The Influences of Micro-Alloying Element Sn and Magnetic Field on the Microstructure Evolution of Al–Bi Immiscible Alloys
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
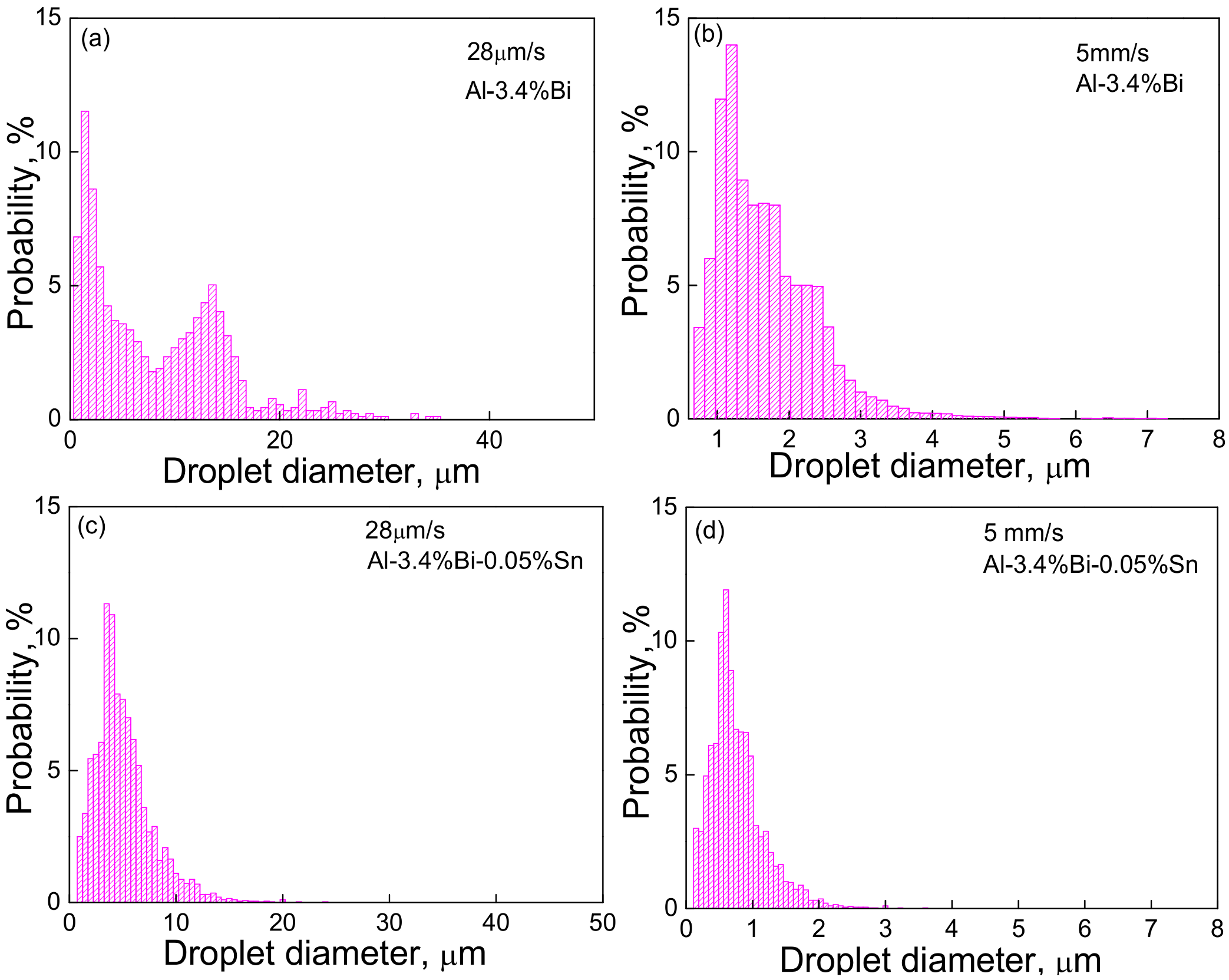
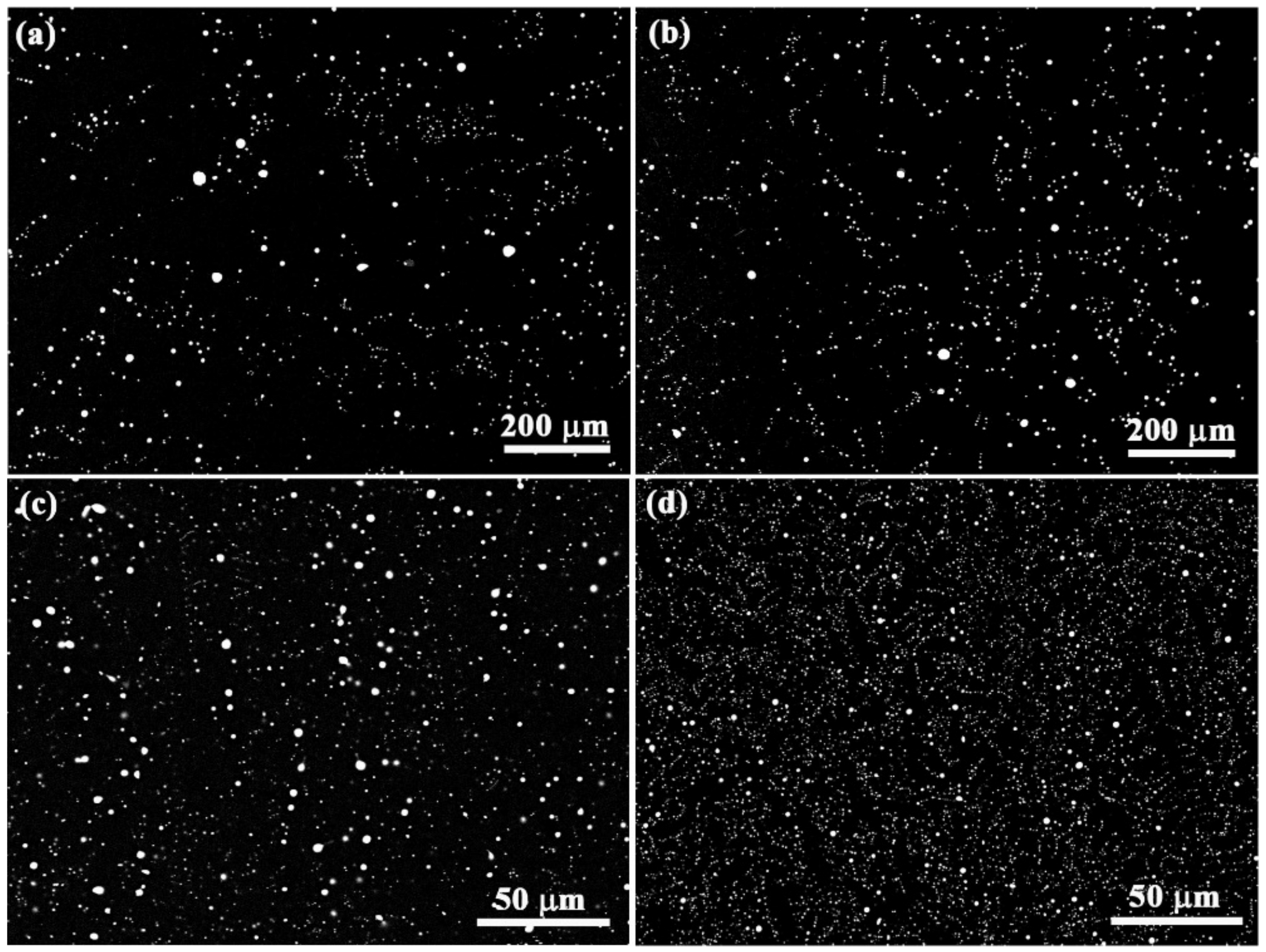
3.1. The Effect of Micro-Alloying Element Sn on the Microstructure of Al–Bi Samples
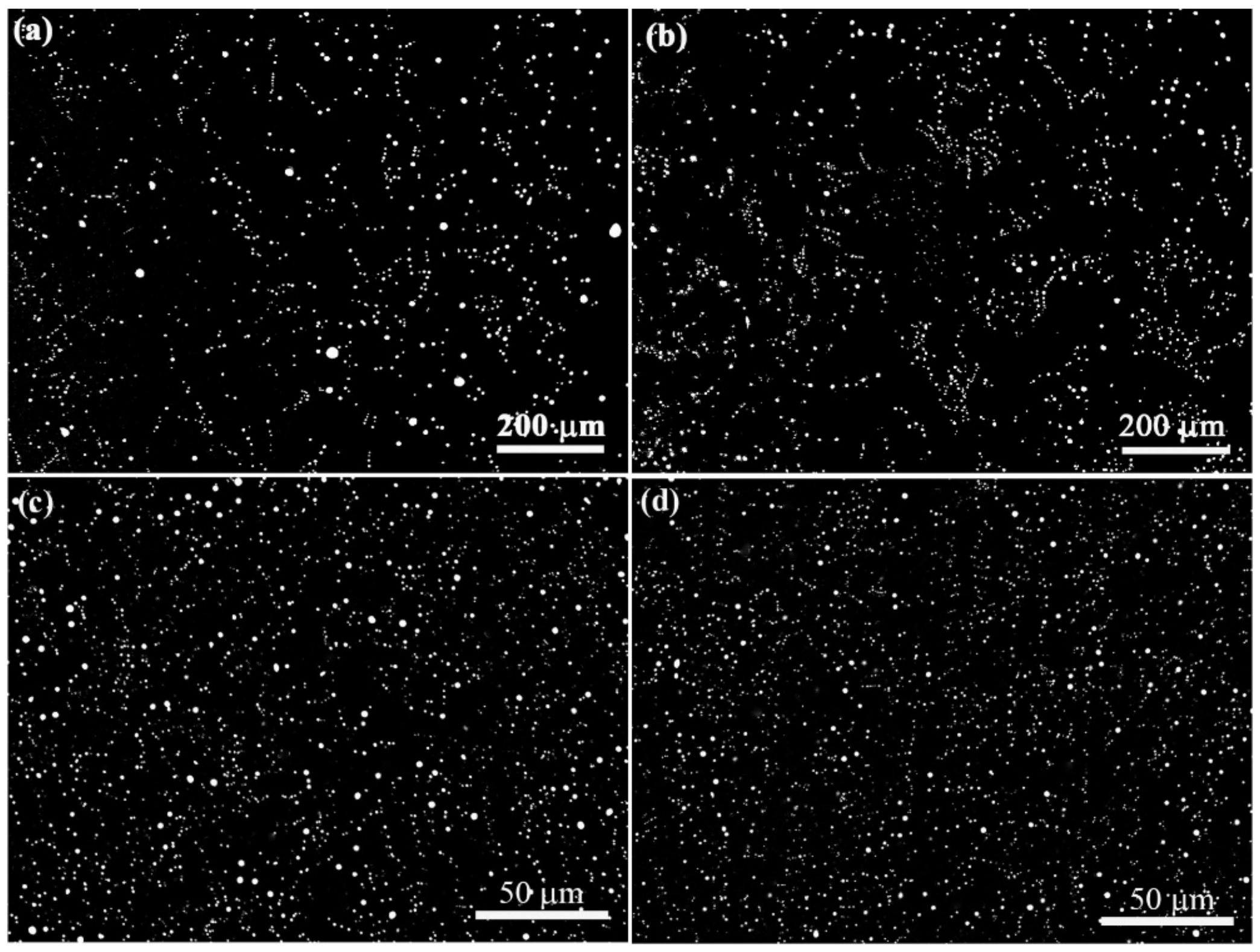
3.2. Microstructure Evolution of Al–Bi–(Sn) Samples Solidified under the Effect of Magnetic
4. Discussions
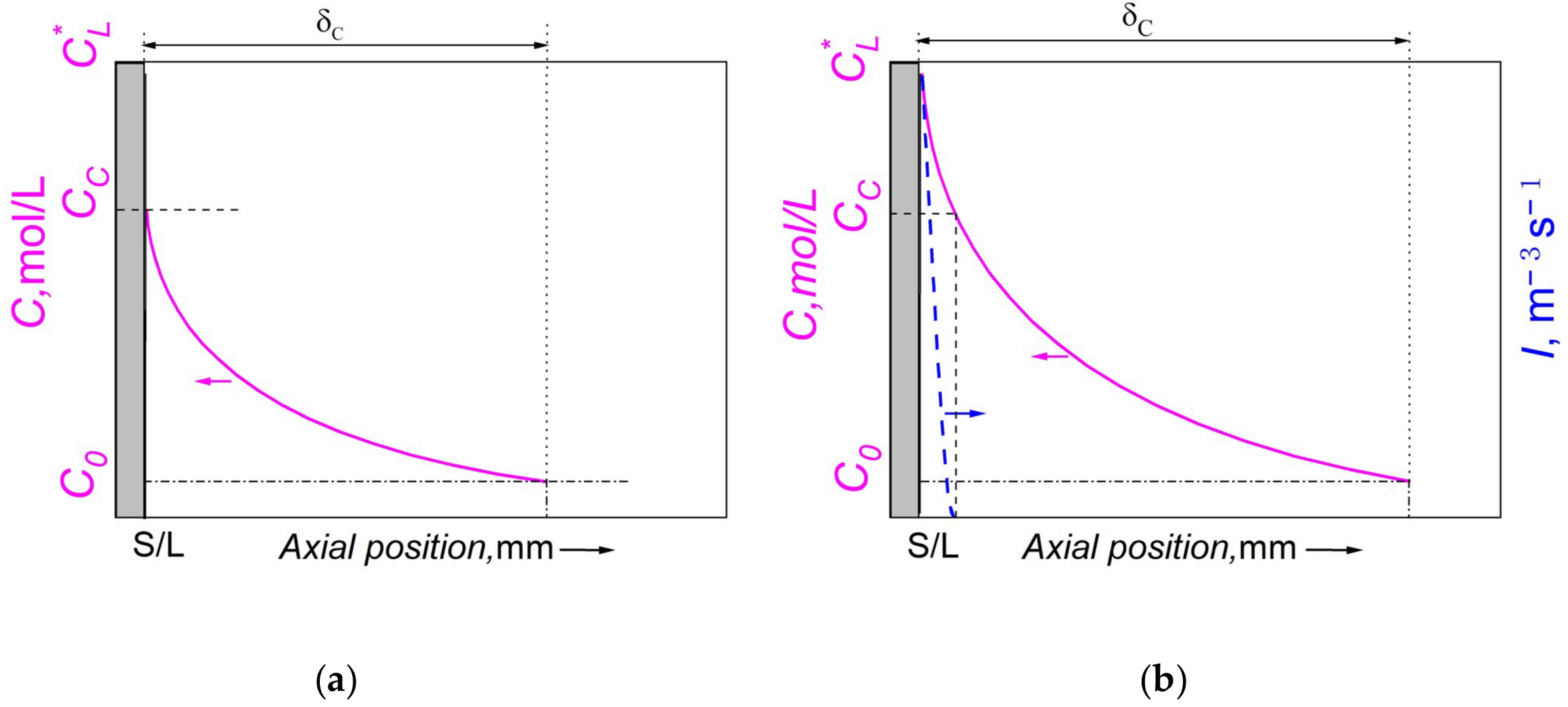
4.1. Microstructure Evolution of Al–Bi Samples
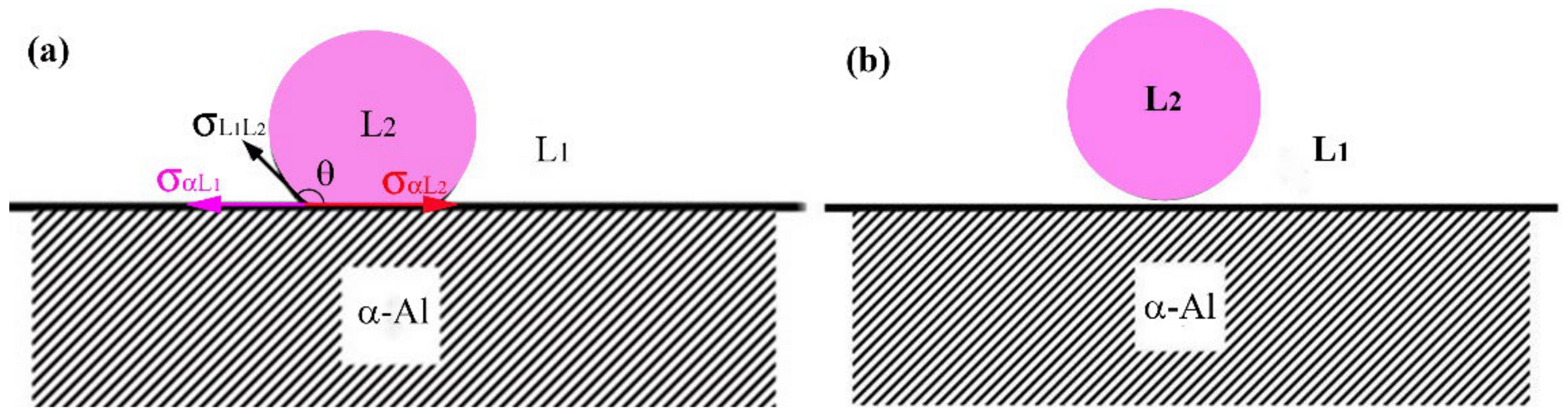
4.2. Effect of Sn on the Microstructure Evolution of Al–Bi Samples
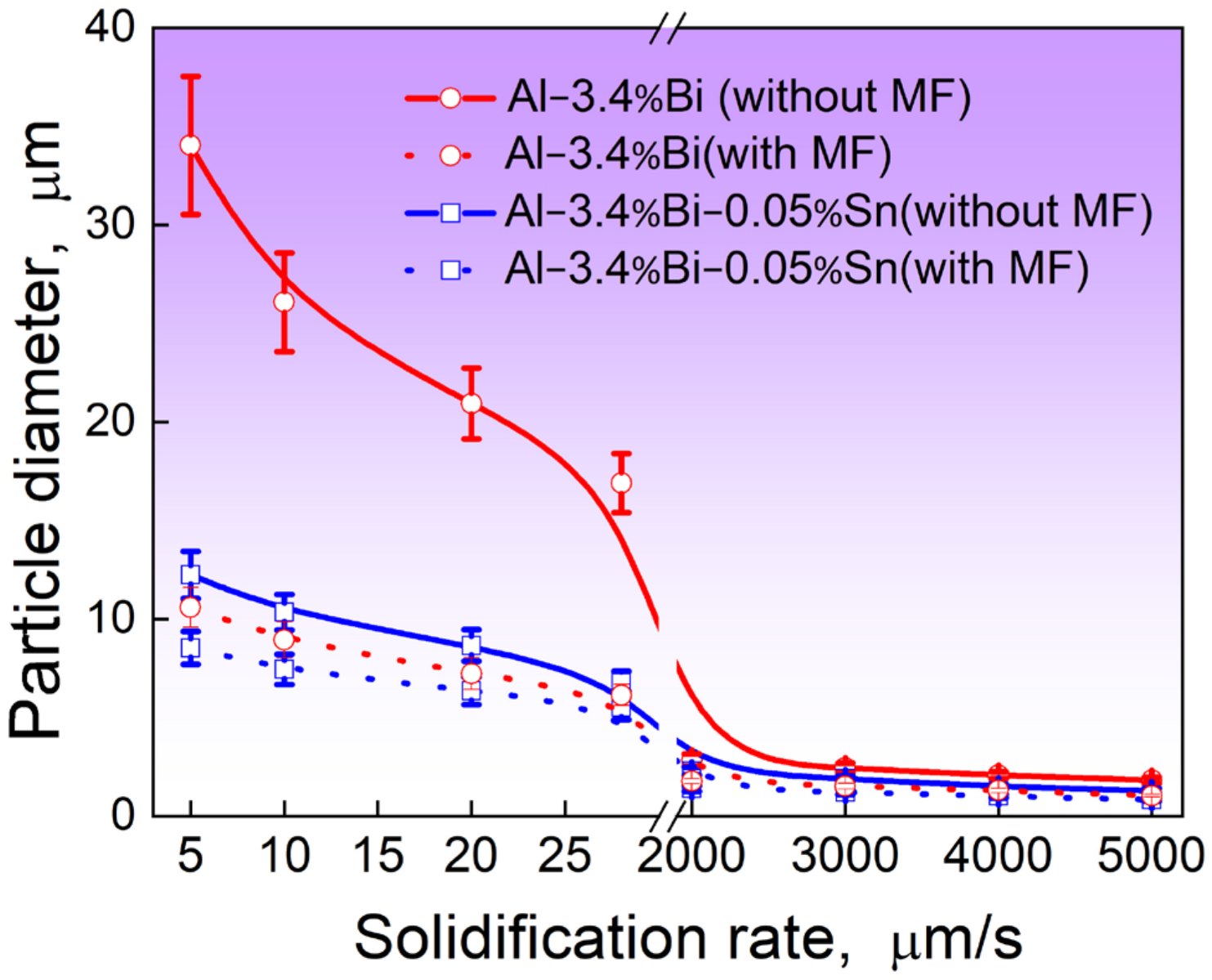
4.3. Effect of Static Magnetic Field on the Microstructure Evolution
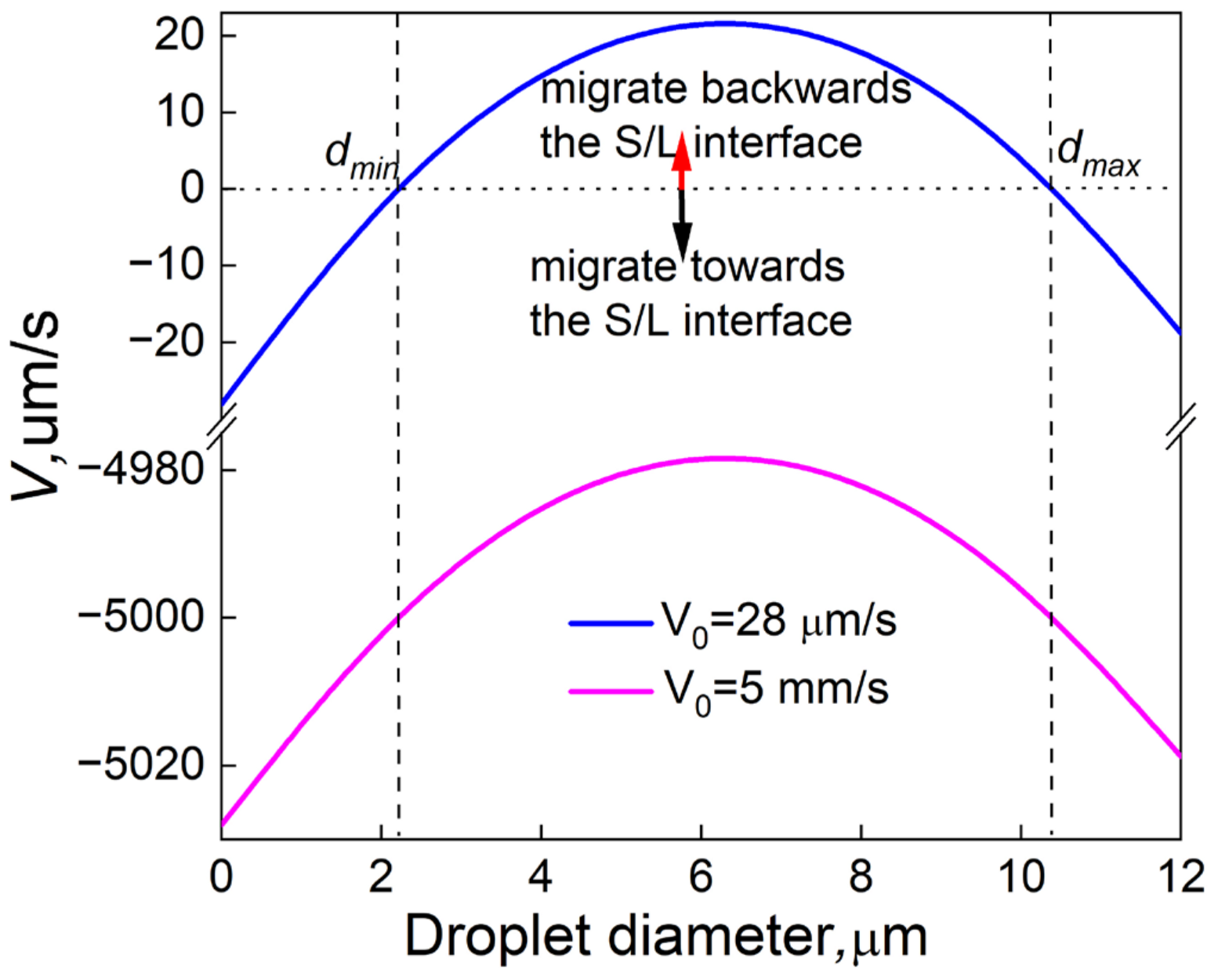
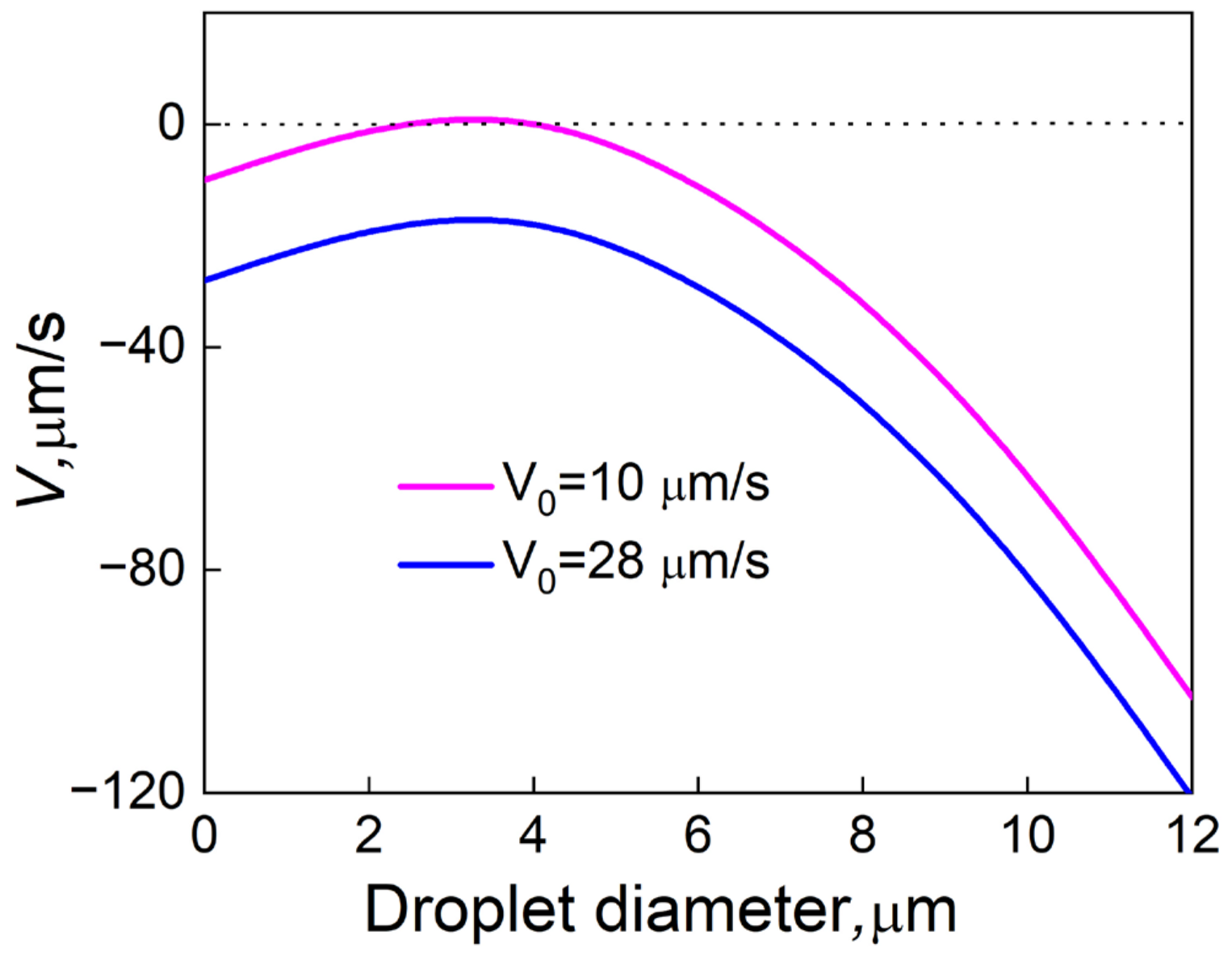
4.4. Effect of Sn and Static Magnetic Field on the Growth of the Droplets
5. Conclusions
- (1)
- The size distribution of the dispersed particles in the low-speed solidified Al–3.4 wt.%Bi alloy shows two peaks, while it only shows one peak when solidified at a relatively high velocity;
- (2)
- The introduction of Sn into the system leads to a reduction in the interfacial tension between the two liquid phases. This reduction has multiple effects: It enhances the nucleation rate and increases the number density of Bi-rich droplets within the sample. Additionally, it decreases the velocity of Marangoni migration and the resultant axial velocity of minority phase droplets near the solidification interface. These changes are beneficial for achieving a smaller average size of the Bi-rich phase and promoting the formation of well-dispersed microstructures in Al–Bi alloys;
- (3)
- By applying a static magnetic field of 0.2 T, it is observed that the number density of dispersed particles increases while the average size and size distribution width of these particles decrease;
- (4)
- In the presence of Sn addition and a static magnetic field, the average radius of the dispersed particles exhibits a strong dependence on the solidification velocity . Specifically, when the alloy is solidified at a relatively low velocity, follows a power function relationship with , given by . On the other hand, when the alloy is solidified at a high velocity, also exhibits a power dependence on , but with a different exponent: .
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, T.; Zhong, Y.; Wang, J.; Ren, Z.; Ren, W.; Lei, Z.; Debray, F.; Beaugnon, E.; Wei, X. Droplet Evolution and Refinement During Liquid–Liquid Decomposition of Zn-6WtPct Bi Immiscible Alloy Under High Static Magnetic Fields. Metall. Mater. Trans. A 2018, 49, 3333–3345. [Google Scholar] [CrossRef]
- Yong, L.; Benjamin, D.; Hariharan, S.; Kamal, K.; Cuiping, W.; Xingjun, L.; Amit, M.; Yunzhi, W. Microstructure development and morphological transition during deposition of immiscible alloy films. Acta Mater. 2021, 220, 117313. [Google Scholar]
- Chen, M.; Jia, P.; Liu, R.; Cui, S.; Geng, H. Solidification of immiscible Al75Bi9Sn16 alloy with different cooling rates. J. Alloys Compd. 2016, 688, 18–22. [Google Scholar]
- Mullis, A.M.; Jegede, O.E.; Bigg, T.D.; Cochrane, R.F. Dynamics of core–shell particle formation in drop-tube processed metastable monotectic alloys. Acta Mater. 2020, 188, 591–598. [Google Scholar] [CrossRef]
- Dong, B.W.; Jie, J.C.; Yao, X.X.; Liu, S.C.; Li, T.J. Effect of Sn addition on morphology evolution of secondary phase in hypomonotectic Cu-Pb-Sn alloy during solidification. J. Alloys Compd. 2019, 791, 936–946. [Google Scholar] [CrossRef]
- Liu, S.C.; Jie, J.C.; Guo, Z.K.; Yin, G.M.; Wang, T.M. Solidification microstructure evolution and its corresponding mechanism of metastable immiscible Cu80Fe20 alloy with different cooling conditions. J. Alloys Compd. 2018, 742, 99–106. [Google Scholar] [CrossRef]
- Wang, W.L.; Wu, Y.H.; Li, L.H.; Yan, N.; Wei, B. Homogeneous granular microstructures developed by phase separation and rapid solidification of liquid Fe-Sn immiscible alloy. J. Alloys Compd. 2017, 693, 650–657. [Google Scholar] [CrossRef]
- Wei, B.; Wang, W.L.; Luo, S.B.; Xia, Z. Phase separation and subsequent solidification of peritectic Fe-Cu-Ge alloys subjected to substantial undercooling processing. J. Alloys Compd. 2017, 717, 190–196. [Google Scholar]
- Wu, Y.H.; Wang, W.L.; Chang, J.; Wei, B. Evolution kinetics of microgravity facilitated spherical macrosegregation within immiscible alloys. J. Alloys Compd. 2018, 763, 808–814. [Google Scholar] [CrossRef]
- Ratke, L.; Diefenbach, S. Liquid immiscible alloys. Mater. Sci. Eng. R 1995, 15, 263–347. [Google Scholar] [CrossRef]
- Jiuzhou, Z.; Ratke, L. Kinetics of Phase Separation in a Hypermonotectic Al–Pb Alloy. Int. J. Mater. Res. 2021, 89, 241–246. [Google Scholar]
- Bünyamin, Ç. Investigations of wear properties of immiscible monotectic Al-10Bi alloy. Philos. Mag. 2023, 103, 137–152. [Google Scholar]
- Sarkar, S.; Srivastava, C.; Chattopadhyay, K. Development of a new class of high strength copper alloy using immiscibility route in Cu-Fe-Si system: Evolution of hierarchical multi-scale microstructure. Mater. Sci. Eng., A 2018, 723, 38–47. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, S.; Zhao, S.; Jin, J.; Chen, D.; Zhang, L. In-situ Fe2P reinforced bulk Cu–Fe immiscible alloy with nanotwinned Cu produced by selective laser melting. J. Alloys Compd. 2020, 838, 155592. [Google Scholar] [CrossRef]
- Peng, S.Y.; Tian, Y.Z.; Yang, Y.; Jiang, M.; Li, H.X.; Wang, J.W.; Li, S.; Qin, G.W. Achieving homogeneous Fe distribution and high strength in Cu-Fe composite consolidated by powder rolling. Mater. Sci. Eng., A 2023, 884, 145563. [Google Scholar] [CrossRef]
- Debaraj, S.; Manas, P.; Atul, S.; Sushil, M. Study on the composition effect upon the microstructure development in Cu-Fe alloys prepared using Aerodynamic Levitation. Mater. Today Commun. 2023, 37, 107288. [Google Scholar]
- Yue, S.; Qu, J.; Li, G.; Liu, S.; Guo, Z.; Jie, J.; Guo, S.; Li, T. A comprehensive investigation of carbon micro-alloying on microstructure evolution and properties of metastable immiscible Cu-Fe alloy. J. Alloys Compd. 2022, 921, 166163. [Google Scholar] [CrossRef]
- Liu, S.; Xu, S.; Jie, J.; Zhang, J.; Dong, Y.; Li, X.; Li, T. Microstructure evolution and magnetic properties of metastable immiscible Cu-Fe alloy with micro-alloying B element. J. Alloys Compd. 2021, 888, 161627. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Q.; Zhao, J. Directional solidification of monotectic composition Al–Bi alloy. Acta Metall. Sin. 2014, 50, 25–31. [Google Scholar]
- Li, H.L.; Zhao, J.Z.; Zhang, Q.X.; He, J. Microstructure Formation in a Directionally Solidified Immiscible Alloy. Metall. Mater. Trans. A 2008, 39, 3308–3316. [Google Scholar] [CrossRef]
- Ratke, L.; Thieringer, W. The influence of particle motion on Ostwald ripening in liquids. Acta Metall. 1985, 33, 1793–1802. [Google Scholar] [CrossRef]
- Rogers, J.; Davis, R. Modeling of collision and coalescence of droplets during microgravity processing of Zn-Bi immiscible alloys. Metall. Trans. A 1990, 21, 59–68. [Google Scholar] [CrossRef]
- Chatain, D.; Vahlas, C. Eustathopoulos, N., Etude des tensions interfaciales liquide-liquide et solide-liquide dans les systemes a monotectique Zn-Pb et Zn-Pb-Sn. Acta Metall. 1984, 32, 227–234. [Google Scholar] [CrossRef]
- Kaban, I.G.; Hoyer, W. Characteristics of liquid-liquid immiscibility in Al–Bi–Cu, Al–Bi–Si, and Al–Bi–Sn monotectic alloys: Differential scanning calorimetry, interfacial tension, and density difference measurements. Phys. Rev. B 2008, 77, 125426. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Galaup, C.; Cooper, K.P. Solidification of Undercooled Monotectic Alloys. MRS Proc. 1981, 9, 491–501. [Google Scholar] [CrossRef]
- Uebber, N.; Ratke, L. Undercooling and nucleation within the liquid miscibility gap of Zn-Pb alloys. Scripta Metall. Mater. 1991, 25, 1133–1137. [Google Scholar] [CrossRef]
- Gránásy, L.; Ratke, L. Homogeneous nucleation within the liquid miscibility gap of Zn-Pb alloys. Scripta Metall. Mater. 1993, 28, 1329–1334. [Google Scholar] [CrossRef]
- Jie, H.; Zhao, J.; Li, H.; Zhang, X.; Zhang, Q. Directional Solidification and Microstructural Refinement of Immiscible Alloys. Metall. Mater. Trans. A 2008, 39, 1174–1182. [Google Scholar]
- Li, H.; Zhao, J. Directional solidification of an Al-Pb alloy in a static magnetic field. Comp. Mater. Sci. 2009, 46, 1069–1075. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Jiang, H.; Zhao, J. The Influences of Micro-Alloying Element Sn and Magnetic Field on the Microstructure Evolution of Al–Bi Immiscible Alloys. Metals 2023, 13, 1867. https://doi.org/10.3390/met13111867
Chen S, Jiang H, Zhao J. The Influences of Micro-Alloying Element Sn and Magnetic Field on the Microstructure Evolution of Al–Bi Immiscible Alloys. Metals. 2023; 13(11):1867. https://doi.org/10.3390/met13111867
Chicago/Turabian StyleChen, Shu, Hongxiang Jiang, and Jiuzhou Zhao. 2023. "The Influences of Micro-Alloying Element Sn and Magnetic Field on the Microstructure Evolution of Al–Bi Immiscible Alloys" Metals 13, no. 11: 1867. https://doi.org/10.3390/met13111867



