Electrophoretic Deposition of Chitosan–Hydroxyapatite Films and Their Electrochemical Behavior in Artificial Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hydroxyapatite
2.2. Electrophoretic Deposition
2.3. Electrochemical Evaluation
3. Results
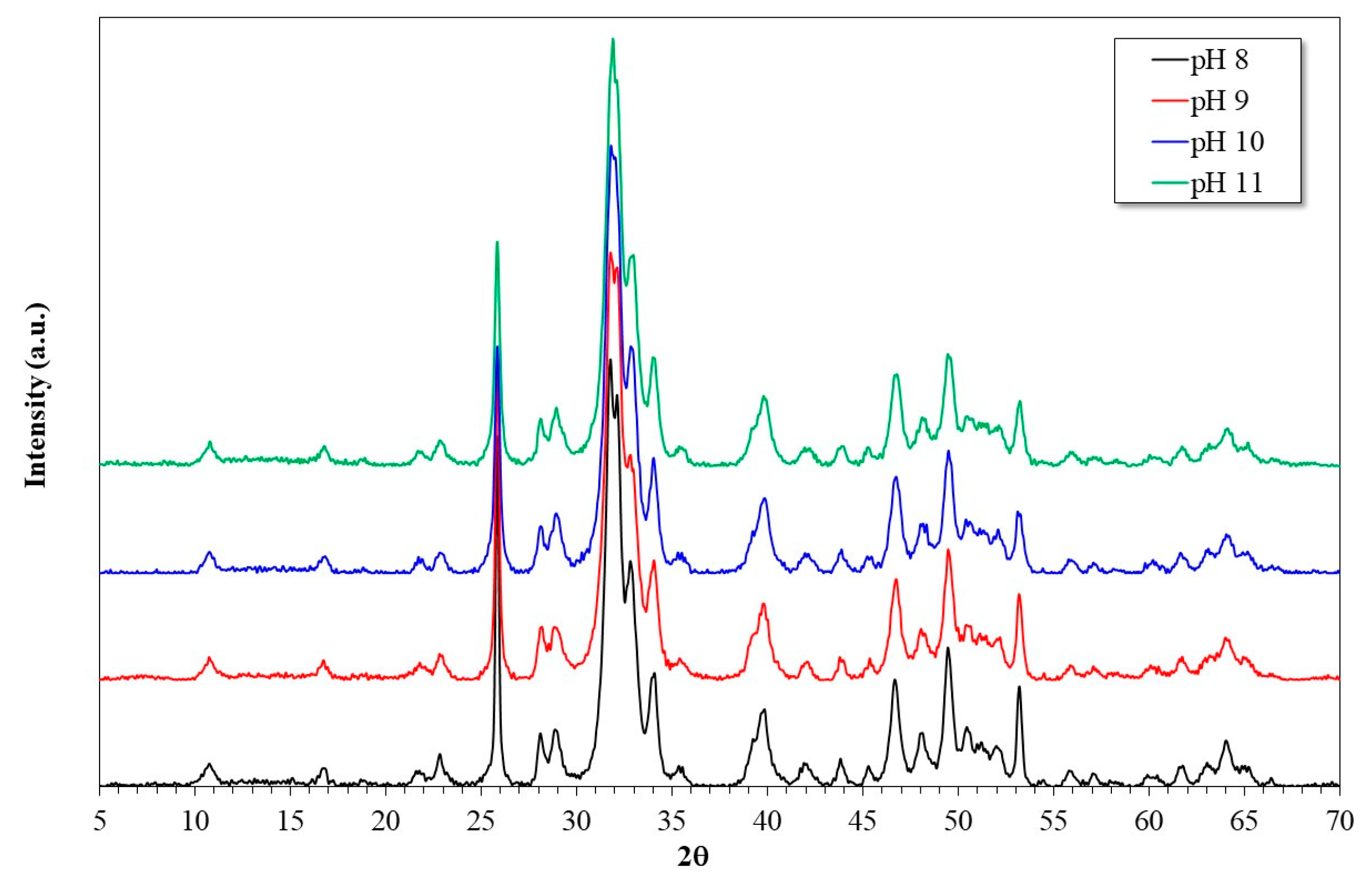
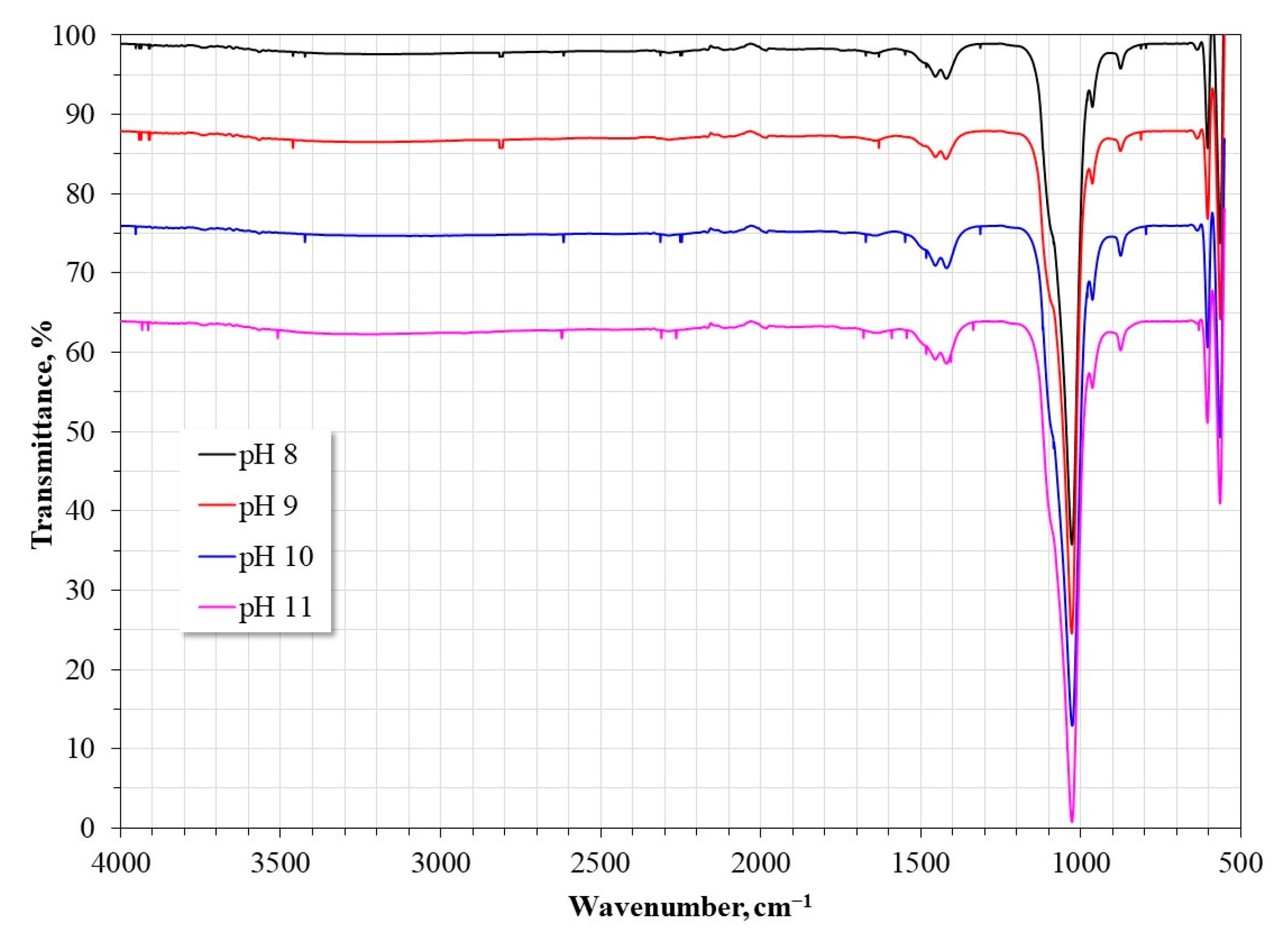
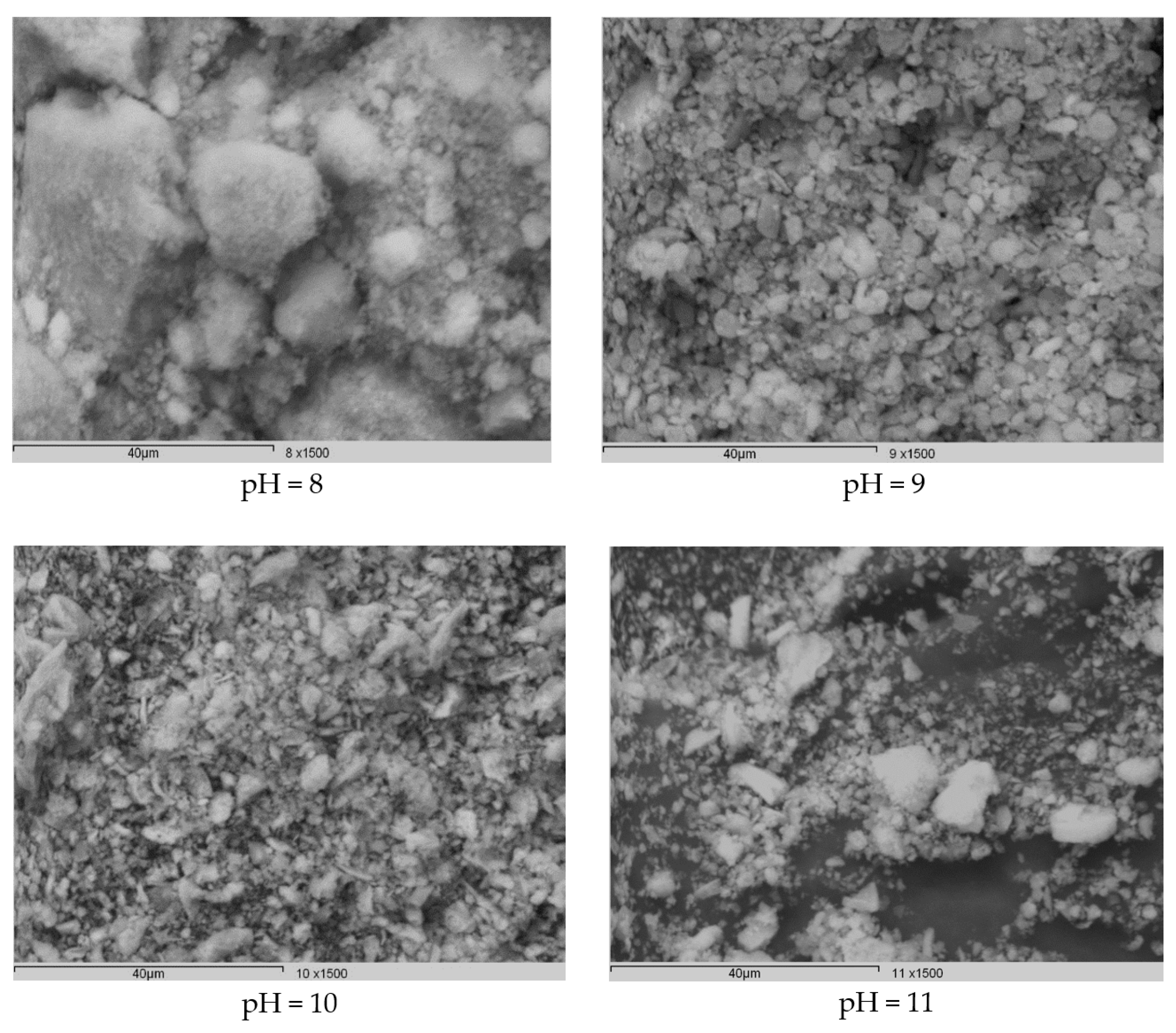
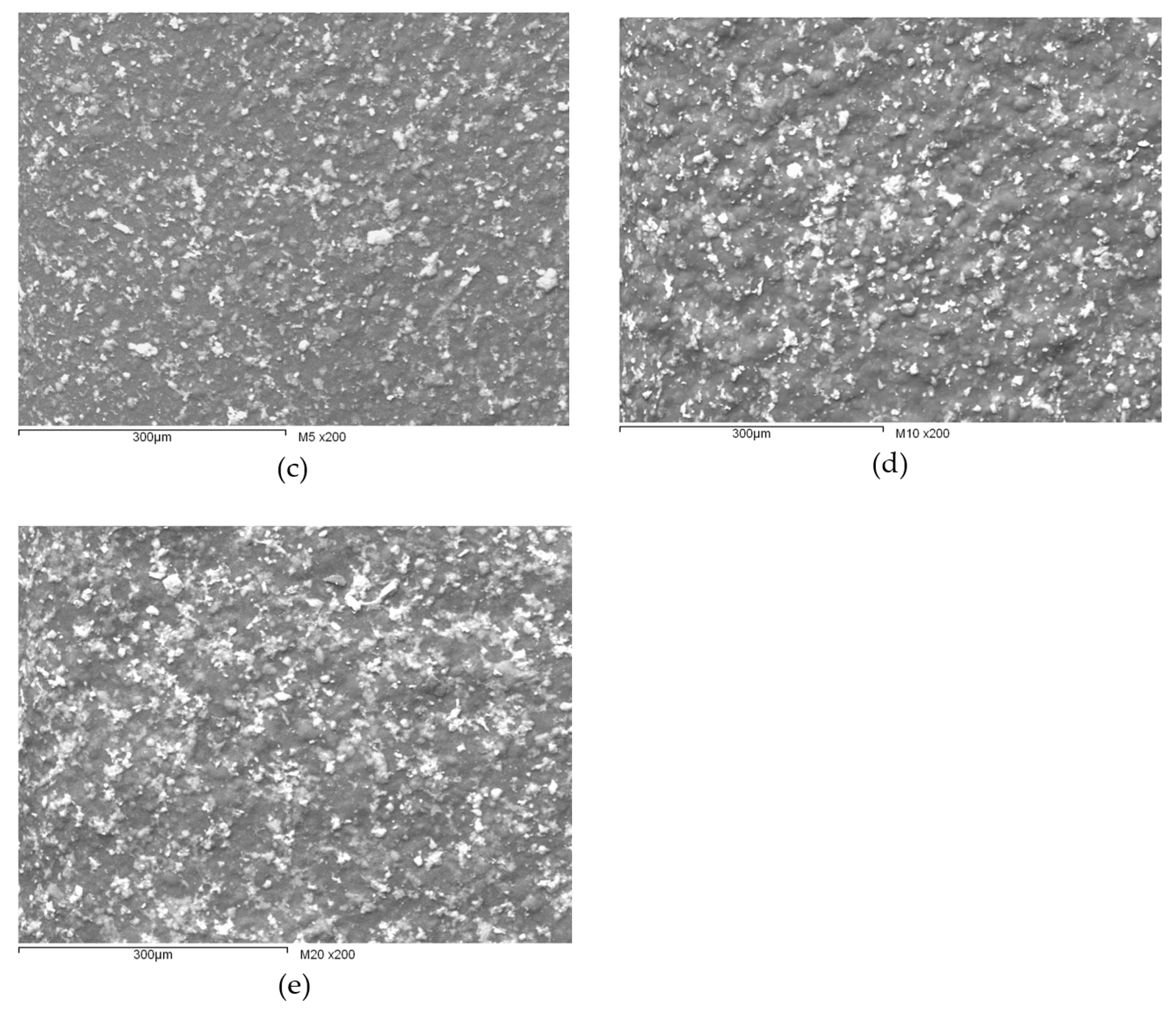
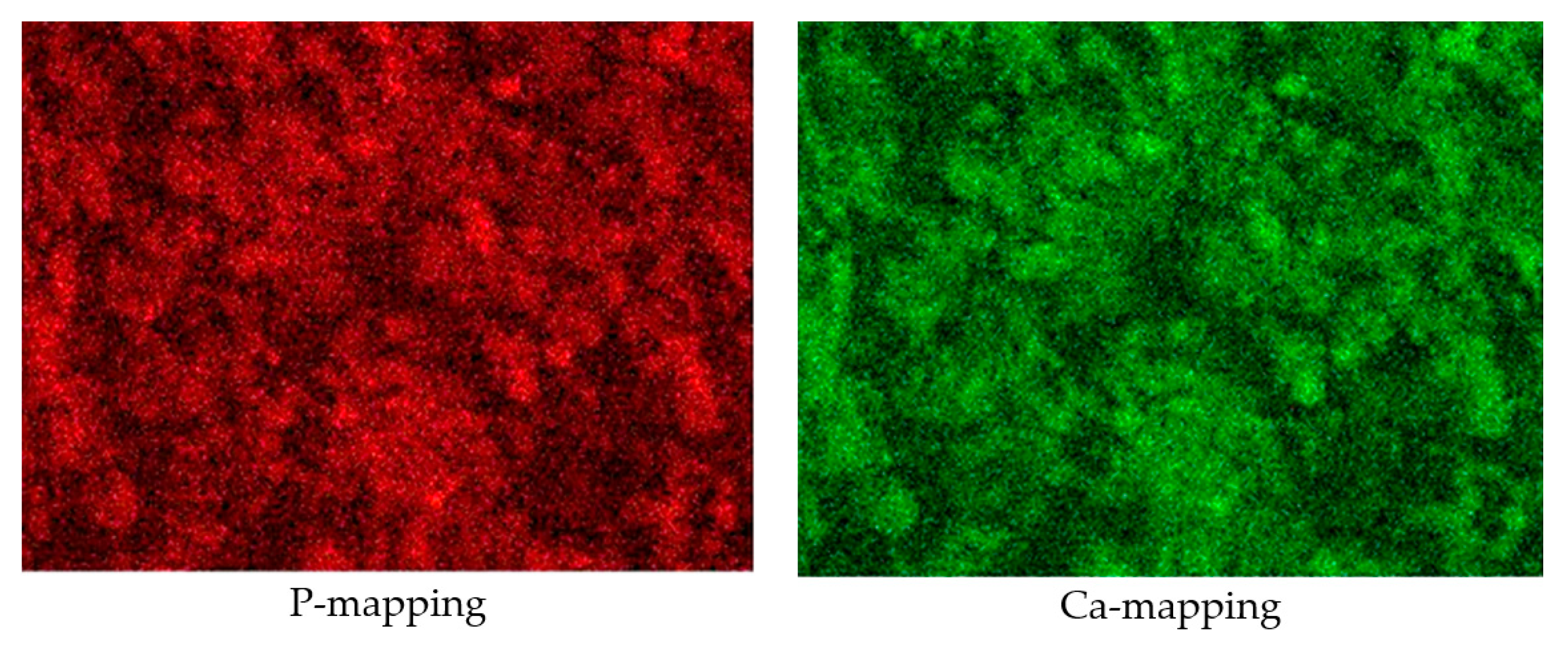
3.1. Characterization of the Synthesized HAp and Deposited Films
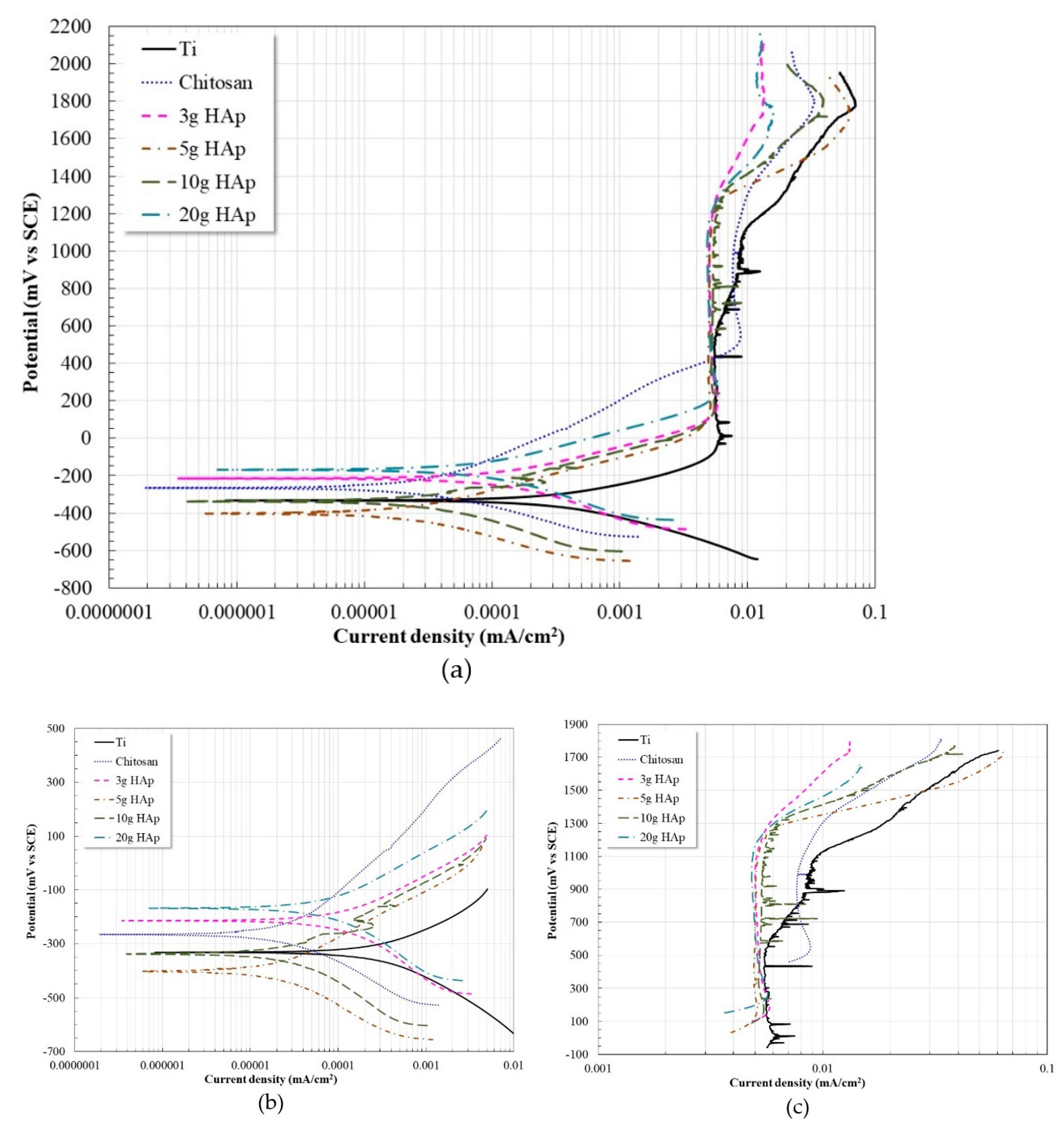
3.2. Potentiodynamic Polarization
3.3. Open Circuit Potential Measurements
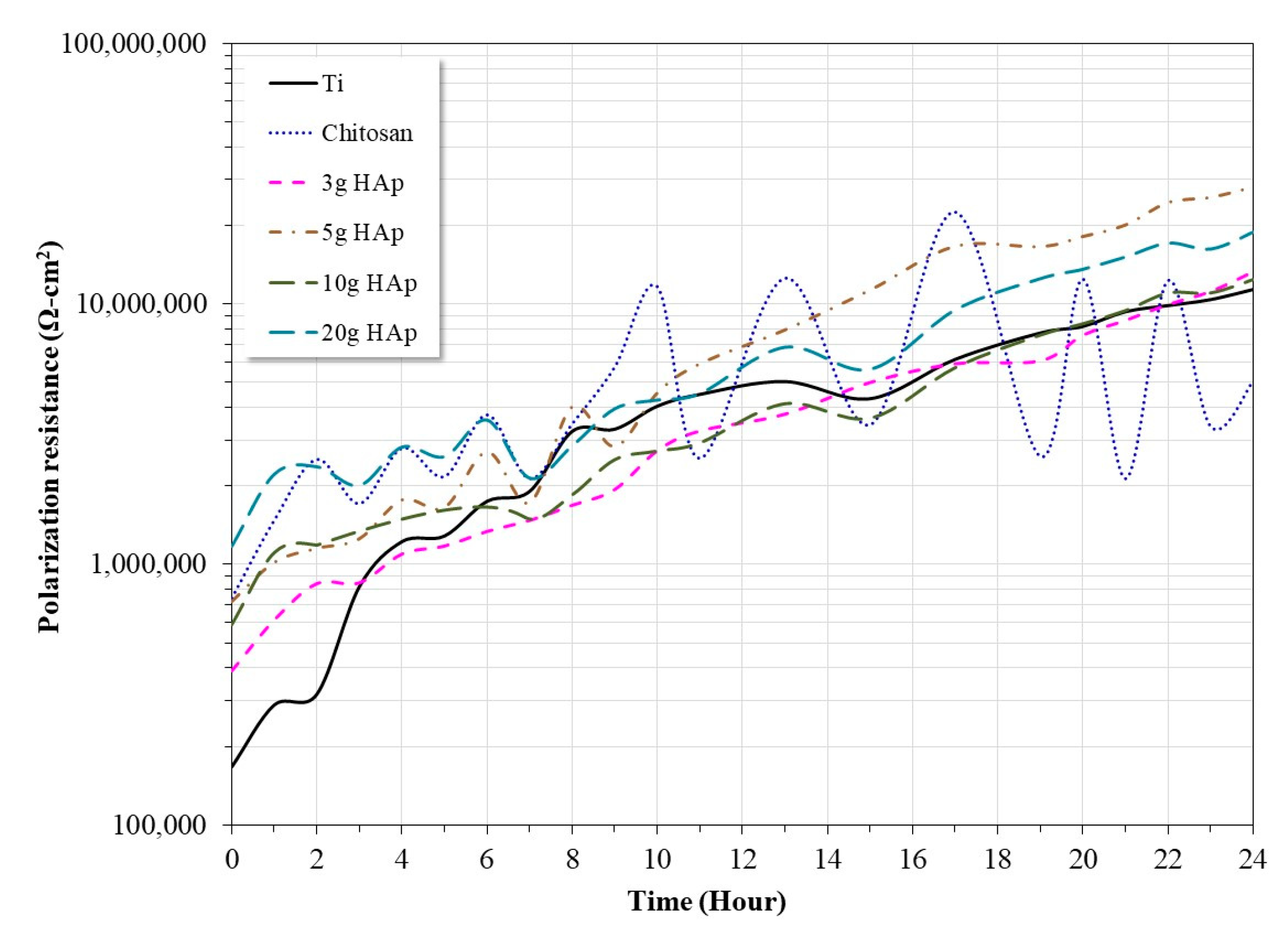
3.4. Polarization Resistance Measurements
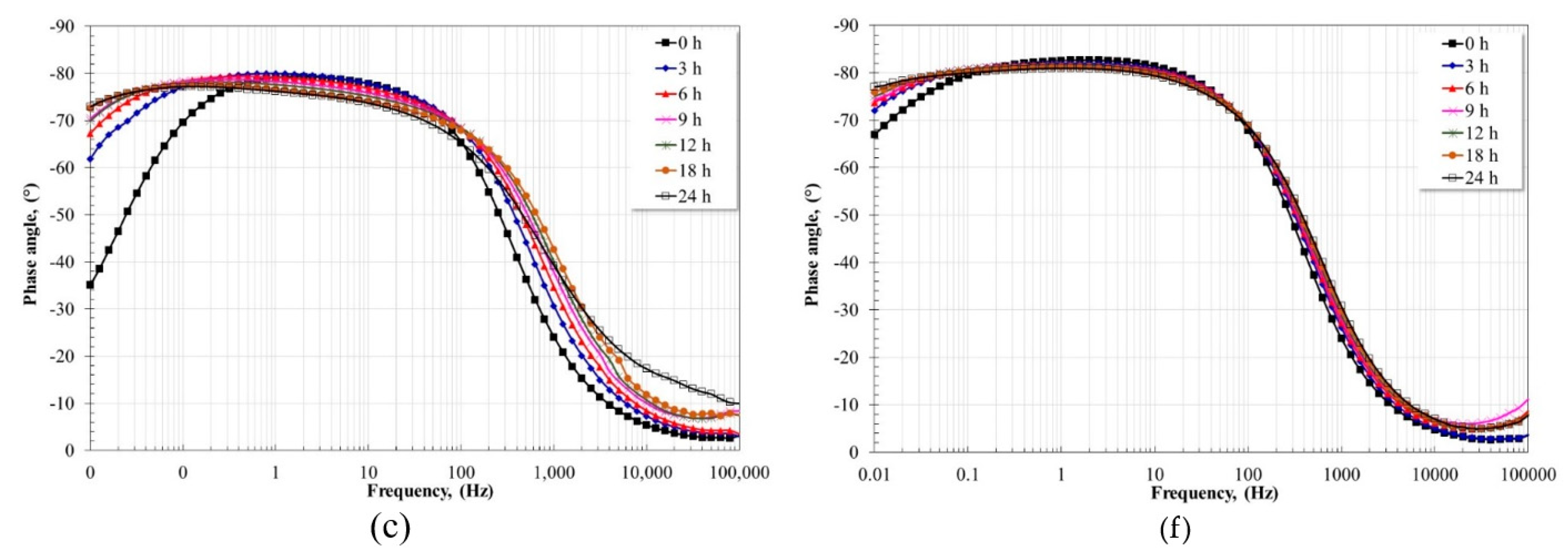
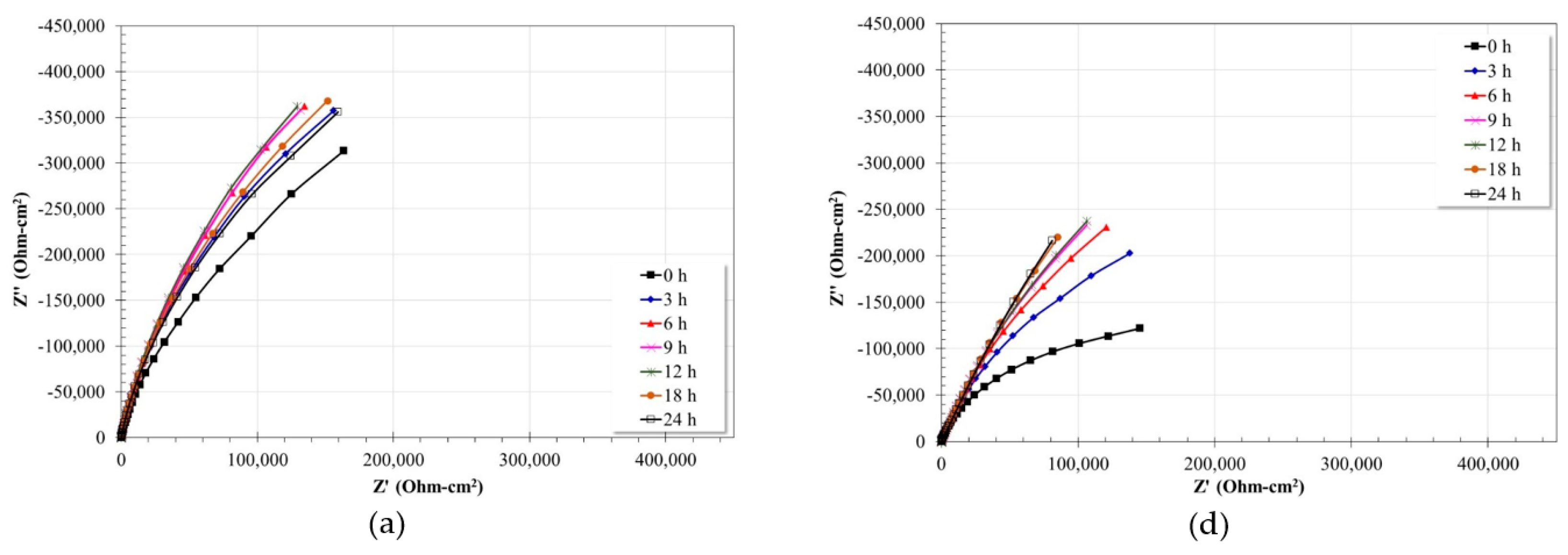
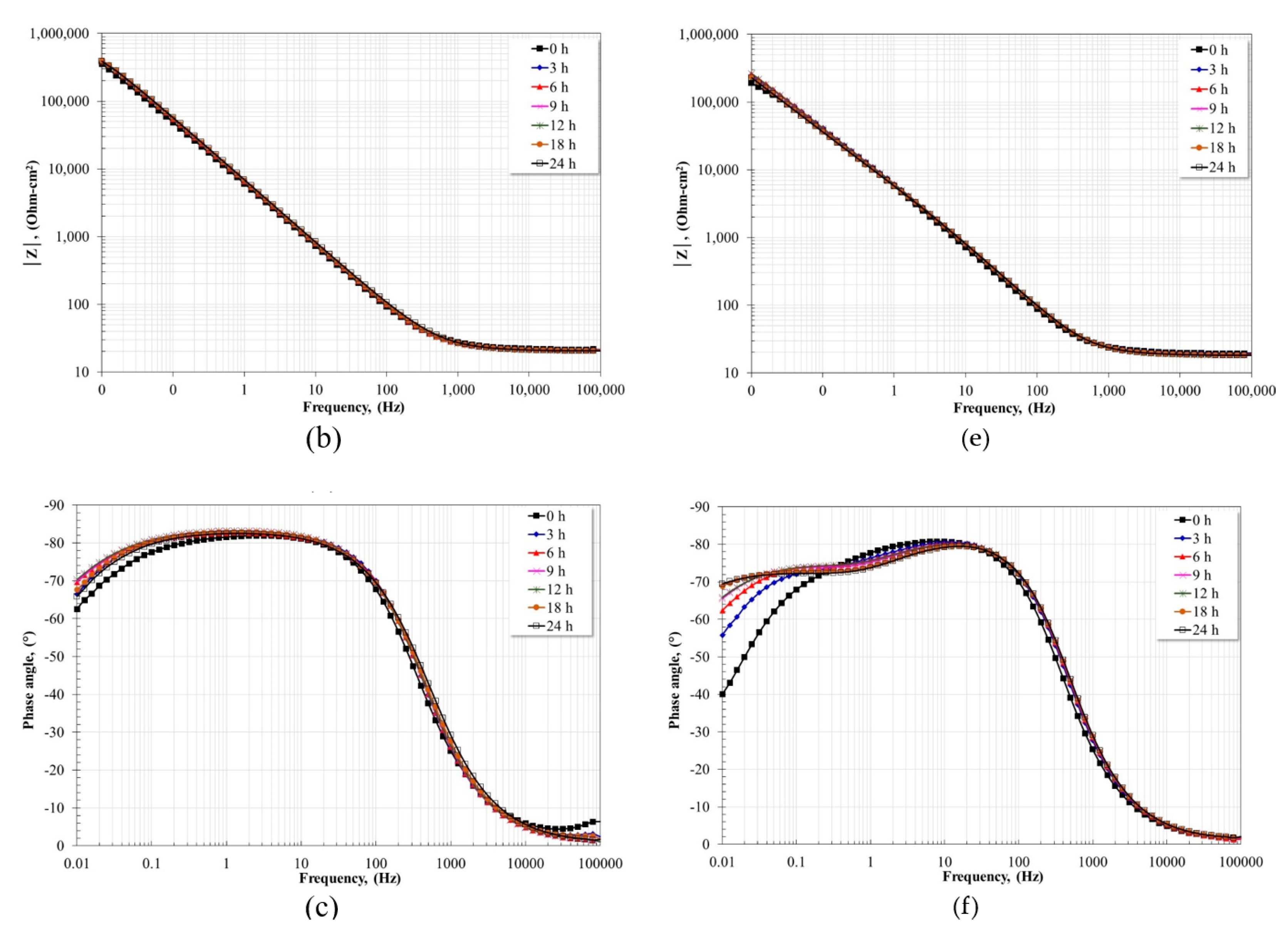
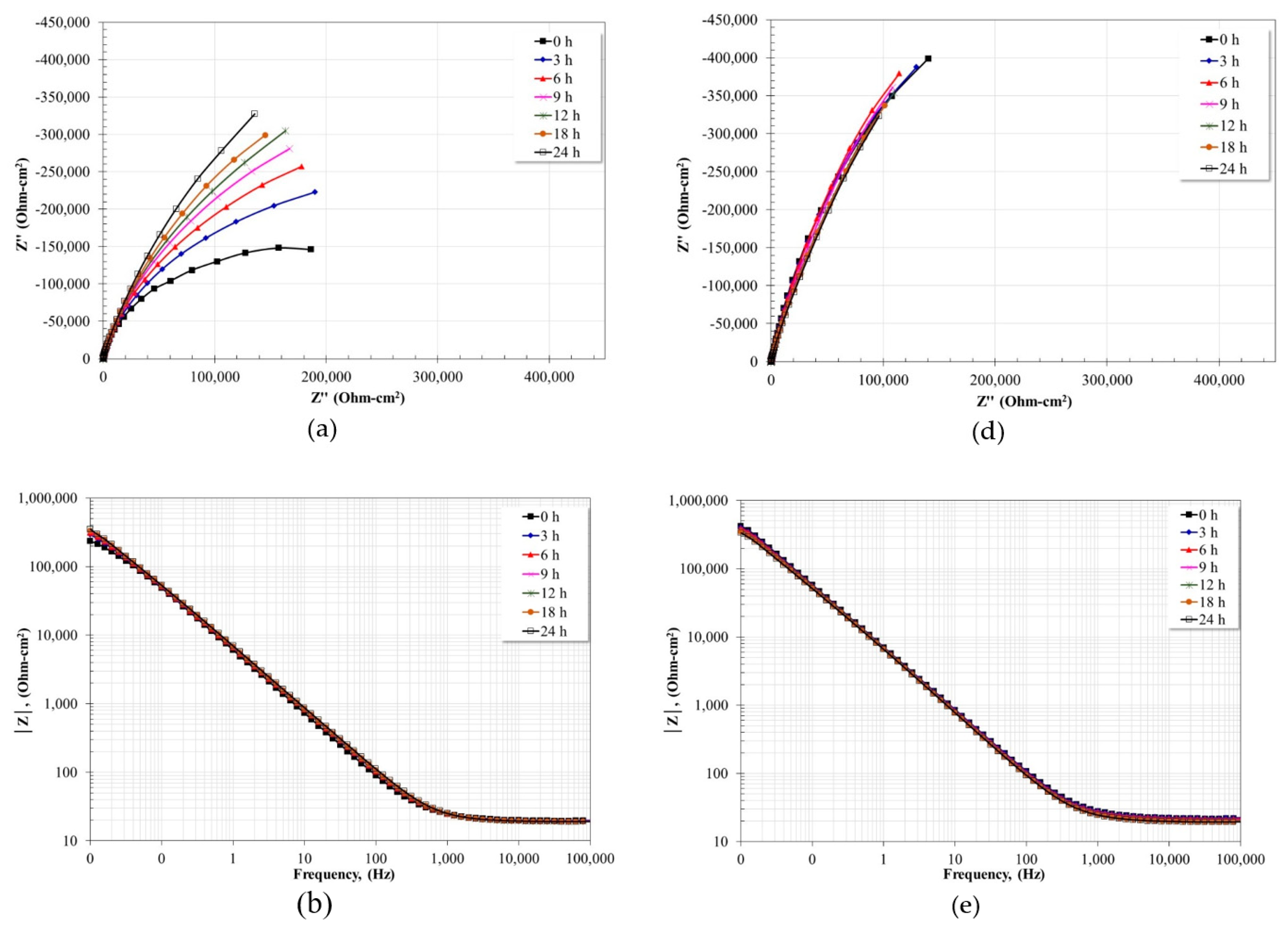
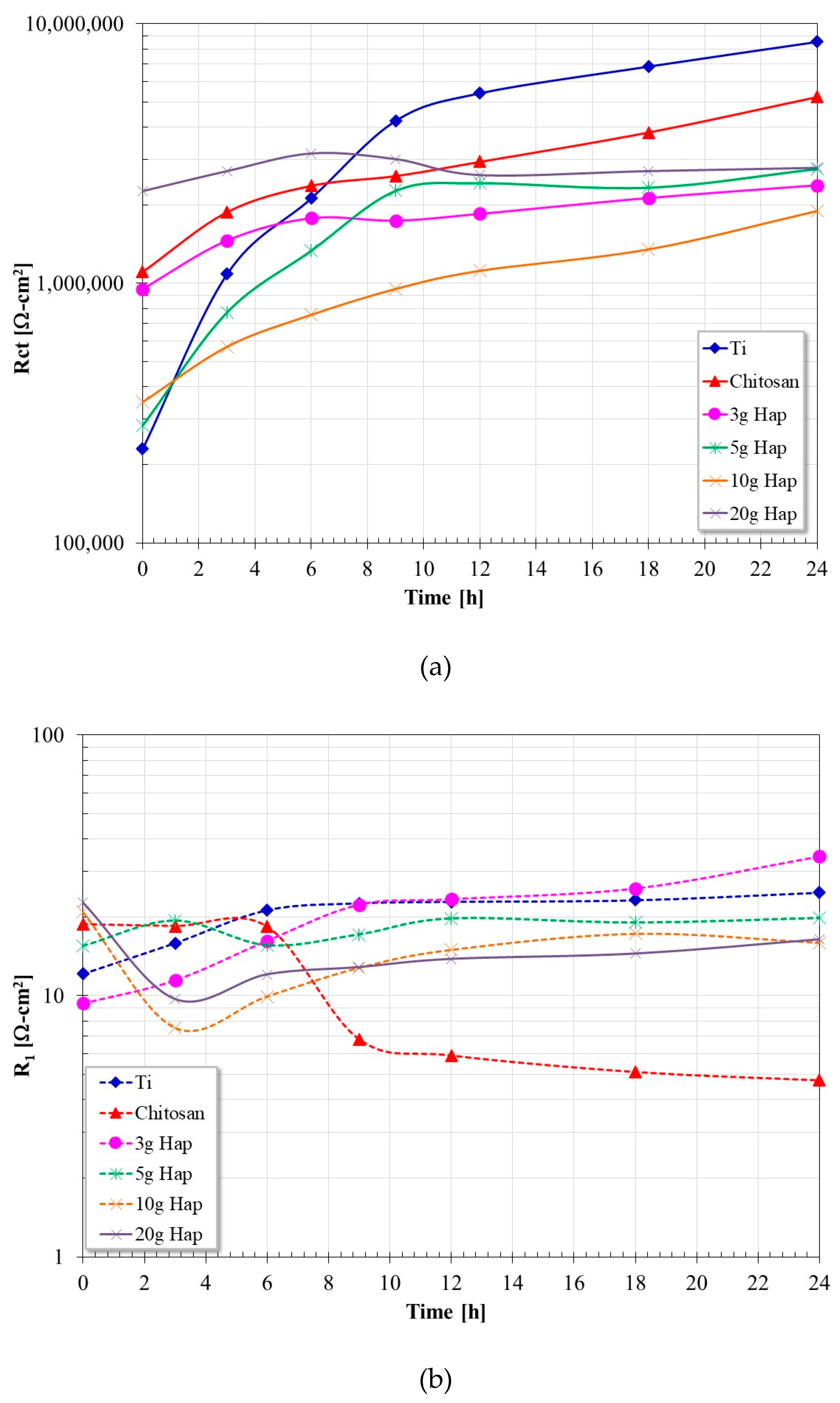
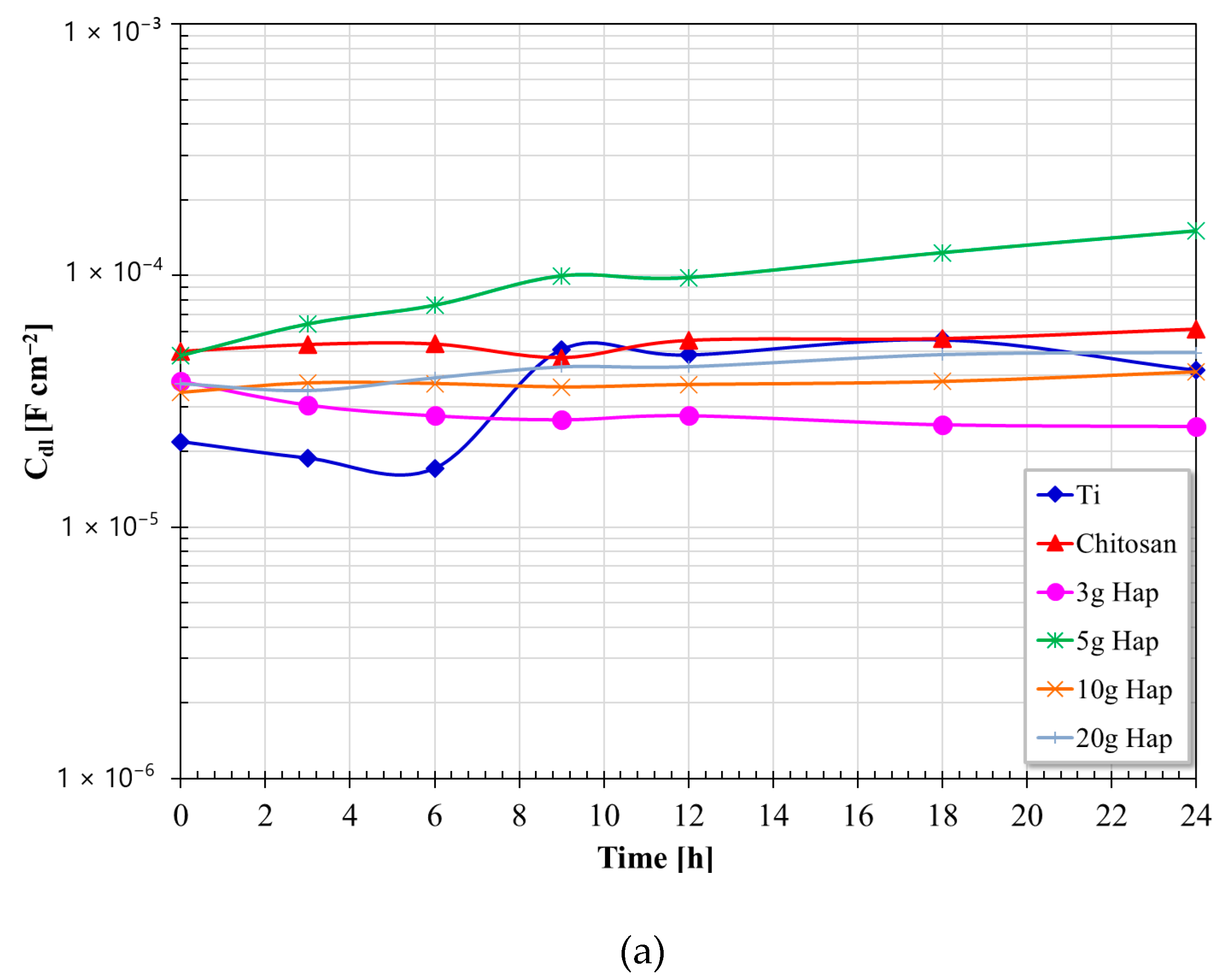
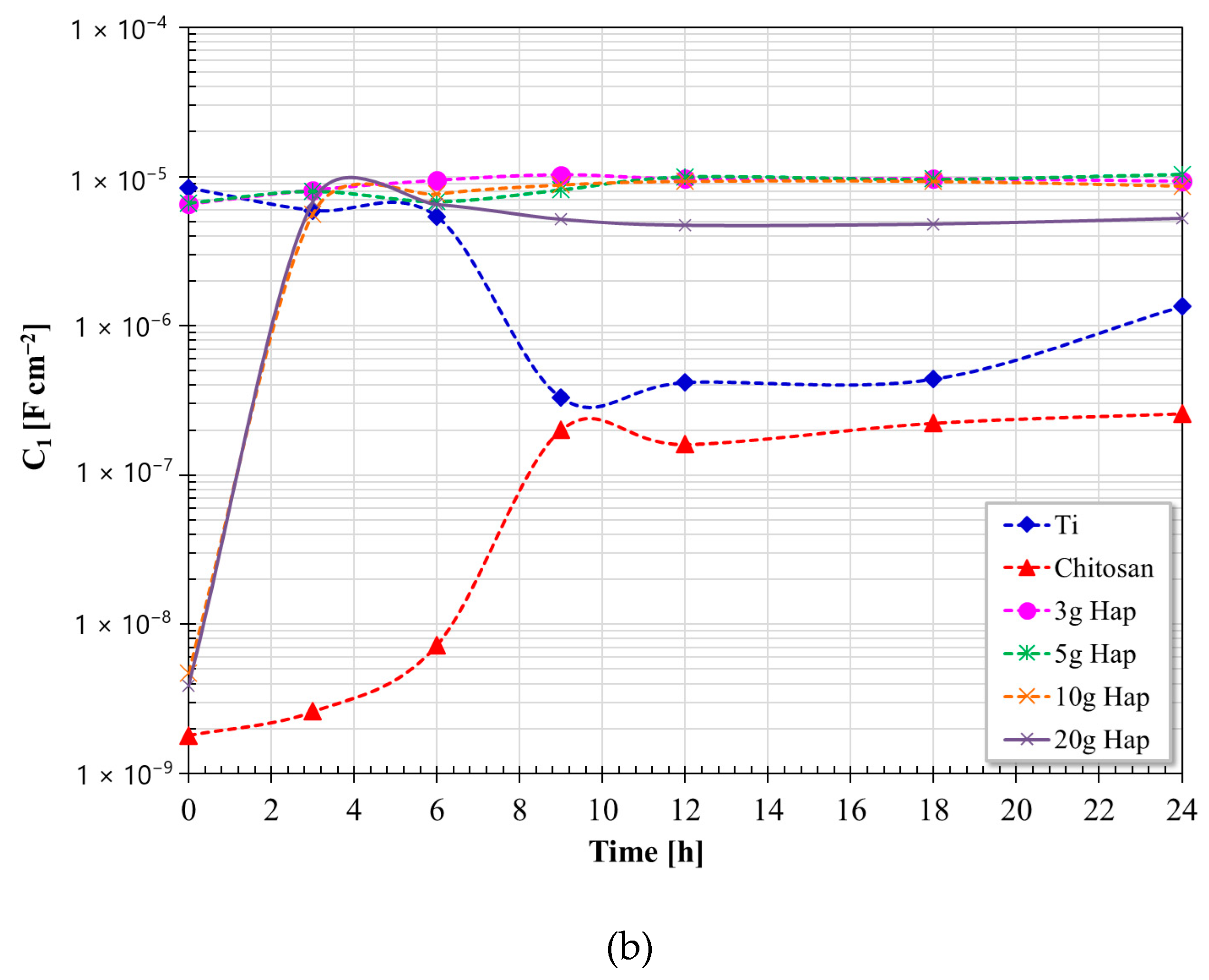
3.5. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porcayo-Palafox, E.; Carrera-Chavez, S.I.; Casolco, S.R.; Porcayo-Calderon, J.; Salinas-Bravo, V.M. Electrochemical Performance of Ti-based Commercial Biomaterials. Adv. Mater. Sci. Eng. 2019, 2019, 4352360. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.M.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.D.; Alexa, O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview. Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.; Khan, I.; Malhotra, R.; Zhu, D. Additive manufacturing and 3D printing of metallic biomaterials. Eng. Regen. 2021, 2, 288–299. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Rahimi, M.; Aghdam, R.M.; Sohi, M.H.; Rezayan, A.H.; Ettelaei, M. Improving biocompatibility and corrosion resistance of anodized AZ31 Mg alloy by electrospun chitosan/mineralized bone allograft (MBA) nanocoatings. Surf. Coat. Technol. 2021, 405, 126627. [Google Scholar] [CrossRef]
- Wathanyu, K.; Tuchinda, K.; Daopiset, S.; Sirivisoot, S. Corrosion resistance and biocompatibility of cold-sprayed titanium on 316L stainless steel. Surf. Coat. Technol. 2022, 445, 128721. [Google Scholar] [CrossRef]
- Lai, K.C.; Chao, S.C.; Tseng, K.K.; Yeh, J.W.; Chen, P.Y. Biocompatible as-cast and homogenized TiNbTaZrMoV high entropy alloys: Mechanical properties, corrosion resistance and in vitro studies. J. Mater. Res. Technol. 2023, 24, 9708–9721. [Google Scholar] [CrossRef]
- Du, P.; Xiang, T.; Cai, Z.; Xie, G. The influence of porous structure on the corrosion behavior and biocompatibility of bulk Ti-based metallic glass. J. Alloys Compd. 2022, 906, 164326. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Hassan, N.; Hendy, A.; Ebrahim, A.; Tamer, T.M. Synthesis and evaluation of novel O-functionalized aminated chitosan derivatives as antibacterial, antioxidant and anticorrosion for 316L stainless steel in simulated body fluid. J. Saudi Chem. Soc. 2021, 25, 101368. [Google Scholar] [CrossRef]
- Witecka, A.; Valet, S.; Basista, M.; Boccaccini, A.R. Electrophoretically deposited high molecular weight chitosan/bioactive glass composite coatings on WE43 magnesium alloy. Surf. Coat. Technol. 2021, 418, 127232. [Google Scholar] [CrossRef]
- Bozkurt, Y.B.; Kavasoğlu, Y.S.; Atik, B.; Kovacı, H.; Uzun, Y.; Çelik, A. Comparison study of corrosion behavior for chitosan coated Ti6Al4V alloy produced by selective laser melting and forging. Prog. Org. Coat. 2023, 182, 107655. [Google Scholar] [CrossRef]
- Wang, J.; Deng, S.; Meng, M.; Tu, W.; Ou, J. Improved corrosion resistance and biocompatibility of AZ31 alloy by acid pickling pretreatment and (H+) hydroxyapatite/chitosan composite coating. Surf. Coat. Technol. 2023, 454, 129157. [Google Scholar] [CrossRef]
- Muñoz-Sanchez, E.R.; Arrieta-Gonzalez, C.D.; Quinto-Hernandez, A.; Garcia-Hernandez, E.; Porcayo-Calderon, J. Synthesis of hydroxyapatite from eggshell and its electrochemical characterization as a coating on Titanium. Int. J. Electrochem. Sci. 2023, 18, 100204. [Google Scholar] [CrossRef]
- Jing, Z.; Cao, Q.; Jun, H. Corrosion, wear and biocompatibility of hydroxyapatite bio-functionally graded coating on titanium alloy surface prepared by laser cladding. Ceram. Int. 2021, 47, 24641–24651. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Ahmad, R.N.; Rosli, N.M.; Manan, M.A.; Marzuki, M.; Wahi, A. Sol gel deposited hydroxyapatite-based coating technique on porous titanium niobium for biomedical applications: A mini review. Mater. Today Proc. 2021, 41, 127–135. [Google Scholar] [CrossRef]
- Lin, M.H.; Chen, Y.C.; Liao, C.C.; Lin, L.W.; Chen, C.F.; Wang, K.K.; Chen, S.-T.; Hsueh, Y.H.; Wu, C.H.; Ou, S.F. Improvement in bioactivity and corrosion resistance of Ti by hydroxyapatite deposition using ultrasonic mechanical coating and armoring. Ceram. Int. 2022, 48, 4999–5008. [Google Scholar] [CrossRef]
- Muthu, D.; Kumar, G.S.; Gowri, M.; Prasath, M.; Viswabaskaran, V.; Kattimani, V.S.; Girija, E.K. Rapid synthesis of eggshell derived hydroxyapatite with nanoscale characteristics for biomedical applications. Ceram. Int. 2022, 48, 1326–1339. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wang, H.-F.; Liou, S.-P.; Ho, W.-F. Synthesis and Characterization of Nano-Hydroxyapatite Obtained from Eggshell via the Hydrothermal Process and the Precipitation Method. Molecules 2023, 28, 4926. [Google Scholar] [CrossRef]
- Goh, K.W.; Wong, Y.H.; Ramesh, S.; Chandran, H.; Krishnasamy, S.; Ramesh, S.; Sidhu, A.; Teng, W.D. Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation. Ceram. Int. 2021, 47, 8879–8888. [Google Scholar] [CrossRef]
- Alhasan, H.S.; Alahmadi, N.; Yasin, S.A.; Khalaf, M.Y.; Ali, G.A.M. Low-Cost and Eco-Friendly Hydroxyapatite Nanoparticles Derived from Eggshell Waste for Cephalexin Removal. Separations 2022, 9, 10. [Google Scholar] [CrossRef]
- Pu’ad, N.A.S.M.; Alipal, J.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis of eggshell derived hydroxyapatite via chemical precipitation and calcination method. Mater. Today Proc. 2021, 42, 172–177. [Google Scholar] [CrossRef]
- Shi, D.; Tong, H.; Lv, M.; Luo, D.; Wang, P.; Xu, X.; Han, Z. Optimization of hydrothermal synthesis of hydroxyapatite from chicken eggshell waste for effective adsorption of aqueous Pb(II). Environ. Sci. Pollut. Res. 2021, 28, 58189–58205. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Jikan, S.S.B.; Adzila, S.; Murni, Z.; Badarulzaman, N.A.; Rosley, R.; Hameed, M.U. Synthesis and Characterizations of Hydroxyapatite using Precursor Extracted from Chicken Egg Shell Waste. Biointerface Res. Appl. Chem. 2022, 12, 5663–5671. [Google Scholar] [CrossRef]
- Baskar, K.; Karthikeyan, B.S.; Gurucharan, I.; Mahalaxmi, S.; Rajkumar, G.; Dhivya, V.; Kishen, A. Eggshell derived nano-hydroxyapatite incorporated carboxymethyl chitosan scaffold for dentine regeneration: A laboratory investigation. Int. Endod. J. 2022, 55, 89–102. [Google Scholar] [CrossRef]
- Sangmala, A.; Naemchanthara, P.; Limsuwan, P.; Naemchanthara, K. Replacement of hydroxyapatite from chicken eggshell waste for ceramic properties improvement. Int. J. Appl. Ceram. 2021, 18, 2132–2142. [Google Scholar] [CrossRef]
- Mardziah, C.M.; Ramesh, S.; Chandran, H.; Sidhu, A.; Krishnasamy, S. Properties of sintered zinc hydroxyapatite bioceramic prepared using waste chicken eggshells as calcium precursor. Ceram. Int. 2023, 49, 12381–12389. [Google Scholar] [CrossRef]
- Mokhtar, M.Z.; Altujjar, A.; Wang, B.; Chen, Q.; Ke, J.C.R.; Cai, R.; Zibouche, N.; Spencer, B.F.; Jacobs, J.; Thomas, A.G.; et al. Spherical hydroxyapatite nanoparticle scaffolds for reduced lead release from damaged perovskite solar cells. Commun. Mater. 2022, 3, 77. [Google Scholar] [CrossRef]
- Martinez-Gomez, M.; Quinto-Hernandez, A.; Flores-Garcia, N.S.; Mayén, J.; Dominguez-Diaz, M.; Martinez, H.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Electrophoretic deposition of chitosan films doped with Nd2Ti2O7 nanoparticles as protective coatings against corrosion in saline solutions. Int. J. Polym. Sci. 2019, 2019, 3864835. [Google Scholar] [CrossRef]
- ISO 10993-15:2001; Part 1—Biological Evaluation of Medical Devices, 10993. ISO: Geneva, Switzerland, 2001.
- Workeneh, K.; Zereffa, E.A.; Segne, T.A.; Eswaramoorthy, R. Eggshell-Derived Nanohydroxyapatite Adsorbent for Defluoridation of Drinking Water from Bofo of Ethiopia. J. Nanomater. 2019, 2019, 2458312. [Google Scholar] [CrossRef]
- Al-Mobarak, N.A.; Al-Swayih, A.A.; Al-Rashoud, F.A. Corrosion Behavior of Ti-6Al-7Nb Alloy in Biological Solution for Dentistry Applications. Int. J. Electrochem. Sci. 2011, 6, 2031–2042. [Google Scholar] [CrossRef]
- Yu, S.Y.; Scully, J.R. Corrosion and Passivity of Ti-13% Nb-13% Zr in Comparison to Other Biomedical Implant Alloys. Corrosion 1997, 53, 965–976. [Google Scholar] [CrossRef]
- Aziz-Kerrzo, M.; Conroy, K.G.; Fenelon, A.M.; Farrell, S.T.; Breslin, C.B. Electrochemical studies on the stability and corrosion resistance of titanium-based implant materials. Biomaterials 2001, 22, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Ibris, N.; Mirza-Rosca, J.C. EIS study of Ti and its alloys in biological media. J. Electroanal. Chem. 2002, 526, 53–62. [Google Scholar] [CrossRef]
- Marino, C.E.B.; Helena-Mascaro, L. EIS characterization of a Ti-dental implant in artificial saliva media: Dissolution process of the oxide barrier. J. Electroanal. Chem. 2004, 568, 115–120. [Google Scholar] [CrossRef]
- Figueira, N.; Silva, T.M.; Carmezim, M.J.; Fernandes, J.C.S. Corrosion behaviour of NiTi alloy. Electrochim. Acta 2009, 54, 921–926. [Google Scholar] [CrossRef]
- Wang, B.L.; Zheng, Y.F.; Zhao, L.C. Electrochemical corrosion behavior of biomedical Ti-22Nb and Ti-22Nb-6Zr alloys in saline medium. Mater. Corros. 2009, 60, 780–794. [Google Scholar] [CrossRef]
- Tual, C.; Espuche, E.; Ecoubes, M.; Domard, A. Transport Properties of Chitosan Membranes: Influence of Crosslinking. J. Polym. Sci. B Polym. Phys. 2000, 38, 1521–1529. [Google Scholar] [CrossRef]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical and barrier properties investigation of chitosan-clay nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Gierszewska-Druyska, M. Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydr. Polym. 2009, 77, 590–598. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Ferrer, J.E.; Victori, L. Oxygen evolution reaction on the iridium electrode in basic medium studied by electrochemical im-pedance spectroscopy. Electrochim. Acta 1994, 39, 581–588. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, T.S.N.S. Corrosion behaviour of Ti–15Mo alloy for dental implant applications. J. Dent. 2008, 36, 500–507. [Google Scholar] [CrossRef]
- de Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Lindholm-Sethson, B.; Ardlin, B.I. Effects of pH and fluoride concentration on the corrosion of titanium. J. Biomed. Mater. Res. A 2008, 86A, 149–159. [Google Scholar] [CrossRef]
- Alvesa, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Gomez-Guzman, N.B.; Canto, J.; Martinez-de-la-Escalera, L.M.; Neri, A.; Porcayo-Calderon, J. Inhibition of X52 Corrosion in CO2-Saturated Brine by a Dialkyl-Diamide from Coffee Bagasse Oil. Molecules 2023, 28, 763. [Google Scholar] [CrossRef]
- Musa, A.Y.; Jalgham, T.T.R.; Mohamad, A.B. Molecular dynamic and quantum chemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros. Sci. 2012, 56, 176–183. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of the inhibition effect of 5-((E)-4-phenylbuta-1, 3-dienylideneamino)-1, 3, 4-thiadiazole-2-thiol Schiff base on mild steel corrosion in hydrochloric acid. Corros. Sci. 2010, 52, 3321–3330. [Google Scholar] [CrossRef]
- Ahamad, I.; Quraishi, M.A. Bis(benzimidazol-2-yl) disulphide: An efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2006–2013. [Google Scholar] [CrossRef]






| Nomenclature | Chitosan–HAp Solutions |
|---|---|
| Chitosan | 0.5% chitosan solution |
| 3 g HAp | 0.5% chitosan and 3% Hap |
| 5 g HAp | 0.5% chitosan and 5% HAp |
| 10 g HAp | 0.5% chitosan and 10% HAp |
| 20 g HAp | 0.5% chitosan and 20% HAp |
| Component | Concentration (g/L) |
|---|---|
| NaCl | 6.8 |
| CaCl2 | 0.2 |
| KCl | 0.4 |
| MgSO4 | 0.1 |
| NaHCO3 | 2.2 |
| NaHPO4 | 0.126 |
| NaH2PO4 | 0.026 |
| Material | Ecorr (mV) | ba (mv/Dec) | bc (mV/Dec) | Icorr (nA/cm2) | Epas (mV) | Ipas (µA/cm2) | Passive Zone (mV) |
|---|---|---|---|---|---|---|---|
| Ti | −333 | 185 | 201 | 398 | 320 | 5–9 | 1000 |
| Chitosan | −265 | 326 | 200 | 36 | 770 | 7–8 | 600 |
| 3 g HAp | −215 | 197 | 254 | 91 | 368 | 5–6 | 1150 |
| 5 g HAp | −401 | 190 | 200 | 25 | 510 | 5–6 | 1160 |
| 10 g HAp | −337 | 192 | 243 | 41 | 460 | 5–6 | 1170 |
| 20 g HAp | −168 | 204 | 231 | 96 | 398 | 5–6 | 1100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Davila, J.P.; Arrieta-Gonzalez, C.D.; Perez-Arizmendi, F.J.; Dorta-Leon, M.A.; Brown-Bojorquez, F.; Porcayo-Calderon, J. Electrophoretic Deposition of Chitosan–Hydroxyapatite Films and Their Electrochemical Behavior in Artificial Plasma. Metals 2023, 13, 1828. https://doi.org/10.3390/met13111828
Mata-Davila JP, Arrieta-Gonzalez CD, Perez-Arizmendi FJ, Dorta-Leon MA, Brown-Bojorquez F, Porcayo-Calderon J. Electrophoretic Deposition of Chitosan–Hydroxyapatite Films and Their Electrochemical Behavior in Artificial Plasma. Metals. 2023; 13(11):1828. https://doi.org/10.3390/met13111828
Chicago/Turabian StyleMata-Davila, J. P., C. D. Arrieta-Gonzalez, F. J. Perez-Arizmendi, M. A. Dorta-Leon, F. Brown-Bojorquez, and J. Porcayo-Calderon. 2023. "Electrophoretic Deposition of Chitosan–Hydroxyapatite Films and Their Electrochemical Behavior in Artificial Plasma" Metals 13, no. 11: 1828. https://doi.org/10.3390/met13111828







