Mechanical Properties of Microporous Copper Powder Compacts Produced by Oxide Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Compacting
2.3. Reduction/Sintering
2.4. Mechanical Testing
2.5. Characterization
3. Results
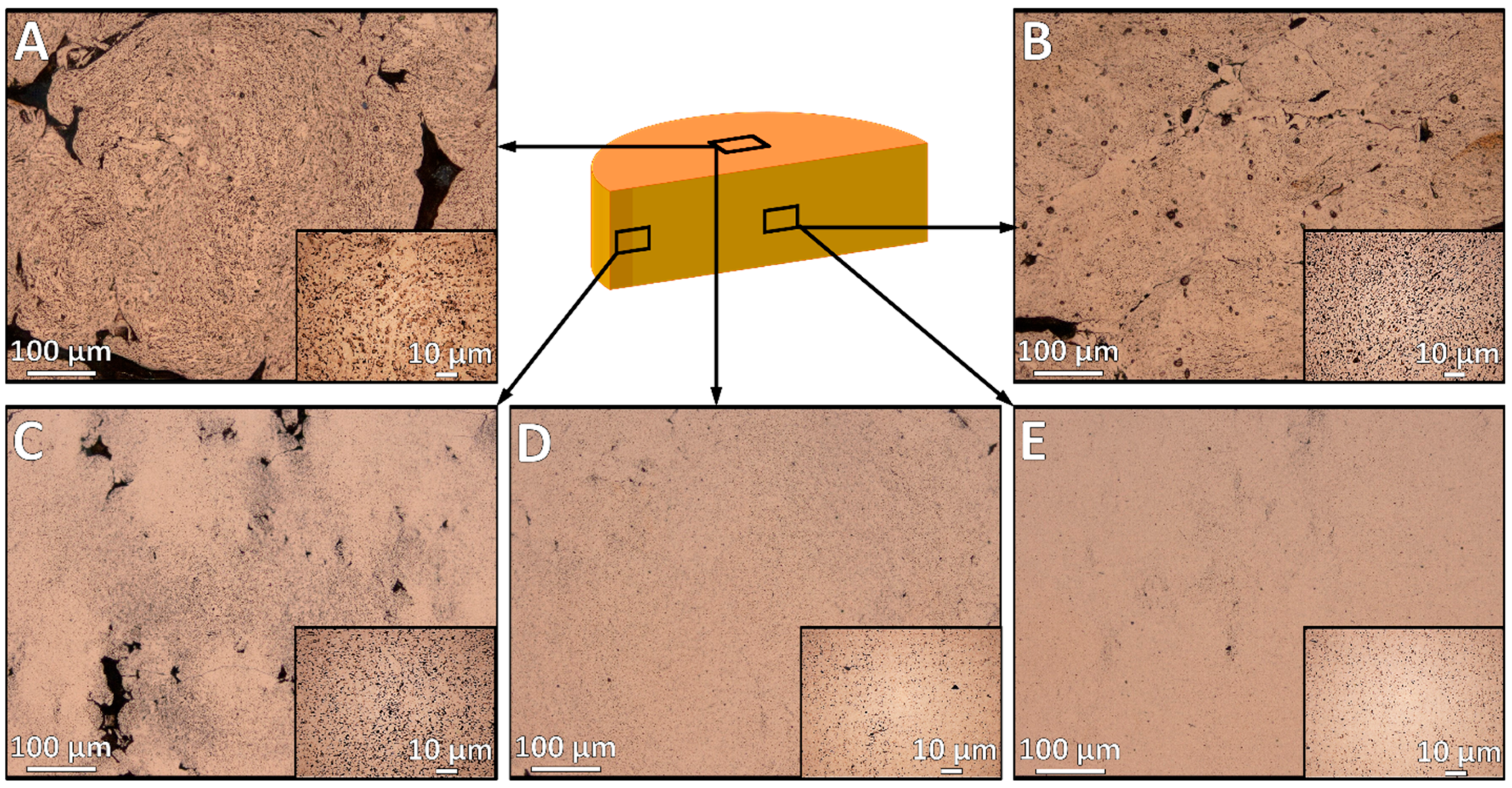
3.1. Effects of Compaction Pressure and Annealing Temperature on Pore Formation
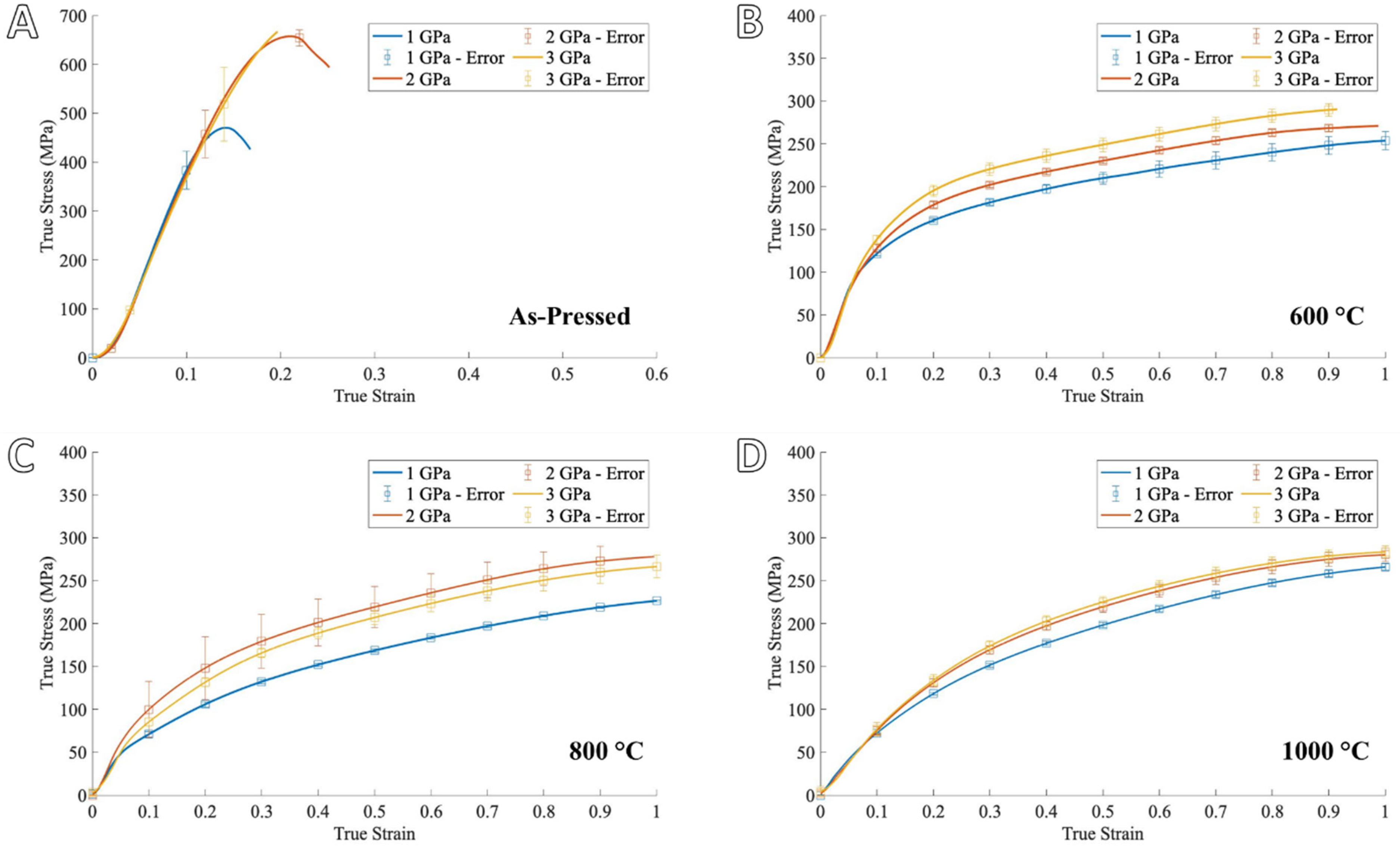
3.2. Processing Effects on Mechanical Properties of Porous Copper
3.3. Mechanical Property Comparison of Porous Cu to Wrought Cu
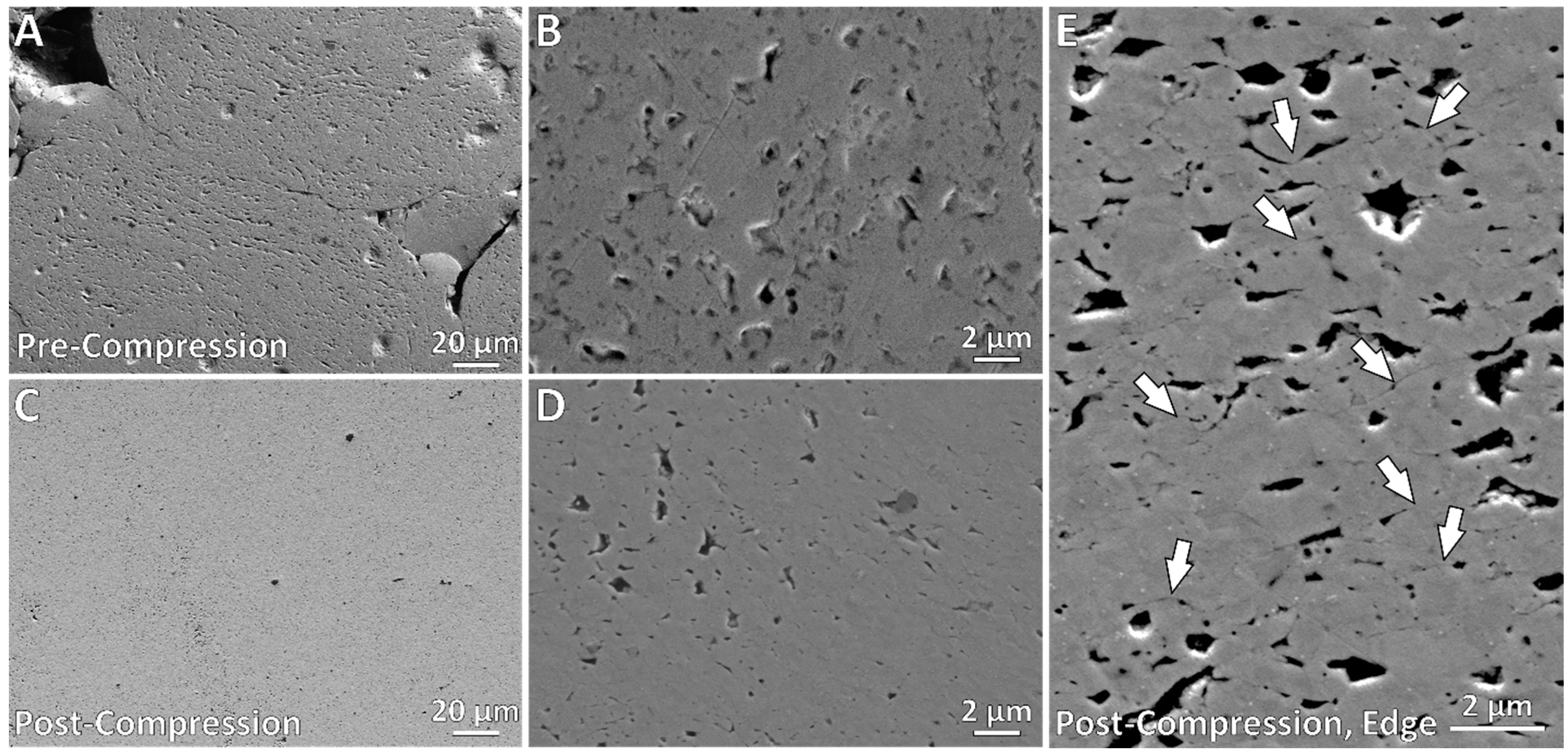
3.4. Pore Characteristics
4. Discussion
4.1. Process-Porosity Relationships
4.1.1. Compaction Pressure
4.1.2. Sintering/Reduction Temperature
4.2. Porosity-Property Relationships
5. Conclusions
- The compaction pressure does not have a strong effect on the formation of porosity, with a maximum of 6% variation across samples pressed at 1, 2, and 3 GPa.
- Higher compaction pressure results in slightly higher density before (~10%) and after (~4% higher) foaming and should be avoided for the highest porosity.
- Higher compaction pressure results in a modest increase in compressive strength (~20% or less improvement).
- Higher foaming temperature results in lower overall porosity and reduced strength (~12% drop in density and ~50% drop in strength)
- The optimum outcome for the highest porosity and good strength tested here is the 1 GPa and 600 °C condition (~40% porosity and ~75 MPa yield stress).
- Even at low sintering temperatures and higher porosity, the foamed samples outperformed wrought Cu on a per-mass basis in both quasi-static and SHPB compression (i.e., similar yield strength with 60% density).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Light-Metal Foams—History of Innovation and Technological Challenges. Adv. Eng. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Banhart, J. Aluminum foams: On the road to real applications. MRS Bull. 2003, 28, 290–295. [Google Scholar] [CrossRef]
- Atwater, M.A.; Guevara, L.N.; Darling, K.A.; Tschopp, M.A. Solid State Porous Metal Production: A Review of the Capabilities, Characteristics, and Challenges. Adv. Eng. Mater. 2018, 20, 33. [Google Scholar] [CrossRef]
- Shapovalov, V.; Boyko, L. Gasar—A new Class of Porous Materials. Adv. Eng. Mater. 2004, 6, 407–410. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, R.-H.; Kwon, H.-S. Preparation of copper foam with 3-dimensionally interconnected spherical pore network by electrodeposition. Electrochem. Commun. 2008, 10, 1148–1151. [Google Scholar] [CrossRef]
- Acierno, A.; Stevens, E.; Sedlar, T.; Kimes, K.; Chmielus, M.; Svihla, K.; Pilz, S.; Dougherty, P.; Spirka, T.A. Optimizing 3D-Printed, Reusable Metal N95 Filters by 3D Characterization and Modeling. AM&P Tech. Artic. 2021, 179, 23–26. [Google Scholar]
- Hoan, T.M.; Toan, N.V.; Hung, N.P.; Trinh, P.V.; Trung, T.B.; Phuong, D.D. Dependence of Particle Size and Geometry of Copper Powder on the Porosity and Capillary Performance of Sintered Porous Copper Wicks for Heat Pipes. Metals 2022, 12, 1716. [Google Scholar] [CrossRef]
- Parvanian, A.M.; Saadatfar, M.; Panjepour, M.; Kingston, A.; Sheppard, A.P. The effects of manufacturing parameters on geometrical and mechanical properties of copper foams produced by space holder technique. Mater. Des. 2014, 53, 681–690. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, B. On the compressive behaviour of sintered porous coppers with low to medium porosities—Part I: Experimental study. Int. J. Mech. Sci. 2005, 47, 744–756. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, E. On the compressive behavior of sintered porous coppers with low-to-medium porosities—Part II: Preparation and microstructure. Int. J. Mech. Sci. 2008, 50, 550–558. [Google Scholar] [CrossRef]
- Sharma, V.M.; Racherla, V.; Pal, S.K. Synthesis of open-cell copper foam using friction sintering. Int. J. Adv. Manuf. Technol. 2019, 103, 3163–3174. [Google Scholar] [CrossRef]
- Najdovski, I.; O’Mullane, A.P. The effect of electrode material on the electrochemical formation of porous copper surfaces using hydrogen bubble templating. J. Electroanal. Chem. 2014, 722, 95–101. [Google Scholar] [CrossRef]
- Zhao, C.; Qi, Z.; Wang, X.; Zhang, Z. Fabrication and characterization of monolithic nanoporous copper through chemical dealloying of Mg–Cu alloys. Corros. Sci. 2009, 51, 2120–2125. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, Z.F.; Zhao, H.X.; Shi, C.H.; Zhao, F. Properties and applications of shape-stabilized phase change energy storage materials based on porous material support—A review. Mater. Today Sustain. 2023, 21, 100336. [Google Scholar] [CrossRef]
- Hayat, M.A.; Ali, H.M.; Janjua, M.M.; Pao, W.; Li, C.; Alizadeh, M. Phase change material/heat pipe and Copper foam-based heat sinks for thermal management of electronic systems. J. Energy Storage 2020, 32, 101971. [Google Scholar] [CrossRef]
- Rudd, J.A.; Hernandez-Aldave, S.; Kazimierska, E.; Hamdy, L.B.; Bain, O.J.; Barron, A.R.; Andreoli, E. Investigation into the Re-Arrangement of Copper Foams Pre-and Post-CO2 Electrocatalysis. Chemistry 2021, 3, 687–703. [Google Scholar] [CrossRef]
- Ingber, T.T.; Bela, M.M.; Püttmann, F.; Dohmann, J.F.; Bieker, P.; Börner, M.; Winter, M.; Stan, M.C. Elucidating the lithium deposition behavior in open-porous copper micro-foam negative electrodes for zero-excess lithium metal batteries. J. Mater. Chem. A 2023, 11, 17828–17840. [Google Scholar] [CrossRef]
- Atwater, M.A.; Darling, K.A.; Tschopp, M.A. Towards Reaching the Theoretical Limit of Porosity in Solid State Metal Foams: Intraparticle Expansion as a Primary and Additive Means to Create Porosity. Adv. Eng. Mater. 2014, 16, 190–195. [Google Scholar] [CrossRef]
- Jung, D.; Boan, B.; Allapatt, N.; Atwater, M. Nanoporous Cu Created by the Reduction of CuO Dispersions. J. Mater. Sci. 2023, 58, 12635–12649. [Google Scholar] [CrossRef]
- Tse Lop Kun, J.E.; Rutherford, A.P.; Learn, R.S.; Atwater, M.A. Parametric Study of Planetary Milling to Produce Cu-CuO Powders for Pore Formation by Oxide Reduction. Materials 2023, 16, 5407. [Google Scholar] [CrossRef]
- ASTM B187/B187M-20; Standard Specification for Copper, Bus Bar, Rod, and Shapes and General Purpose Rod, Bar, and Shapes. ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–9.
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- ASTM. E9 (2019) Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature; ASTM International: West Conshohocken, PA, USA, 2019; p. E9. [Google Scholar]
- Elzey, D.M.; Wadley, H.N.G. The Limits Of Solid State Foaming. Acta Mater. 2001, 49, 849–859. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
- ASM International. Annealing and Recrystallization of Coppers. In ASM Handbook; Totten, G., MacKenzie, D., Eds.; ASM International: Materials Park, OH, USA, 2016; Volume 4, pp. 325–334. [Google Scholar]
- Li, P.; Chen, C.-G.; Qin, Q.; Lu, T.-X.; Shao, Y.-R.; Yang, F.; Hao, J.-J.; Guo, Z.-M. Sintering microstructure and properties of copper powder prepared by electrolyzation and atomization. J. Cent. South Univ. 2021, 28, 1966–1977. [Google Scholar] [CrossRef]
- Atwater, M.A.; Darling, K.A.; Tschopp, M.A. Solid-State Foaming by Oxide Reduction and Expansion: Tailoring the Foamed Metal Microstructure in the Cu–CuO System with Oxide Content and Annealing Conditions. Adv. Eng. Mater. 2016, 18, 83–95. [Google Scholar] [CrossRef]
- Rajak, D.K.; Linul, E. Crushing response of Composite Metallic Foams: Density and High Strain Rate effects. J. Alloys Compd. 2021, 871, 159614. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Staf, H.; Larsson, P.-L. Evaluation of an advanced powder-die frictional model. Powder Technol. 2020, 363, 569–574. [Google Scholar] [CrossRef]
- Bina, E.P.; Haghighat, H. Limit analysis of plane strain compression of cylindrical billets between flat dies. Int. J. Adv. Manuf. Technol. 2021, 116, 3561–3570. [Google Scholar] [CrossRef]
- Meyers, M.; Chawla, K. Mechanical Behavior of Materials, 2nd ed.; Cambridge University Press: New York, NY, USA, 2009; p. 187. [Google Scholar]
- Dixit, M.; Srivastava, R. Effect of compaction pressure on microstructure, density and hardness of Copper prepared by Powder Metallurgy route. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Mechanical, Materials and Renewable Energy, Sikkim, India, 8–10 December 2017; IOP Publishing Ltd.: Bristol, UK, 2017; p. 012209. [Google Scholar]
- Fischmeister, H.F. Powder compaction: Fundamentals and recent developments. Proc. Inst. Mech. Eng. 1982, 196, 105–121. [Google Scholar] [CrossRef]
- Abedi, M.; Asadi, A.; Vorotilo, S.; Mukasyan, A.S. A critical review on spark plasma sintering of copper and its alloys. J. Mater. Sci. 2021, 56, 19739–19766. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Pérez, M.; Frenkel, A.I. Reduction of CuO in H2: In situ time-resolved XRD studies. Catal. Lett. 2003, 85, 247–254. [Google Scholar] [CrossRef]
- Atwater, M.A.; Corcoran, S.G. Using Powder Metallurgy and Oxide Reduction to Produce Eco-Friendly Bulk Nanoporous Nickel. Adv. Eng. Mater. 2023, 25, 2201629. [Google Scholar] [CrossRef]
- Taylor, S.L.; Jakus, A.E.; Shah, R.N.; Dunand, D.C. Iron and Nickel Cellular Structures by Sintering of 3D-Printed Oxide or Metallic Particle Inks. Adv. Eng. Mater. 2017, 19, 1600365. [Google Scholar] [CrossRef]
- Ashby, M.F. Mechnical properties of cellular solids. Metall. Trans. A 1983, 14A, 1755–1769. [Google Scholar] [CrossRef]
- Gibson, L. Mechanical behavior of metallic foams. Annu. Rev. Mater. Sci. 2000, 30, 191–227. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Li, W.; He, J.; Li, C.; Dai, S.; Chen, J.; Ren, Y. Study on the Compression Properties and Deformation Failure Mechanism of Open-Cell Copper Foam. Adv. Eng. Mater. 2017, 19, 1600861. [Google Scholar] [CrossRef]
- El-Hadek, M.A.; Kaytbay, S. Mechanical and physical characterization of copper foam. Int. J. Mech. Mater. Des. 2008, 4, 63–69. [Google Scholar] [CrossRef]
- Simone, A.; Gibson, L. The compressive behaviour of porous copper made by the GASAR process. J. Mater. Sci. 1997, 32, 451–457. [Google Scholar] [CrossRef]
- Li, Z.; Yang, T.; Jin, Q.; Li, Z.; Jiang, Y.; Zhou, R. Compressive behaviours of lotus-type porous copper fabricated by Gasar process. Procedia Eng. 2012, 31, 337–342. [Google Scholar] [CrossRef]
- Marx, J.; Portanova, M.; Rabiei, A. Performance of Composite Metal Foam Armors against Various Threat Sizes. J. Compos. Sci. 2020, 4, 176. [Google Scholar] [CrossRef]
- Movahedi, N.; Fiedler, T.; Taşdemirci, A.; Murch, G.E.; Belova, I.V.; Güden, M. Impact loading of functionally graded metal syntactic foams. Mater. Sci. Eng. A 2022, 839, 142831. [Google Scholar] [CrossRef]
- Liu, X.-H.; Huang, H.-Y.; Xie, J.-X. Effect of strain rate on the compressive deformation behaviors of lotus-type porous copper. Int. J. Miner. Metall. Mater. 2014, 21, 687–695. [Google Scholar] [CrossRef]
- Iqbal, N.; Xue, P.; Liao, H.J.; Wang, B.; Li, Y.L. Material characterization of porous bronze at high strain rates. Mater. Sci. Eng. A 2011, 528, 4408–4412. [Google Scholar] [CrossRef]
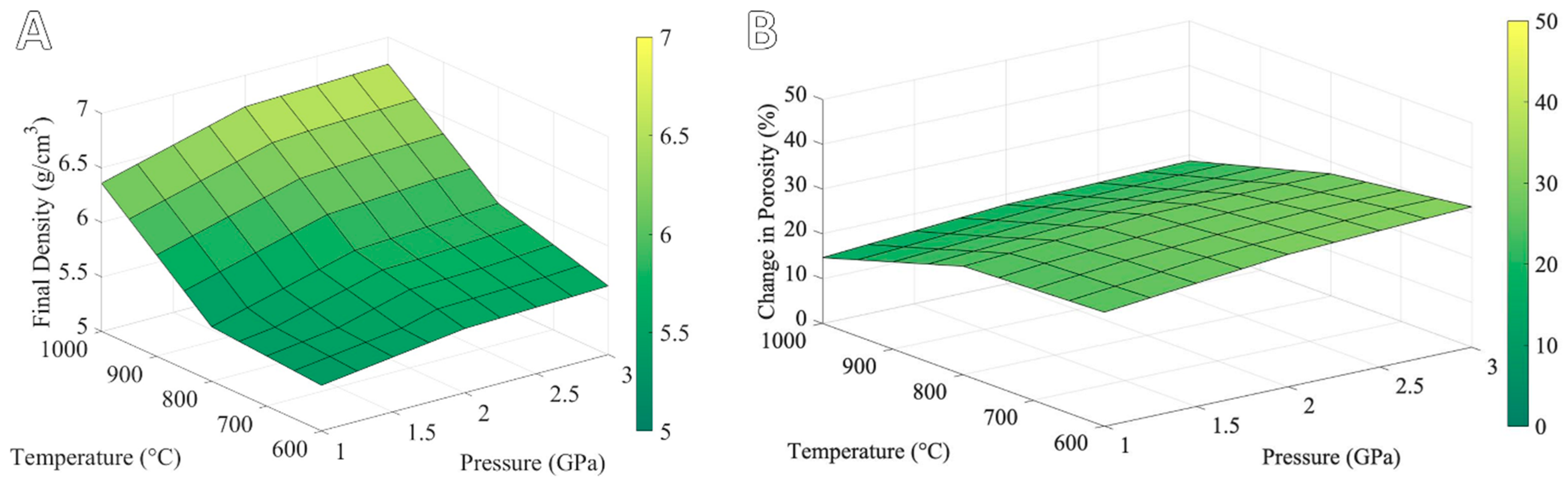



| Pressure (GPa) | Pore Formation Temperature (°C) | Density, before (g/cm3) | Density, after (g/cm3) | Change in Porosity (%) |
|---|---|---|---|---|
| 1 | 600 | 7.69 ± 0.02 | 5.42 ± 0.06 | 25.40 ± 0.46 |
| 800 | 7.67 ± 0.04 | 5.50 ± 0.06 | 24.21 ± 0.99 | |
| 1000 | 7.67 ± 0.02 | 6.36 ± 0.05 | 14.66 ± 0.44 | |
| 2 | 600 | 8.23 ± 0.01 | 5.59 ± 0.05 | 29.52 ± 0.60 |
| 800 | 8.26 ± 0.05 | 5.85 ± 0.04 | 26.94 ± 0.08 | |
| 1000 | 8.30 ± 0.06 | 6.71 ± 0.05 | 17.72 ± 0.20 | |
| 3 | 600 | 8.44 ± 0.02 | 5.63 ± 0.04 | 31.92 ± 0.60 |
| 800 | 8.38 ± 0.04 | 5.93 ± 0.03 | 27.26 ± 0.28 | |
| 1000 | 8.42 ± 0.01 | 6.75 ± 0.07 | 18.68 ± 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse Lop Kun, J.; Patterson, E.; Learn, R.; Atwater, M. Mechanical Properties of Microporous Copper Powder Compacts Produced by Oxide Reduction. Metals 2023, 13, 1942. https://doi.org/10.3390/met13121942
Tse Lop Kun J, Patterson E, Learn R, Atwater M. Mechanical Properties of Microporous Copper Powder Compacts Produced by Oxide Reduction. Metals. 2023; 13(12):1942. https://doi.org/10.3390/met13121942
Chicago/Turabian StyleTse Lop Kun, Julian, Emma Patterson, Ryan Learn, and Mark Atwater. 2023. "Mechanical Properties of Microporous Copper Powder Compacts Produced by Oxide Reduction" Metals 13, no. 12: 1942. https://doi.org/10.3390/met13121942





