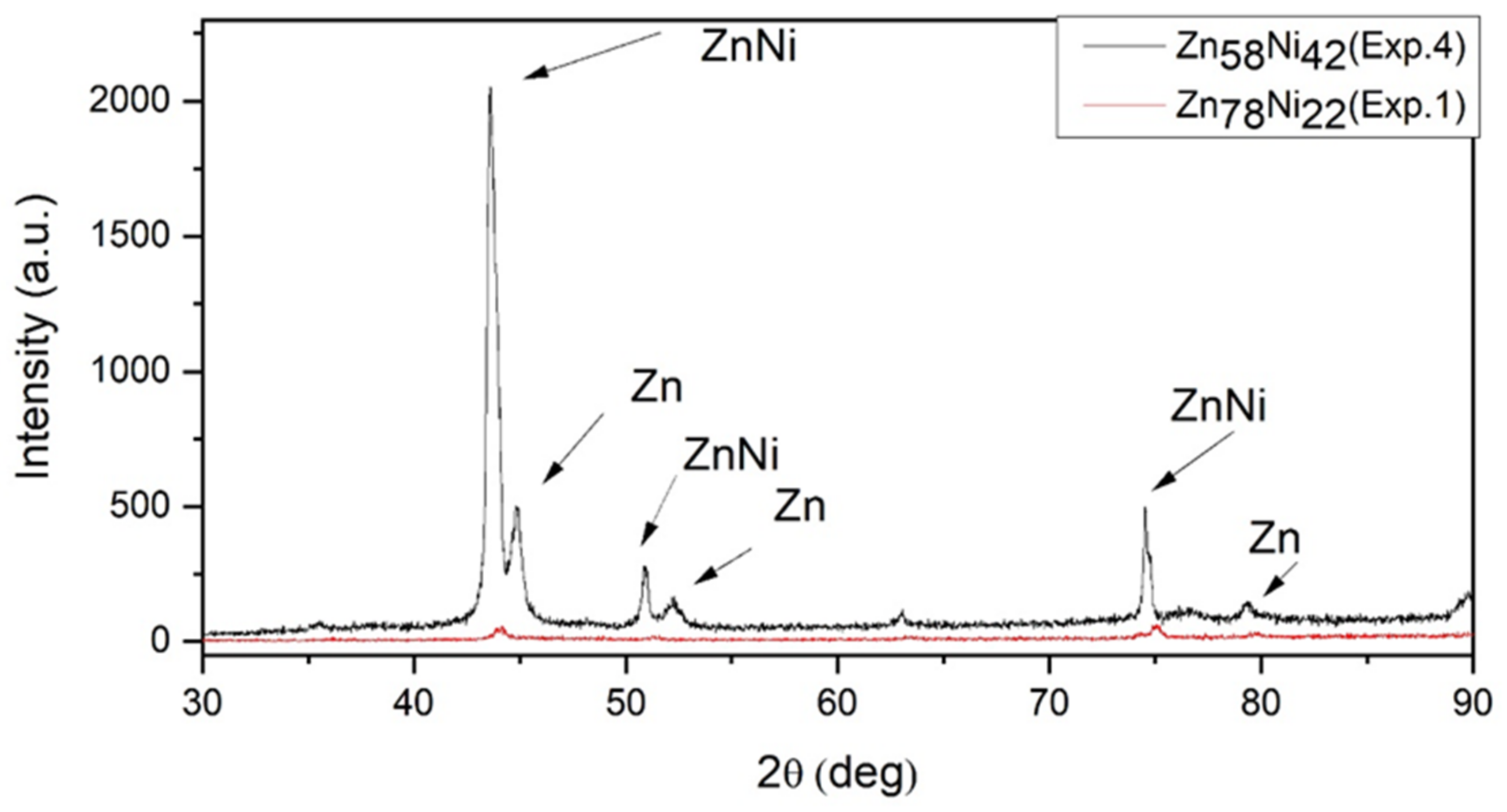




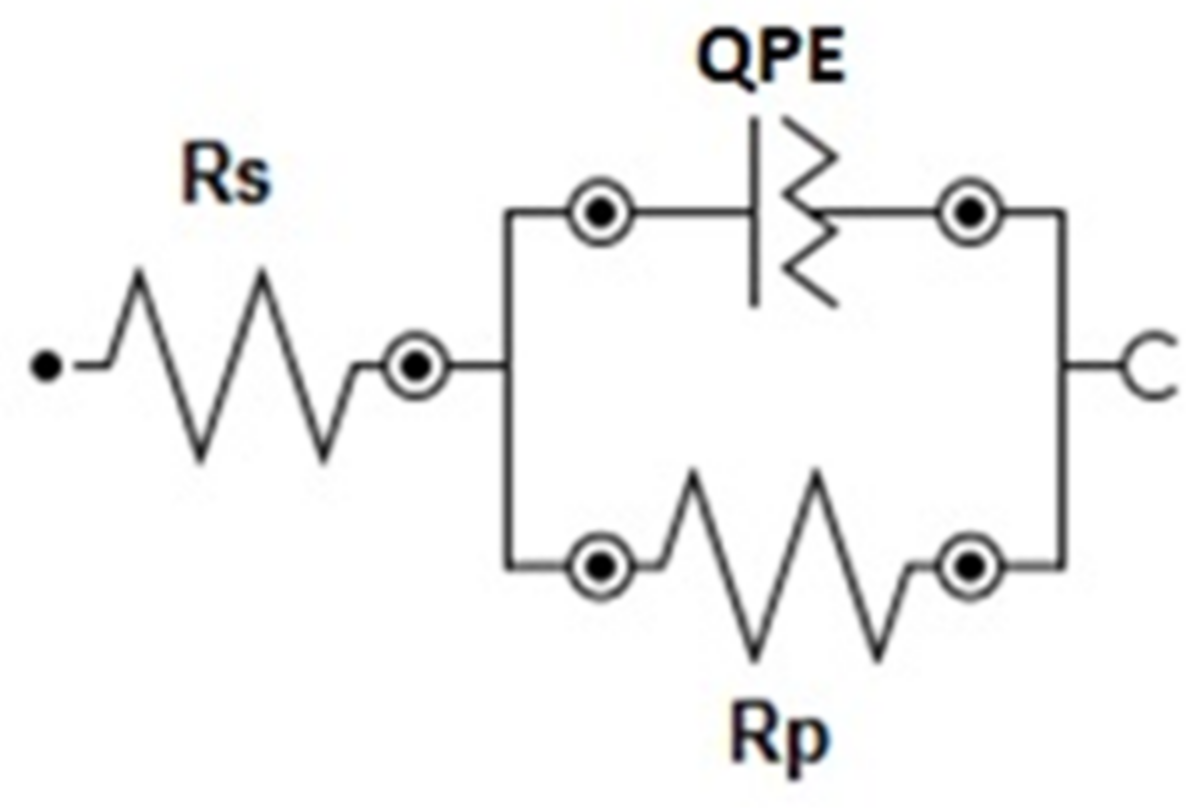
| Factors | Code | |||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Current density (mA/cm2) | X1 | 20 | 50 | 80 |
| Bath temperature (°C) | X2 | 30 | 45 | 60 |
| Run | J (mA/cm²) | T (°C) | Ecorr (V) | Icorr (µA/cm²) | Rp (k.cm2) | Ni %wt. | Zn %wt. |
|---|---|---|---|---|---|---|---|
| 1 | −(20) | −(30) | −1.2340 | 84.584 | 0.4519 | 22 | 78 |
| 2 | +(80) | −(30) | −0.990 | 59.966 | 1.2291 | 26 | 74 |
| 3 | −(20) | +(60) | −0.929 | 77.127 | 0.5732 | 23 | 77 |
| 4 | +(80) | +(60) | −0.789 | 6.848 | 4.1363 | 42 | 58 |
| 5 | 0 (50) | 0 (45) | −0.992 | 15.306 | 1.9390 | 27 | 73 |
| 6 | 0 (50) | 0 (45) | −0.983 | 14.020 | 2.1380 | 28 | 72 |
| Factors | Quadratic Sum | Degree of Freedom | Quadratic Average | F | p |
|---|---|---|---|---|---|
| Sodium tungstate (M) | 132.2500 | 1 | 132.2500 | 211.6000 | 0.004693 |
| Nickel sulfate (M) | 72.2500 | 1 | 72.2500 | 115.6000 | 0.008540 |
| Iteration | 56.2500 | 1 | 56.2500 | 90.0000 | 0.010929 |
| Error | 1.2500 | 2 | 0.6250 | ||
| Total quadratic sum | 262.0000 | 5 |
| Coating | Rs (Ω cm2) | Rp (Ω cm2) | Y (S sα cm−2) | α |
|---|---|---|---|---|
| Zn78Ni22 (Exp. 1) | 37.62 | 564 | 0.00029 | 0.70 |
| Zn58Ni42 (Exp. 4) | 38.95 | 2924 | 0.00017 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.D.; Almeida, A.F.; Santana, R.A.C.; Campos, A.R.N.; Oliveira, J.A.M.; Alves, J.J.N.; Santos, T.F.A.; Silva, A.A.; Prasad, S.; Silva, P.C.S.; et al. Effects of Current Density and Bath Temperature on the Morphological and Anticorrosive Properties of Zn-Ni Alloys. Metals 2023, 13, 1808. https://doi.org/10.3390/met13111808
Costa JD, Almeida AF, Santana RAC, Campos ARN, Oliveira JAM, Alves JJN, Santos TFA, Silva AA, Prasad S, Silva PCS, et al. Effects of Current Density and Bath Temperature on the Morphological and Anticorrosive Properties of Zn-Ni Alloys. Metals. 2023; 13(11):1808. https://doi.org/10.3390/met13111808
Chicago/Turabian StyleCosta, Josiane D., Arthur F. Almeida, Renato A. C. Santana, Ana R. N. Campos, José A. M. Oliveira, José J. N. Alves, Tiago F. A. Santos, Antônio A. Silva, Shiva Prasad, Paulo C. S. Silva, and et al. 2023. "Effects of Current Density and Bath Temperature on the Morphological and Anticorrosive Properties of Zn-Ni Alloys" Metals 13, no. 11: 1808. https://doi.org/10.3390/met13111808
APA StyleCosta, J. D., Almeida, A. F., Santana, R. A. C., Campos, A. R. N., Oliveira, J. A. M., Alves, J. J. N., Santos, T. F. A., Silva, A. A., Prasad, S., Silva, P. C. S., Souza, E. L. S., Delgado, J. M. P. Q., & Lima, A. G. B. (2023). Effects of Current Density and Bath Temperature on the Morphological and Anticorrosive Properties of Zn-Ni Alloys. Metals, 13(11), 1808. https://doi.org/10.3390/met13111808









