Recent Development of Low-Cost β-Ti Alloys for Biomedical Applications: A Review
Abstract
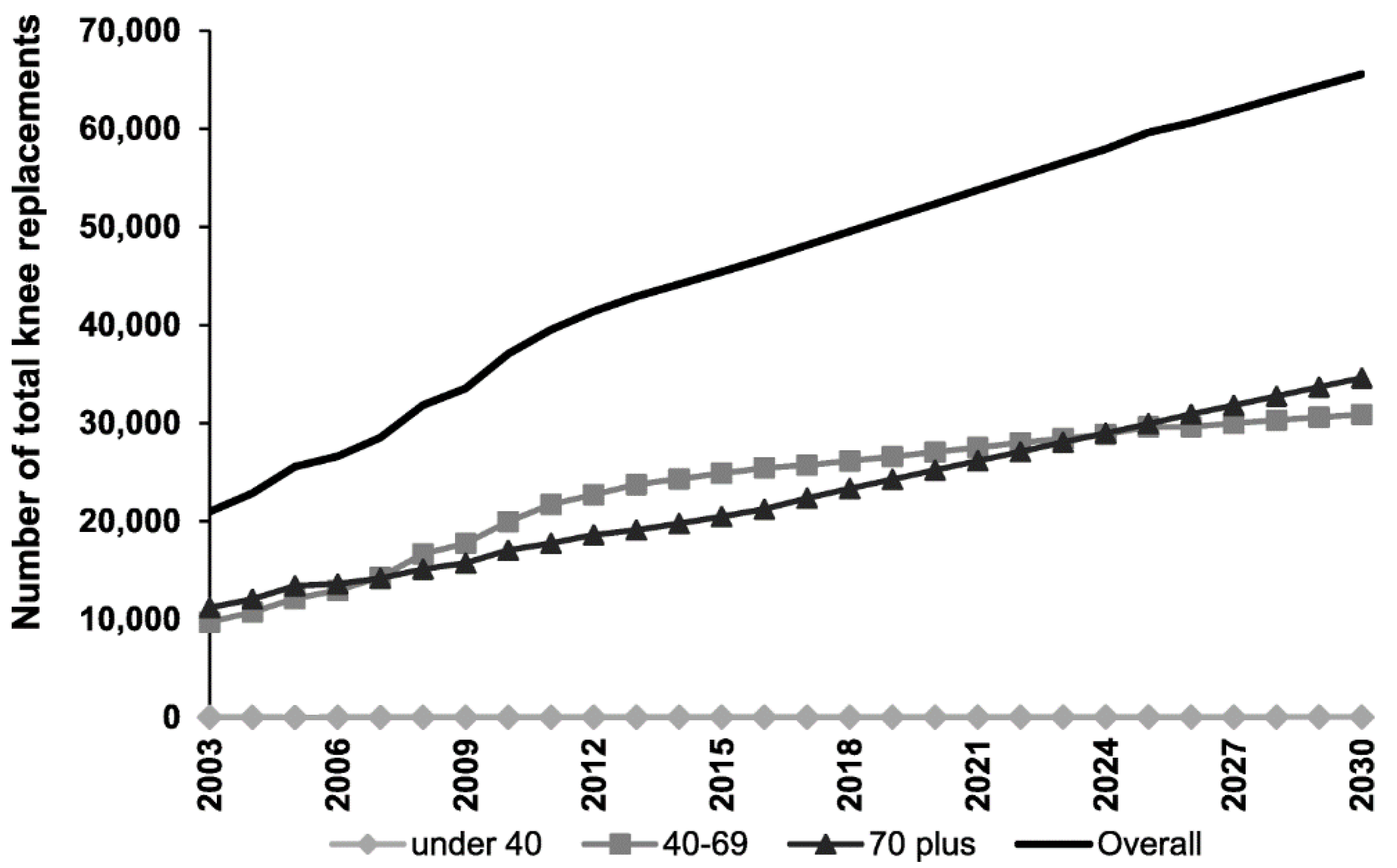
1. Introduction
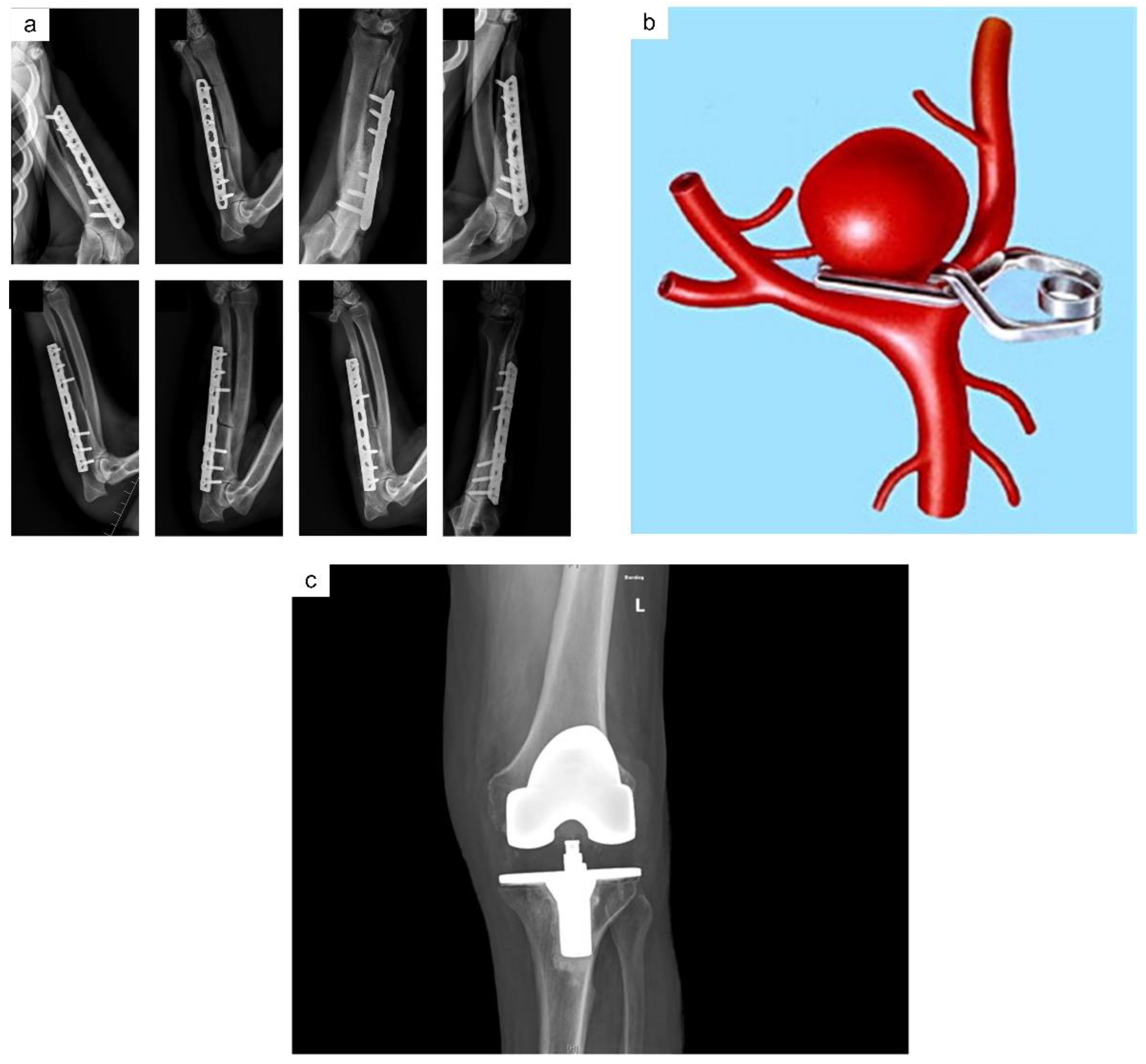
2. Titanium Alloys—A Highly Desirable Biomaterial for Implants
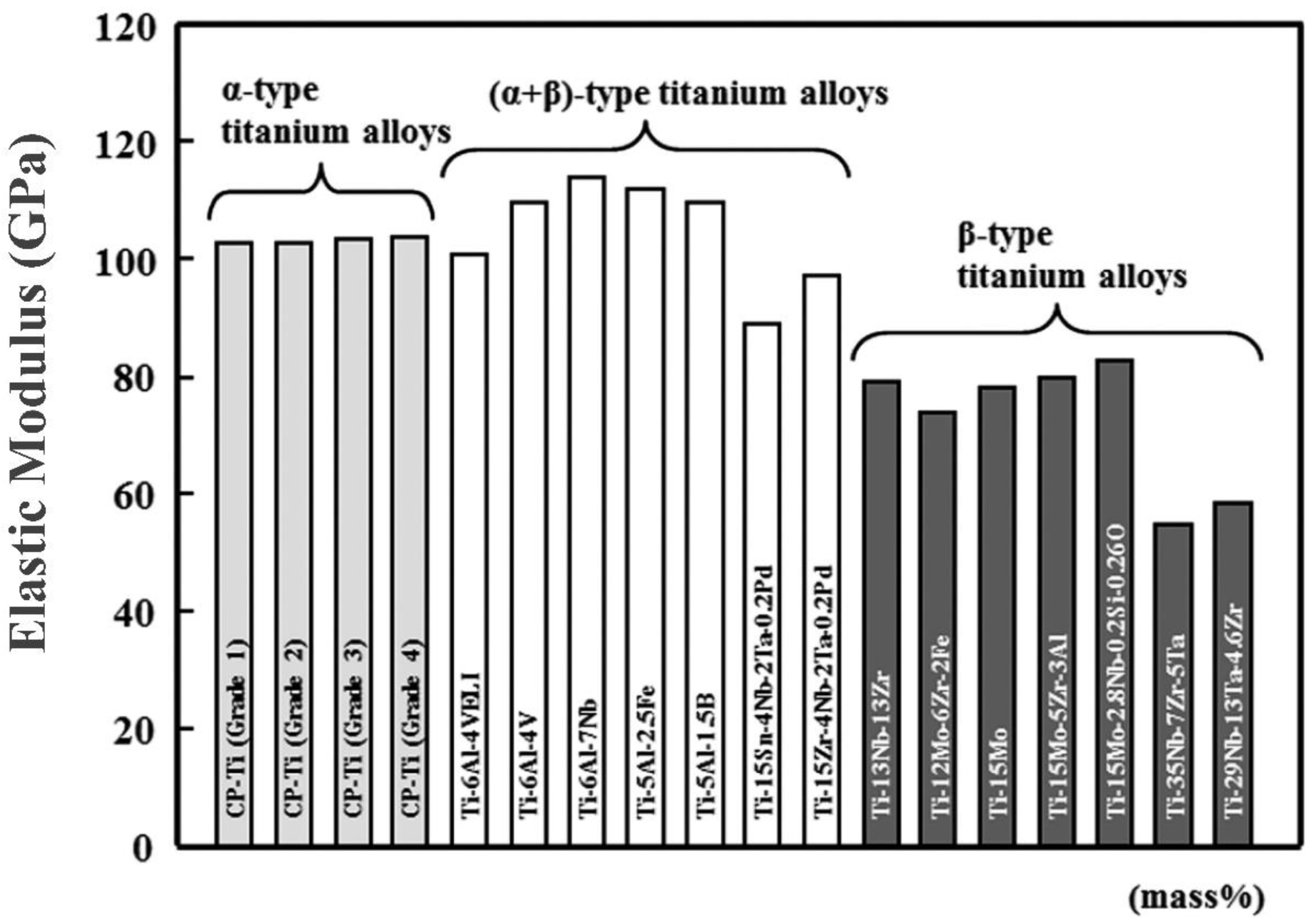
2.1. α-Alloy
2.2. β-Alloy
2.3. α + β. Ti Alloy
3. Low-Cost Alloying Elements
3.1. Molybdenum (Mo)
| Alloy | Process | Phase Constituent | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|---|
| Tensile/Compressive/Bending Strength (MPa) | Yield Strength (MPa) | Hardness (HV) | E (GPa) | ||||
| Ti6Al4V | Forged | α + β | 895–930 | 825–860 | 110 | [65] | |
| Ti-7.5Mo | Casting | α” | 1395 | - | 263 | 55 | [66] |
| Ti-15Mo | Casting | β | 921 | 745 | 84 | [60] | |
| Ti-12Mo-3Nb | Solution Treated | β | 747 | 449 | - | 105 | [67] |
| Ti-12Mo-5Ta | Solution Treated | β | 74 | [68] | |||
| Ti-20Zr-15Mo | As cast | β | 801 | - | 300 | 67 | [69] |
| Ti-20Zr-10Mo | TMT | β | - | - | 375–380 | 79 | [69] |
| Ti-15Mo-1Si | β | 181 | 18 | [70] | |||
| Ti-6Mo-3Sn | Solution Treated | ω + β + α” | 320 | - | [61] | ||
3.2. Iron (Fe)
3.3. Manganese (Mn)
3.4. Zirconium (Zr)
3.5. Copper (Cu)
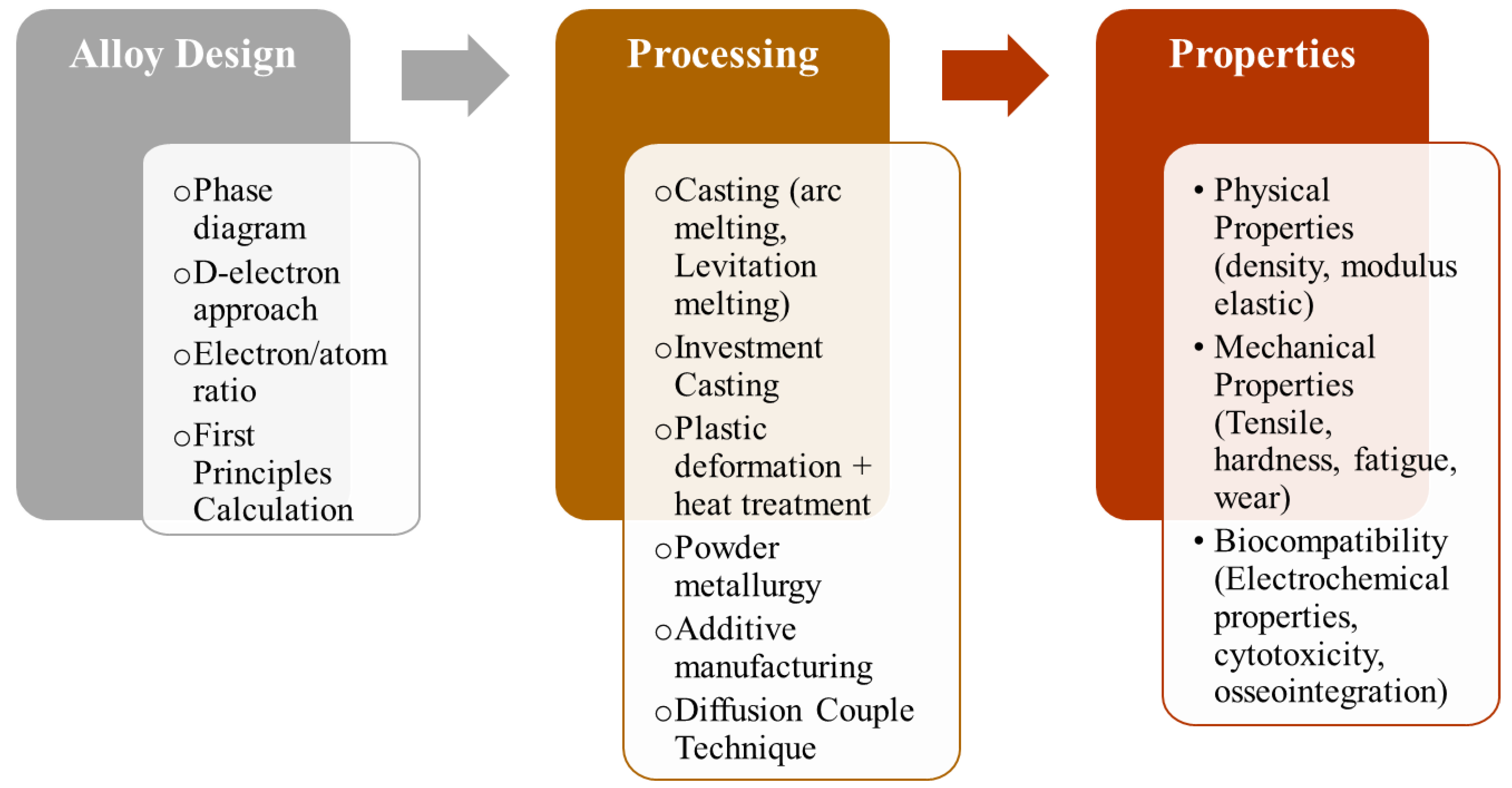
4. Processing Routes to Fabricate β Ti Alloy Biomedical Components
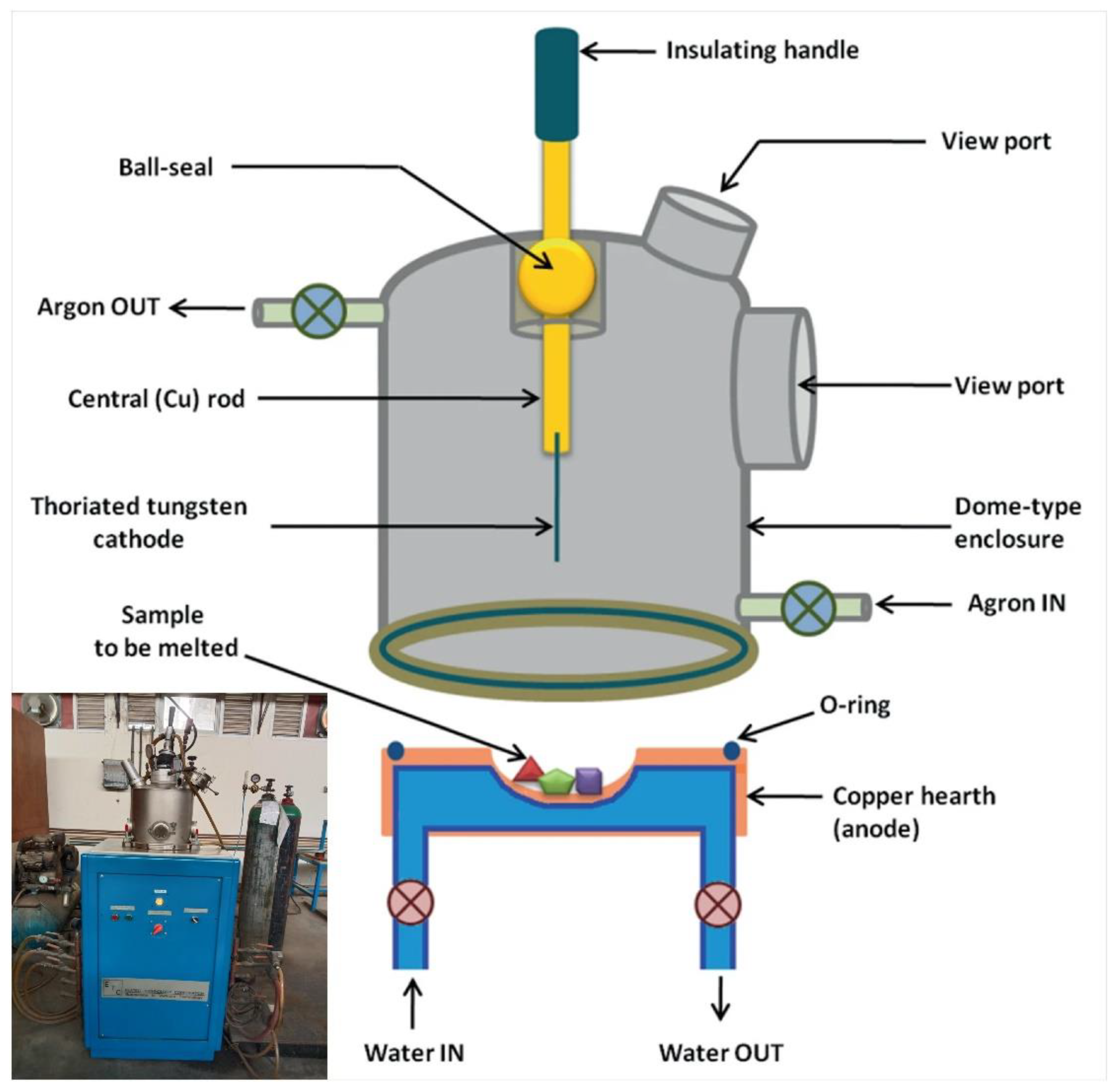
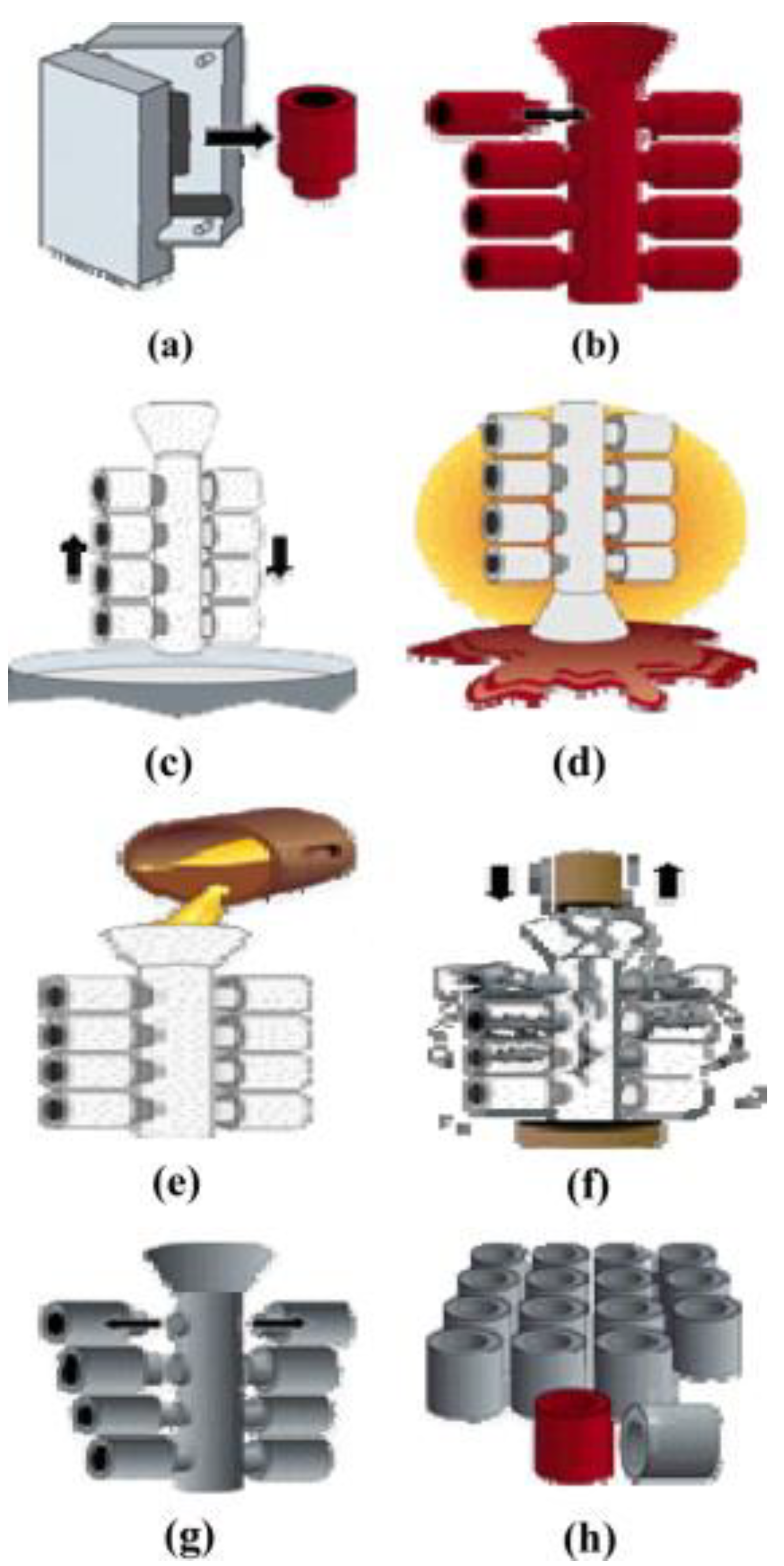
4.1. Arc Melting
4.2. Thermomechanical Processing
4.3. Investment Casting
4.4. Powder Metallurgy
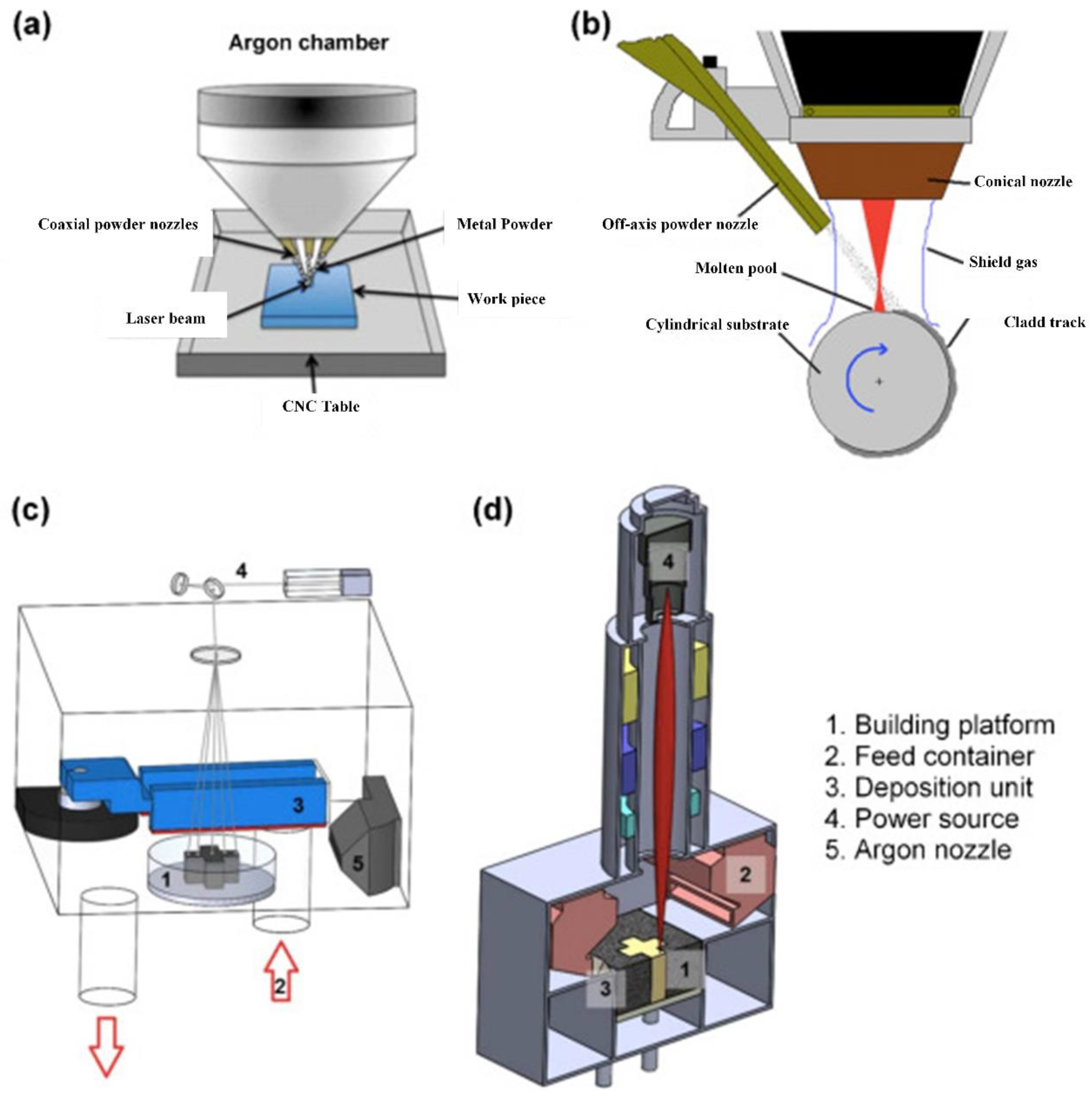
4.5. Additive Manufacturing
4.6. Diffusion Couple
5. Properties of Ti Alloys with Low-Cost Alloying Elements
6. Summary and Future Works
- In previous studies, most alloy designs were based on the calculation of Mo equivalent. A high Mo equivalent does not necessarily produce titanium alloys with high mechanical strength and low elastic modulus. Titanium alloy design studies with different approaches, such as density functional theory, can be carried out to complement existing alloy design methods, such as the Mo equivalent and the Bo and Md methods and compare the results with experiments to give a better understanding of the relationship between alloying elements, processing, and final properties.
- Additive manufacturing is the most advanced manufacturing process today. However, the resulting mechanical properties can still not compete with conventional thermomechanical treatment processes that offer flexibility for tailoring microstructure and mechanical properties. Combining the additive manufacturing process and the heat treatment process can be an interesting idea to optimize the microstructure of the additive manufacturing results to produce better mechanical properties.
- Each alloying element added to a titanium alloy has its characteristics, for example, melting point. The large gap in melting point between titanium and its alloying element will affect the alloying process and manufacturing method. The difference in atomic size between Ti and its alloying elements will also affect the final crystal structure formed. Studying the effect of alloying elements with different characteristics on the manufacturing process is necessary to understand further what process is most appropriate for a particular type of alloy.
- Most previous studies only focused on mechanical properties in the form of tensile or compressive strength and elastic modulus. A fatigue test is required for permanent bone implants because the implant material will be used for a very long time, up to many years. Thus, future studies on fatigue tests on low-cost titanium materials are needed to understand the relationship between microstructure, processing, tensile strength, elastic modulus, and fatigue properties of biomedical implant material.
- Titanium for biomaterial applications will be used in the body environment. Mechanical testing such as fatigue in a system that simulates body conditions with the addition of simulated body fluid and temperature suitable for the human body can provide an overview of the mechanical properties of biomaterials when applied within the human body.
- Additional research is to be conducted to develop surface modification processes which can improve biocompatibility and osseointegration between implants and bone.
- Most in-vitro studies on cells are only carried out over a few days, making it difficult to understand the interactions between implants and cells. It is necessary to add an immersion test for a more extended period of up to several months to determine whether ions are released from the implant surface and how far this affects the toxicity of the implant material.
- Further studies on the bioactivity of cells formed after in-vitro tests are required. Observations of genetic changes in cells can be carried out to find out whether there is an interaction between cells and implant material.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| TKR | Total knee replacement |
| UTS | Ultimate tensile strength |
| HCP | Hexagonal closed-packed |
| BCC | Body centred cubic |
| PM | Powder metallurgy |
| ELI | Extra low interstitial |
| EBM | Electron beam melting |
| SLM | Selective laser melting |
| DED | Direct energy deposition |
| SME | Shape memory effect |
| TNTZ | Ti-29Nb-13Ta-4.6Zr |
| VAR | Vacuum arc remelting |
| CP | Commercially pure |
| V | Vanadium |
| Al | Aluminium |
| Ti | Titanium |
References
- Gepreel, M.A.H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J. Titanium Today: Medical Edition. In Titanium Today; International Titanium Association: Northglenn, CO, USA, 2020. [Google Scholar]
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; de Steiger, R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 20 October 2022).
- Ronoh, F.M.K.; Dabees, S.; Sobola, D. Advances in Sustainable Grinding of Different Types of the Titanium biomaterials for Medical Applications: A Review. Biomed. Eng. Adv. 2022, 4, 100047. [Google Scholar] [CrossRef]
- Zheng, B.W.; Dong, F.Y.; Zhang, Y.; Huang, H.J.; Yuan, X.G.; Zuo, X.J.; Luo, L.S.; Su, Y.Q.; Wang, L.; Han, B.S.; et al. Microstructure, mechanical properties and deformation behavior of new V-free low-cost Ti-6Al-xFe-yCr alloys. Mater. Res. Express 2019, 6, 026551. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.H. A review on alloy design, biological response, and strengthening of beta-titanium alloys as biomaterials. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 121, 16. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa, P.N.; Pessoa, J.C. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Gouda, M.K.; Salman, S.A.; Ebied, S.; Ashmawy, A.M.; Gepreel, M.A.H.; Chiba, A. Biocompatibility and corrosion resistance of low-cost Ti-14Mn-Zr alloys. J. Mater. Res. 2021, 36, 4883–4893. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Liu, H.H.; Cho, K.; Nakai, M.; Itoh, Y.; Narushima, T.; Ikeda, M. Fabrication of low-cost beta-type Ti-Mn alloys for biomedical applications by metal injection molding process and their mechanical properties. J. Mech. Behav. Biomed. Mater. 2016, 59, 497–507. [Google Scholar] [CrossRef]
- Lin, J.H.C.; Fu, Y.H.; Chen, Y.C.; Peng, Y.P.; Ju, C.P. Solution treatment-delayed zirconium-strengthening behavior in Ti-7.5Mo-xZr alloy system. Mater. Res. Express 2018, 5, 9. [Google Scholar] [CrossRef]
- Santhosh, R.; Geetha, M.; Rao, M.N. Recent Developments in Heat Treatment of Beta Titanium Alloys for Aerospace Applications. Trans. Indian Inst. Met. 2017, 70, 1681–1688. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Sutowo, C.; Senopati, G.; Pramono, A.W.; Supriadi, S.; Suharno, B. Microstructures, mechanical properties, and corrosion behavior of novel multi-component Ti-6Mo-6Nb-xSn-xMn alloys for biomedical applications. Aims Mater. Sci. 2020, 7, 192–202. [Google Scholar] [CrossRef]
- Pope, J.J.; Calvert, E.L.; Weston, N.S.; Jackson, M. FAST-DB: A novel solid-state approach for diffusion bonding dissimilar titanium alloy powders for next generation critical components. J. Mater. Process. Technol. 2019, 269, 200–207. [Google Scholar] [CrossRef]
- Rashid, R.A.R.; Palanisamy, S.; Attar, H.; Bermingham, M.; Dargusch, M.S. Metallurgical features of direct laser-deposited Ti6Al4V with trace boron. J. Manuf. Process. 2018, 35, 651–656. [Google Scholar] [CrossRef]
- Liang, S.X. Review of the Design of Titanium Alloys with Low Elastic Modulus as Implant Materials. Adv. Eng. Mater. 2020, 22, 2000555. [Google Scholar] [CrossRef]
- Sarraf, M.; Ghomi, E.R.; Alipour, S.; Ramakrishna, S.; Sukiman, N.L. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2022, 5, 371–395. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Gurappa, I. Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact. 2002, 49, 73–79. [Google Scholar] [CrossRef]
- Behrens, B.A.; Wirth, C.J.; Windhagen, H.; Nolte, I.; Meyer-Lindenberg, A.; Bouguecha, A. Numerical investigations of stress shielding in total hip prostheses. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2008, 222, 593–600. [Google Scholar] [CrossRef]
- Cristofolini, L.; Juszczyk, M.; Taddei, F.; Field, R.E.; Rushton, N.; Viceconti, M. Stress shielding and stress concentration of contemporary epiphyseal hip prostheses. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2009, 223, 27–44. [Google Scholar] [CrossRef]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone Loss and Reduced Bone Quality of the Human Femur after Total Hip Arthroplasty under Stress-Shielding Effects by Titanium-Based Implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef]
- Chanlalit, C.; Shukla, D.R.; Fitzsimmons, J.S.; An, K.N.; O’Driscoll, S.W. Stress Shielding Around Radial Head Prostheses. J. Hand Surg. 2012, 37, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.C.; Xu, H.T.; Ding, H.L.; Qin, H.; An, Z.Q. Comparison of the effect on bone healing process of different implants used in minimally invasive plate osteosynthesis: Limited contact dynamic compression plate versus locking compression plate. Sci. Rep. 2016, 6, 37902. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Barry, M.; Akkielah, L.; Askar, M.A.; Bin Nasser, A.S. Miliary tuberculosis with delayed-onset total knee arthroplasty Mycobacteria tuberculosis infection successfully treated with medical therapy alone: A case report and literature review. Knee 2019, 26, 1152–1158. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Albrektsson, B.C.T.; Mölne, J.; Wennerberg, A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Eur. J. Oral Implantol. 2018, 11, 37–46. [Google Scholar]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Leyens, M.P.C. Titanium and Titanium Alloys; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Bailor, J.; Li, T.; Prima, F.; Boehlert, C.J.; Devaraj, A. A review of the metastable omega phase in beta titanium alloys: The phase transformation mechanisms and its effect on mechanical properties. Int. Mater. Rev. 2022, 67, 1–20. [Google Scholar] [CrossRef]
- Cotton, J.D.; Briggs, R.D.; Boyer, R.R.; Tamirisakandala, S.; Russo, P.; Shchetnikov, N.; Fanning, J.C. State of the Art in Beta Titanium Alloys for Airframe Applications. Jom 2015, 67, 1281–1303. [Google Scholar] [CrossRef]
- Lu, J.W.; Ge, P.; Zhao, Y.Q. Recent Development of Effect Mechanism of Alloying Elements in Titanium Alloy Design. Rare Metal Mat. Eng. 2014, 43, 775–779. [Google Scholar] [CrossRef]
- Froes, F.H. Titanium—Physical Metallurgy, Processing, and Applications; ASM International: Materials Park, OH, USA, 2015. [Google Scholar]
- Bolzoni, L.; Raynova, S.; Yang, F. Strengthening mechanisms of Ti via Al addition. J. Alloys Compd. 2020, 820, 153447. [Google Scholar] [CrossRef]
- Xu, X.J.; Lin, J.P.; Han, D.D. Effect of Al on Microstructures and Properties of Ti-45Al-8.5Nb-0.2B-0.2W Alloy. In Proceedings of Chinese Materials Congress (CMC 2012), Taiyuan, China, 13–18 July 2012; pp. 44–49. [Google Scholar]
- Najafizadeh, M.; Bozorg, M.; Bahadoran, A.; Liang, J.M.; Zhang, D.L. Compressive and biocorrosion properties of Ti-XAl-2Fe-3Cu alloys fabricated by powder metallurgy. J. Alloys Compd. 2021, 884, 161079. [Google Scholar] [CrossRef]
- Alshammari, Y.; Manogar, B.; Raynova, S.; Yang, F.; Bolzoni, L. Behaviour of novel low-cost blended elemental Ti-5Fe-xAl alloys fabricated via powder metallurgy. J. Mech. Behav. Biomed. Mater. 2020, 110, 103865. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.S.; Araujo, R.O.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. Influence of Oxygen Content and Microstructure on the Mechanical Properties and Biocompatibility of Ti-15 wt%Mo Alloy Used for Biomedical Applications. Materials 2014, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wong, S.M.; Wong, H.M.; Wu, S.L.; Hu, T.; Yeung, K.W.K.; Chu, P.K. Effects of Carbon and Nitrogen Plasma Immersion Ion Implantation on In vitro and In vivo Biocompatibility of Titanium Alloy. ACS Appl. Mater. Interfaces 2013, 5, 1510–1516. [Google Scholar] [CrossRef]
- Cui, W.F.; Qin, G.W.; Duan, J.Z.; Wang, H. A graded nano-TiN coating on biomedical Ti alloy: Low friction coefficient, good bonding and biocompatibility. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 520–528. [Google Scholar] [CrossRef]
- Mat-Baharin, N.H.; Razali, M.; Mohd-Said, S.; Syarif, J.; Muchtar, A. Influence of alloying elements on cellular response and in-vitro corrosion behavior of titanium-molybdenum-chromium alloys for implant materials. J. Prosthodont. Res. 2020, 64, 490–497. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Singh, S.B.; Chakraborty, M. Elastic modulus of biomedical titanium alloys by nano-indentation and ultrasonic techniques—A comparative study. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2008, 489, 419–425. [Google Scholar] [CrossRef]
- Hon, Y.H.; Wang, J.Y.; Pan, Y.N. Composition/phase structure and properties of titanium-niobium alloys. Mater. Trans. 2003, 44, 2384–2390. [Google Scholar] [CrossRef]
- Anene, F.A.; Jaafar, C.N.A.; Zainol, I.; Hanim, M.A.A.; Suraya, M.T. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. Part C-J. Mech. Eng. Sci. 2021, 235, 3792–3805. [Google Scholar] [CrossRef]
- Bondy, S.C. The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology 2010, 31, 575–581. [Google Scholar] [CrossRef]
- Verstraeten, S.; Aimo, L.; Oteiza, P. Aluminium and lead: Molecular mechanisms of brain toxicity. Arch. Toxicol. 2008, 82, 789–802. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium and tungsten derivatives as antidiabetic agents—A review of their toxic effects. Biol. Trace Elem. Res. 2002, 88, 97–112. [Google Scholar] [CrossRef]
- Byrne, A.R.; Kosta, L. Vanadium in Foods and in Human-Body Fluids and Tissues. Sci. Total Environ. 1978, 10, 17–30. [Google Scholar] [CrossRef]
- Nilaco. Pure Metals. Available online: https://shop.nilaco.jp/en/order/?large_category=1 (accessed on 11 October 2022).
- Ahmed, M.; Wexler, D.; Casillas, G.; Ivasishin, O.M.; Pereloma, E.V. The influence of beta phase stability on deformation mode and compressive mechanical properties of Ti-10V-3Fe-3Al alloy. Acta Mater. 2015, 84, 124–135. [Google Scholar] [CrossRef]
- Guo, S.F.; Liu, Z.; Chan, K.C.; Chen, W.; Zhang, H.; Wang, J.F.; Yu, P. A plastic Ni-free Zr-based bulk metallic glass with high specific strength and good corrosion properties in simulated body fluid. Mater. Lett. 2012, 84, 81–84. [Google Scholar] [CrossRef]
- Hezil, N.; Aissani, L.; Fellah, M.; Samad, M.A.; Obrosov, A.; Timofei, C.; Marchenko, E. Structural, and tribological properties of nanostructured alpha plus beta type titanium alloys for total hip. J. Mater. Res. Technol.-JMRT 2022, 19, 3568–3578. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cui, Y.W.; Zhang, L.C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Holden, F.C.; Ogden, H.R.; Jaffee, R.I. Heat Treatment and Mechanical Properties of Ti-Mo Alloys. Trans. Am. Inst. Min. Metall. Eng. 1956, 206, 1388–1393. [Google Scholar] [CrossRef]
- Ho, W.F. A comparison of tensile properties and corrosion behavior of cast Ti-7.5Mo with c.p. Ti, Ti-15Mo and Ti-6Al-4V alloys. J. Alloys Compd. 2008, 464, 580–583. [Google Scholar] [CrossRef]
- Hashmi, M.L.; Wadood, A. Microstructural, mechanical and shape memory characterizations of Ti-Mo-Sn alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 688–700. [Google Scholar] [CrossRef]
- Biesiekierski, J.W.A.; Gepreel, M.A.-H.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Lee, E.B.; Han, M.K.; Kim, B.J.; Song, H.J.; Park, Y.J. Effect of molybdenum on the microstructure, mechanical properties and corrosion behavior of Ti alloys. Int. J. Mater. Res. 2014, 105, 847–853. [Google Scholar] [CrossRef]
- Lourenco, M.L.; Cardoso, G.C.; Sousa, K.D.J.; Donato, T.A.G.; Pontes, F.M.L.; Grandini, C.R. Development of novel Ti-Mo-Mn alloys for biomedical applications. Sci. Rep. 2020, 10, 6298. [Google Scholar] [CrossRef]
- ASTM F1472; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications. ASTM: West Conshohocken, PA, USA, 2020.
- Ho, W.F.; Ju, C.P.; Lin, J.H.C. Structure and properties of cast binary Ti-Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Panaino, J.V.P.; Santos, I.D.; Araujo, L.S.; Mei, P.R.; de Almeida, L.H.; Nunes, A. Characterization of a new beta titanium alloy, Ti-12Mo-3Nb, for biomedical applications. J. Alloys Compd. 2012, 536, S208–S210. [Google Scholar] [CrossRef]
- Gordin, D.M.; Gloriant, T.; Nemtoi, G.; Chelariu, R.; Aelenei, N.; Guillou, A.; Ansel, D. Synthesis, structure and electrochemical behavior of a beta Ti-12Mo-5Ta alloy as new biomaterial. Mater. Lett. 2005, 59, 2936–2941. [Google Scholar] [CrossRef]
- Qi, M.X.; Chen, B.H.; Xia, C.Q.; Liu, Y.; Liu, S.G.; Zhong, H.; Zou, X.R.; Yang, T.; Li, Q. Microstructure, mechanical properties and biocompatibility of novel Ti-20Zr-xMo alloys. J. Alloys Compd. 2021, 888, 161478. [Google Scholar] [CrossRef]
- Verestiuc, L.; Spataru, M.C.; Baltatu, M.S.; Butnaru, M.; Solcan, C.; Sandu, A.V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti-Mo-Si materials for bone prosthesis applications. J. Mech. Behav. Biomed. Mater. 2021, 113, 104198. [Google Scholar] [CrossRef]
- Eleanor Whitney, S.R.R.; Crowe, T.; Cameron-Smith, D.; Walsh, A. Understanding Nutrition; Cengage Learning: Victoria, Australia, 2013. [Google Scholar]
- Polonis, D.H.; Parr, J.G. Martensite Formation in Powders and Lump Specimens of Ti-Fe Alloys. Trans. Am. Inst. Min. Metall. Eng. 1955, 203, 64–72. [Google Scholar] [CrossRef]
- Chen, B.Y.; Hwang, K.S.; Ng, K.L. Effect of cooling process on the alpha phase formation and mechanical properties of sintered Ti-Fe alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2011, 528, 4556–4563. [Google Scholar] [CrossRef]
- Lin, D.J.; Lin, J.H.C.; Ju, C.P. Structure and properties of Ti-7.5Mo-xFe alloys. Biomaterials 2002, 23, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Abdelrhman, Y.; Gepreel, M.A.H.; Kobayashi, S.; Okano, S.; Okamoto, T. Biocompatibility of new low-cost (alpha plus beta)-type Ti-Mo-Fe alloys for long-term implantation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 552–562. [Google Scholar] [CrossRef]
- Ho, W.F.; Pan, C.H.; Wu, S.C.; Hsu, H.C. Mechanical properties and deformation behavior of Ti-5Cr-xFe alloys. J. Alloys Compd. 2009, 472, 546–550. [Google Scholar] [CrossRef]
- Nakajima, H.; Ohshida, S.; Nonaka, K.; Yoshida, Y.; Fujita, F.E. Diffusion of iron in beta Ti-Fe alloys. Scr. Mater. 1996, 34, 949–953. [Google Scholar] [CrossRef]
- Bak, G.R.; Won, J.W.; Choe, H.J.; Park, C.H.; Hyun, Y.T. Effect of iron content on beta -> alpha phase transformation behavior of Ti-5Al-xFe (x=1, 2.5, 4) alloys during continuous cooling. J. Mater. Res. Technol.-JMRT 2019, 8, 2887–2897. [Google Scholar] [CrossRef]
- Raynova, S.; Yang, F.; Bolzoni, L. The effect of thermomechanical treatments on the properties of powder metallurgy Ti-5Fe alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2021, 801, 140389. [Google Scholar] [CrossRef]
- Qi, P.; Li, B.L.; Wang, T.B.; Zhou, L.; Nie, Z.R. Microstructure and properties of a novel ternary Ti-6Zr-xFe alloy for biomedical applications. J. Alloys Compd. 2021, 854, 157119. [Google Scholar] [CrossRef]
- Niu, J.Z.; Guo, Y.H.; Li, K.; Liu, W.J.; Dan, Z.H.; Sun, Z.G.; Chang, H.; Zhou, L. Improved mechanical, bio-corrosion properties and in vitro cell responses of Ti-Fe alloys as candidate dental implants. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 122, 111917. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Niinomi, M.; Nakai, M.; Liu, H.H.; Santos, P.F.; Itoh, Y.; Ikeda, M.; Gepreel, M.A.H.; Narushima, T. Improvement in mechanical strength of low-cost beta-type Ti-Mn alloys fabricated by metal injection molding through cold rolling. J. Alloys Compd. 2016, 664, 272–283. [Google Scholar] [CrossRef]
- Kim, J.W.; Hwang, M.J.; Han, M.K.; Kim, Y.G.; Song, H.J.; Park, Y.J. Effect of manganese on the microstructure, mechanical properties and corrosion behavior of titanium alloys. Mater. Chem. Phys. 2016, 180, 341–348. [Google Scholar] [CrossRef]
- Alshammari, Y.; Yang, F.; Bolzoni, L. Mechanical properties and microstructure of Ti-Mn alloys produced via powder metallurgy for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 91, 391–397. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Jiang, H.; Liu, M.; Wang, C.H.; Cao, G.H. Microstructures and mechanical properties of Mn modified, Ti-Nb-based alloys. J. Alloys Compd. 2017, 723, 1091–1097. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Cho, K.; Nakai, M.; Liu, H.H.; Ohtsu, N.; Hirano, M.; Ikeda, M.; Narushima, T. Microstructures, mechanical properties and cytotoxicity of low cost beta Ti-Mn alloys for biomedical applications. Acta Biomater. 2015, 26, 366–376. [Google Scholar] [CrossRef]
- Nomura, N. Zirconium Alloys for Orthopedic Applications. In Advances in Metallic Biomaterials: Tissues, Materials and Biological Reactions; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mehjabeen, A.; Song, T.T.; Xu, W.; Tang, H.P.; Qian, M. Zirconium Alloys for Orthopaedic and Dental Applications. Adv. Eng. Mater. 2018, 20, 1800207. [Google Scholar] [CrossRef]
- Awad, A.H.; Gepreel, M.A.H. Basic characterization of new Ti-Mn-Zr alloys. In Proceedings of 10th International Conference on Key Engineering Materials (ICKEM), Univ Complutense Madrid, Electr Network, Madrid, Spain, 26–29 March 2020; pp. 1904–1908. [Google Scholar]
- Kuroda, P.A.B.; Quadros, F.D.; Afonso, C.R.M.; Grandini, C.R. The Effect of Solution Heat Treatment Temperature on Phase Transformations, Microstructure and Properties of Ti-25Ta-xZr Alloys Used as a Biomaterial. J. Mater. Eng. Perform. 2020, 29, 2410–2417. [Google Scholar] [CrossRef]
- Jiang, B.B.; Wang, Q.; Wen, D.H.; Xu, F.; Chen, G.Q.; Dong, C.; Sun, L.X.; Liaw, P.K. Effects of Nb and Zr on structural stabilities of Ti-Mo-Sn-based alloys with low modulus. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2017, 687, 1–7. [Google Scholar] [CrossRef]
- Akimoto, T.U.T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. Evaluation of corrosion resistance of implant-use Ti-Zr binary alloys with a range of compositions. Rep. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 73–79. [Google Scholar] [CrossRef]
- Ou, P.; Hao, C.; Liu, J.; He, R.; Wang, B.; Ruan, J. Cytocompatibility of Ti-xZr alloys as dental implant materials. J. Mater. Sci.-Mater. Med. 2021, 32, 50. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, L.; Alqattan, M.; Peters, L.; Alshammari, Y.; Yang, F. Ternary Ti alloys functionalised with antibacterial activity. Sci. Rep. 2020, 10, 22201. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ma, Z.; Li, M.; Zhang, Y.; Liu, W.Q.; Liao, Z.H.; Yang, K. Antibacterial Properties of Ti-6Al-4V-xCu Alloys. J. Mater. Sci. Technol. 2014, 30, 699–705. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.B.; Liu, C.; Wang, H.Y.; Ren, B.R.; Yang, K.; Zhang, E.L. Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 35, 392–400. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.M.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, L.; Liu, R.; Yang, K.; Zhang, Y.; Liao, Z.H.; Liu, W.Q.; Qi, M.; Misra, R.D.K. Effect of Heat Treatment on Cu Distribution, Antibacterial Performance and Cytotoxicity of Ti-6Al-4V-5Cu Alloy. J. Mater. Sci. Technol. 2015, 31, 723–732. [Google Scholar] [CrossRef]
- Osorio, W.R.; Cremasco, A.; Andrade, P.N.; Garcia, A.; Caram, R. Electrochemical behavior of centrifuged cast and heat treated Ti-Cu alloys for medical applications. Electrochim. Acta 2010, 55, 759–770. [Google Scholar] [CrossRef]
- Zhang, E.L.; Ren, J.; Li, S.Y.; Yang, L.; Qin, G.W. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of as-cast Ti-Cu alloys. Biomed. Mater. 2016, 11, 065001. [Google Scholar] [CrossRef]
- Alshammari, Y.; Yang, F.; Bolzoni, L. Low-cost powder metallurgy Ti-Cu alloys as a potential antibacterial material. J. Mech. Behav. Biomed. Mater. 2019, 95, 232–239. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Luo, R.D.; Ren, J.K.; Zhang, L.; Jiang, Y.H.; He, Z.Y. Design of a new Ti-Mo-Cu alloy with excellent mechanical and antibacterial properties as implant materials. Mater. Lett. 2022, 306, 130875. [Google Scholar] [CrossRef]
- Xu, W.; Hou, C.J.; Mao, Y.X.; Yang, L.; Tamaddon, M.; Zhang, J.L.; Qu, X.H.; Liu, C.Z.; Su, B.; Lu, X. Characteristics of novel Ti-10Mo-xCu alloy by powder metallurgy for potential biomedical implant applications. Bioact. Mater. 2020, 5, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, Y.; Yang, F.; Bolzoni, L. Fabrication and characterisation of low-cost powder metallurgy Ti-xCu-2.5Al alloys produced for biomedical applications. J. Mech. Behav. Biomed. Mater. 2022, 126, 105022. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive review on alloy design, processing, and performance of beta Titanium alloys as biomedical materials. Int. Mater. Rev. 2021, 66, 114–139. [Google Scholar] [CrossRef]
- Nakano, T. Physical and mechanical properties of metallic biomaterials. In Metals for Biomedical Devices, 2nd ed.; Niinomi, M., Ed.; ELsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Morinaga, M. The molecular orbital approach and its application to biomedical titanium alloy design. In Titanium in Medical and Dental Applications; Froes, F.H.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tane, M.; Akita, S.; Nakano, T.; Hagihara, K.; Umakoshi, Y.; Niinomi, M.; Nakajima, H. Peculiar elastic behavior of Ti-Nb-Ta-Zr single crystals. Acta Mater. 2008, 56, 2856–2863. [Google Scholar] [CrossRef]
- Hu, Q.M.; Yang, R. Mechanical properties of structural materials from first-principles. Curr. Opin. Solid State Mater. Sci. 2006, 10, 19–25. [Google Scholar] [CrossRef]
- Fang, H.Z.; Chen, R.R.; Yang, Y.; Su, Y.Q.; Ding, H.S.; Guo, J.J.; Fu, H.Z. Role of graphite on microstructural evolution and mechanical properties of ternary TiAl alloy prepared by arc melting method. Mater. Des. 2018, 156, 300–310. [Google Scholar] [CrossRef]
- Saidi, M.; Walha, S.; Hlil, E.K.; Bessais, L.; Jemmali, M. Effect of chromium substitution on structural, magnetic and magnetocaloric properties of GdFe12-xCrx intermetallic compounds, Mossbauer spectrometry and ab initio calculations. J. Solid State Chem. 2021, 297, 122019. [Google Scholar] [CrossRef]
- Litzbarski, L.S.; Winiarski, M.J.; Skokowski, P.; Klimczuk, T.; Andrzejewski, B. Investigation of magnetic order in a new intermetallic compound Nd2PtGe3. J. Magn. Magn. Mater. 2021, 521, 167494. [Google Scholar] [CrossRef]
- Supriadi, S.; Immanuel, T.; Sutowo, C.; Lucky, G.; Senopati, G.; Suharno, B. Effect of Mn in New beta Titanium Alloy Ti-6Nb-6Mo on Corrosion Behavior and Mechanical Properties. In Proceedings of 3rd International Seminar on Metallurgy and Materials (ISMM)—Exploring New Innovation in Metallurgy and Materials, Tangerang Selatan, Indonesia, 23–24 October 2020. [Google Scholar]
- Senopati, G.; Sutowo, C.; Kartika, I.; Suharno, B. The Effect of Solution Treatment on Microstructure and Mechanical Properties of Ti-6Mo-6Nb-8Sn Alloy. In Proceedings of 6th International Conference on Advanced Materials Science and Technology (ICAMST), Semarang, Indonesia, 9–10 October 2015; pp. 224–228. [Google Scholar]
- Jain, D.; Sudarsan, V.; Tyagi, A.K. Synthesis of Metallic Materials by Arc Melting Technique. In Handbook on Synthesis Strategies for Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Nishiyama, H.; Sawada, T.; Takana, H.; Tanaka, M.; Ushio, M. Computational simulation of arc melting process with complex interactions. ISIJ Int. 2006, 46, 705–711. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Furuhara, T.; Maki, T.; Makino, T. Microstructure control by thermomechanical processing in beta-Ti-15-3 alloy. J. Mater. Process. Technol. 2001, 117, 318–323. [Google Scholar] [CrossRef]
- Hua, K.; Zhang, Y.; Tong, Y.; Zhang, F.; Kou, H.; Li, X.; Wang, H.; Li, J. Enhanced mechanical properties of a metastable β titanium alloy via optimized thermomechanical processing. Mater. Sci. Eng. A 2022, 840, 142997. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Stechschulte, J.; Fraser, H.L. Comparison of microstructural evolution in Ti-Mo-Zr-Fe and Ti-15Mo biocompatible alloys. J. Mater. Sci.-Mater. Med. 2005, 16, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.V.; Borborema, S.; Araújo, L.S.; Malet, L.; Dille, J.; Almeida, L.H.d. Influence of thermo-mechanical processing on structure and mechanical properties of a new metastable beta Ti-29Nb-2Mo-6Zr alloy with low Young’s modulus. J. Alloys Compd. 2020, 820, 153078. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.X.; Li, Y.C.; Zhang, Y.W.; Munir, K.; Jiang, H.W.; Wen, C.E. Deformation mechanism and mechanical properties of a thermomechanically processed beta Ti-28Nb-35.4Zr alloy. J. Mech. Behav. Biomed. Mater. 2018, 78, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.L.; Pontes, F.M.L.; Grandini, C.R. The Influence of Thermomechanical Treatments on the Structure, Microstructure, and Mechanical Properties of Ti-5Mn-Mo Alloys. Metals 2022, 12, 527. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Quadros, F.D.; de Araujo, R.O.; Afonso, C.R.M.; Grandini, C.R. Effect of Thermomechanical Treatments on the Phases, Microstructure, Microhardness and Young’s Modulus of Ti-25Ta-Zr Alloys. Materials 2019, 12, 3210. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.X.; Weng, W.J.; Zhang, Y.W.; Li, Y.C.; Wen, C.E. Effect of thermomechanical treatment on the mechanical and microstructural evolution of a beta-type Ti-40.7Zr-24.8Nb alloy. Bioact. Mater. 2019, 4, 303–311. [Google Scholar] [CrossRef]
- Rajabi, F.; Hanzaki, A.Z.; Abedi, H.R.; Farghadany, E. Corrosion behavior of thermo-mechanically processed biomedical Ti-29Nb-13Ta-4.6Zr. J. Alloys Compd. 2017, 725, 23–31. [Google Scholar] [CrossRef]
- Lu, J.W.; Ge, P.; Li, Q.; Zhang, W.; Huo, W.T.; Hu, J.J.; Zhang, Y.S.; Zhao, Y.Q. Effect of microstructure characteristic on mechanical properties and corrosion behavior of new high strength Ti-1300 beta titanium alloy. J. Alloys Compd. 2017, 727, 1126–1135. [Google Scholar] [CrossRef]
- Liu, C.H.; Jin, S.; Lai, X.M.; He, B.; Li, F. Influence of complex structure on the shrinkage of part in investment casting process. Int. J. Adv. Manuf. Technol. 2015, 77, 1191–1203. [Google Scholar] [CrossRef]
- Yuan, C.; Cheng, X.; Holt, G.S.; Shevchenko, D.; Withey, P.A. Investment casting of Ti-46Al-8Nb-1B alloy using moulds with CaO-stabilized zirconia face coat at various mould pre-heat temperatures. Ceram. Int. 2015, 41, 4129–4139. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process-A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Prayoga, B.T.; Dharmastiti, R.; Akbar, F.; Suyitno. Microstructural characterization, defect and hardness of titanium femoral knee joint produced using vertical centrifugal investment casting. J. Mech. Sci. Technol. 2018, 32, 149–156. [Google Scholar] [CrossRef]
- Nastac, L.; Gungor, M.N.; Ucok, I.; Klug, K.L.; Tack, W.T. Advances in investment casting of Ti-6Al-4V alloy: A review. Int. J. Cast Met. Res. 2006, 19, 73–93. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Reda, R.; Adel, A. Heat Treatment of Low Cost Beta Titanium Alloy Produced by Investment Casting. J. Pet. Min. Eng. 2020, 22, 35–41. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Zhang, B.; Liu, C.C.; Lv, S.M.; Yang, S.D.; Qu, X.H. Effects of Porosity on Mechanical Properties and Corrosion Resistances of PM-Fabricated Porous Ti-10Mo Alloy. Metals 2018, 8, 188. [Google Scholar] [CrossRef]
- Annur, D.; Kartika, I.; Supriadi, S.; Suharno, B. Titanium and titanium based alloy prepared by spark plasma sintering method for biomedical implant applications-a review. Mater. Res. Express 2021, 8, 012001. [Google Scholar] [CrossRef]
- Zou, L.M.; Yang, C.; Long, Y.; Xiao, Z.Y.; Li, Y.Y. Fabrication of biomedical Ti-35Nb-7Zr-5Ta alloys by mechanical alloying and spark plasma sintering. Powder Metall. 2012, 55, 65–70. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Qiu, D.; Gibson, M.A.; Zheng, Y.F.; Fraser, H.L.; StJohn, D.H.; Easton, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, P.; Rashid, R.A.R.; Masood, S.H.; Ruan, D.; Palanisamy, S. Mechanical Properties of SLM-Printed Aluminium Alloys: A Review. Materials 2020, 13, 4301. [Google Scholar] [CrossRef]
- Liu, S.Y.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Cottam, R.; Palanisamy, S.; Avdeev, M.; Jarvis, T.; Henry, C.; Cuiuri, D.; Balogh, L.; Rashid, R.A.R. Diffraction Line Profile Analysis of 3D Wedge Samples of Ti-6Al-4V Fabricated Using Four Different Additive Manufacturing Processes. Metals 2019, 9, 60. [Google Scholar] [CrossRef]
- Schwab, H.; Palm, F.; Kuhn, U.; Eckert, J. Microstructure and mechanical properties of the near-beta titanium alloy Ti-5553 processed by selective laser melting. Mater. Des. 2016, 105, 75–80. [Google Scholar] [CrossRef]
- Zhou, L.B.; Yuan, T.C.; Tang, J.Z.; He, J.J.; Li, R.D. Mechanical and corrosion behavior of titanium alloys additively manufactured by selective laser melting—A comparison between nearly beta titanium, alpha titanium and alpha plus beta titanium. Opt. Laser Technol. 2019, 119, 105625. [Google Scholar] [CrossRef]
- Maimaitiyili, T.; Mosur, K.; Kurzynowski, T.; Casati, N.; Van Swygenhoven, H. Phase Studies of Additively Manufactured Near Beta Titanium Alloy-Ti55511. Materials 2020, 13, 1723. [Google Scholar] [CrossRef]
- Nartu, M.; Flannery, D.; Mazumder, S.; Mantri, S.A.; Joshi, S.S.; Ayyagari, A.V.; McWilliams, B.; Cho, K.; Dahotre, N.B.; Banerjee, R. Influence of Process Parameters on Mechanical and Corrosion Behavior of DED-Processed Biomedical Ti-35Nb-7Zr-5Ta Alloy. Jom 2021, 73, 1819–1827. [Google Scholar] [CrossRef]
- Chen, Y.; Kou, H.C.; Cheng, L.; Zhang, Y.L.; Yu, Y.; Lu, Y.L. Kinetic Diffusion Couple for Mapping Microstructural and Mechanical Data on Ti-Al-Mo Titanium Alloys. Materials 2018, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xue, Y.; Shi, F. Intermetallic Formation and Mechanical Properties of Ni-Ti Diffusion Couples. Mater. Des. 2017, 130, 175–182. [Google Scholar] [CrossRef]
- Fang, Z.G.Z.; Paramore, J.D.; Sun, P.; Chandran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder metallurgy of titanium—Past, present, and future. Int. Mater. Rev. 2018, 63, 407–459. [Google Scholar] [CrossRef]
- Wang, H.; Luo, H.L.; Chen, J.Q.; Tang, J.C.; Yao, X.Y.; Zhou, Y.H.; Yan, M. Cost-affordable, biomedical Ti-5Fe alloy developed using elemental powders and laser in-situ alloying additive manufacturing. Mater. Charact. 2021, 182, 111526. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Attar, H.; Okulov, I.V.; Dargusch, M.S.; Kent, D. Microstructural evolution and mechanical properties of bulk and porous low-cost Ti-Mo-Fe alloys produced by powder metallurgy. J. Alloys Compd. 2021, 853, 11. [Google Scholar] [CrossRef]
- Lee, T.; Lee, S.; Kim, I.S.; Moon, Y.H.; Kim, H.S.; Park, C.H. Breaking the limit of Young’s modulus in low-cost Ti-Nb-Zr alloy for biomedical implant applications. J. Alloys Compd. 2020, 828, 154401. [Google Scholar] [CrossRef]
- Xu, Y.D.; Gao, J.H.; Huang, Y.H.; Rainforth, W.M. A low-cost metastable beta Ti alloy with high elastic admissible strain and enhanced ductility for orthopaedic application. J. Alloys Compd. 2020, 835, 155391. [Google Scholar] [CrossRef]
- Tsao, L.C.; Hsieh, M.J.; Yu, Y.C. Effects of Sn additions on microstructure and corrosion resistance of heat-treated Ti-Cu-Sn titanium alloys. Corros. Eng. Sci. Technol. 2018, 53, 252–258. [Google Scholar] [CrossRef]
- Gunawarman, B.; Niinomi, M.; Akahori, T.; Souma, T.; Ikeda, M.; Toda, H. Mechanical properties and microstructures of low cost beta titanium alloys for healthcare applications. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2005, 25, 304–311. [Google Scholar] [CrossRef]
- Nocivin, A.; Raducanu, D.; Vasile, B.; Trisca-Rusu, C.; Cojocaru, E.M.; Dan, A.D.; Irimescu, R.; Cojocaru, V.D. Tailoring a Low Young Modulus for a Beta Titanium Alloy by Combining Severe Plastic Deformation with Solution Treatment. Materials 2021, 14, 3467. [Google Scholar] [CrossRef]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti-24Nb-4Zr-8Sn alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Attar, H.; Dargusch, M.S.; Kent, D. Microstructure, phase composition and mechanical properties of new, low cost Ti-Mn-Nb alloys for biomedical applications. J. Alloys Compd. 2019, 787, 570–577. [Google Scholar] [CrossRef]
- Azizi, H.; Zurob, H.; Bose, B.; Ghiaasiaan, S.R.; Wang, X.; Coulson, S.; Duz, V.; Phillion, A.B. Additive manufacturing of a novel Ti-Al-V-Fe alloy using selective laser melting. Addit. Manuf. 2018, 21, 529–535. [Google Scholar] [CrossRef]
- Alshammari, Y.; Jia, M.; Yang, F.; Bolzoni, L. The effect of alpha plus beta forging on the mechanical properties and microstructure of binary titanium alloys produced via a cost-effective powder metallurgy route. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2020, 769, 138496. [Google Scholar] [CrossRef]
- Xu, R.J.; Liu, B.; Yan, Z.Q.; Chen, F.; Guo, W.M.; Liu, Y. Low-cost and high-strength powder metallurgy Ti-Al-Mo-Fe alloy and its application. J. Mater. Sci. 2019, 54, 12049–12060. [Google Scholar] [CrossRef]
- Dong, Y.P.; Tang, J.C.; Wang, D.W.; Wang, N.; He, Z.D.; Li, J.; Zhao, D.P.; Yan, M. Additive manufacturing of pure Ti with superior mechanical performance, low cost, and biocompatibility for potential replacement of Ti-6Al-4V. Mater. Des. 2020, 196, 109142. [Google Scholar] [CrossRef]
- Li, G.C.; Li, J.; Tian, X.J.; Cheng, X.; He, B.; Wang, H.M. Microstructure and properties of a novel titanium alloy Ti-6Al-2V-1.5Mo-0.5Zr-0.3Si manufactured by laser additive manufacturing. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2017, 684, 233–238. [Google Scholar] [CrossRef]
- Calvert, E.; Wynne, B.; Weston, N.; Tudball, A.; Jackson, M. Thermomechanical processing of a high strength metastable beta titanium alloy powder, consolidated using the low-cost FAST-forge process. J. Mater. Process. Technol. 2018, 254, 158–170. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, S.J.; Obbard, E.G.; Wang, H.; Wang, S.C.; Hao, Y.L.; Yang, R. Elastic properties of Ti-24Nb-4Zr-8Sn single crystals with bcc crystal structure. Acta Mater. 2011, 59, 3081–3090. [Google Scholar] [CrossRef]
- Ahmed, M.; Savvakin, D.G.; Ivasishin, O.M.; Pereloma, E.V. The effect of thermo-mechanical processing and ageing time on microstructure and mechanical properties of powder metallurgy near beta titanium alloys. J. Alloys Compd. 2017, 714, 610–618. [Google Scholar] [CrossRef]



| Alloy | Phase | Density (g/cm3) | UTS (MPa) | Raw Materials Price (USD/Kg) | Ref. |
|---|---|---|---|---|---|
| Ti6Al4V | α + β | 4.43 | 1250 | 26.568 | [7] |
| Ti-5Al-2Sn-2Zr-4Cr-4Mo-1Fe | β | 4.69 | 1723 | 13.366 | |
| Ti-15V-3Sn-3Al-3Cr | β | 4.76 | 1335 | 69.771 | |
| Ti-8V-4Mo-3Al-6Cr-4Zr | β | 4.82 | 1503 | 44.712 | |
| Ti-11.5Mo-6Zr-4.5Sn | β | 5.06 | 1352 | 17.545 | |
| Ti-12Mo-6Zr-2Fe | β | 5 | 1100 | 16.815 | |
| Ti-14Mn | β | 4.9 | 1950 | 10.566 |
| Material | Tensile Strength (MPa) | Yield Strength (MPa) | Elastic Modulus (GPa) | Ref. |
|---|---|---|---|---|
| Bone (cortical) | 70–150 | 30–70 | 15–30 | [26,28] |
| Stainless steel | 490–1350 | 190–690 | 200–210 | |
| Cobalt alloy | 655–1793 | 310–1586 | 210–253 | |
| Titanium alloy | 690–1100 | 585–1060 | 55–110 | |
| Magnesium alloy | 100–250 | - | 40–45 |
| Material | Item Number | Size/Specification | Price (USD/Kg) |
|---|---|---|---|
| Titanium | TI-454100 | ≤45 μ; 10 g; 99.98% | 2610 |
| Vanadium | V-474100 | <75 μ; 10 g; 99.5% | 17,400 |
| Niobium | NB-324100 | <45 μ; 10 g; 99.9% | 7830 |
| Tantalum | TA-414051 | −325 mesh; 10 g; 99.9% | 7308 |
| Manganese | MN-284101 | <45 μ; 99.9%; 100 g | 957 |
| Molybdenum | MO-294250 | <100 μ; 250 g; 99.9+ % | 870 |
| Zircon | ZR-490100 | 0.8–35 mm; 99.6%; 1 kg | 489 |
| Aluminium | AL-014120 | <30 μ; 100 g; 99.8% | 435 |
| Iron | FE-224510 | 100 mesh; 50 g; 99+ % | 331 |
| Copper | CU-114111 | −200 mesh; 1 kg; 99.8% | 70 |
| Alloy | Process | Phase Constituent | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|---|
| Tensile/Compressive/Bending Strength (MPa) | Yield Strength (MPa) | Hardness (HV) | E (GPa) | ||||
| Ti6Al4V | Forged | α + β | 895–930 | 825–860 | 110 | [65] | |
| Ti-7Fe | Sintered | α + β | 916 | - | 270 | - | [73] |
| Ti-7.Mo-2Fe | 2453 | - | 403.1 | 92 | [74] | ||
| Ti-Mo-Fe | Casting + TMT | α + β | 949 | - | 422 | 84.5 | [75] |
| Ti-5Fe | PM + TMT | α + β | 1030 | 950 | - | - | [79] |
| Ti-6Zr-5Fe | Casting | α + β | 800–900 | - | 300 | 93 | [80] |
| Alloy | Process | Phase Constituent | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|---|
| Tensile/Compressive/Bending Strength (MPa) | Yield Strength (MPa) | Hardness (HV) | E (GPa) | ||||
| Ti6Al4V | Forged | α + β | 895–930 | 825–860 | 110 | [65] | |
| Ti-6Mo-6Nb-4Sn-4Mn | Casting + Homogenization | β | - | - | 320 | 94 | [14] |
| Ti-9Mn | MIM | β | 1046 | 992 | 300 | 89 | [10] |
| Ti-5Mn | Casting + Heat treatment | α + β | - | - | 457.2 | 129.9 | [83] |
| Ti-5Mn | PM + Forged | α + β | 1224 | 1154 | 415.7 | 97 | [84] |
| Ti-16Nb-7Mn | CR + ST | β | 695 | 646 | - | 77 | [85] |
| Alloy | Process | Phase Constituent | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|---|
| Tensile/Compressive/Bending Strength (MPa) | Yield Strength (MPa) | Hardness (HV) | E (GPa) | ||||
| Ti6Al4V | Forged | α + β | 895–930 | 825–860 | 110 | [65] | |
| Ti-25Ta-40Zr | Casting + TMT | α” + β | - | - | 310 | 60 | [90] |
| Ti-7.5Mo-9Zr | Casting + HT | α” + β | 971 | 563 | - | 89 | [11] |
| Ti-5.1Mo-6.3Sn-9.9Nb-4.9Zr | α” + β + ω | 762 | 275 | - | 59 | [91] | |
| Alloy | Process | Phase Constituent | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|---|
| Tensile/Compressive/Bending Strength (MPa) | Yield Strength (MPa) | Hardness (HV) | E (GPa) | ||||
| Ti6Al4V | Forged | α + β | 895–930 | 825–860 | 110 | [65] | |
| Ti-15Mo-10Cu | Casting | α” + β + Ti2Cu | 1200 | 1141 | - | 67 | [102] |
| Ti-10Mo-3Cu | PM | α” + β + Ti2Cu | 1098 | - | - | 71.1 | [103] |
| Ti-5Cu | PM + Forged | β + Ti2Cu | 901 | 757 | 302 | 98 | [101] |
| Ti-5Cu-2.5Al | PM | β + Ti2Cu | 800 | 750 | 250 | - | [104] |
| Low-Cost Titanium Alloy | Processing Route | Properties | Ref. |
|---|---|---|---|
| Ti-2.5Al-xCu (x = 0.5–5 wt.%) | Powder Mixing | An increase in the Cu content from 0.5 to 5 wt.% is accompanied by an increase in hardness from 195 HV to 250 HV, yield strength from 596 to 755 MPa and ultimate tensile strength from 645 to 800 MPa. This is due to the refinement of the lamella microstructure and the presence of Ti2Cu intermetallic compound and solid solution strengthening effect. | [104] |
| Compaction | |||
| Sintering | |||
| Ti-14Mn-xZr (x = 1–6 wt.%) | Arc melting | The tensile strength of Ti-14Mn was increase with increasing of Zr content. The highest tensile strength was obtained with To-14Mn-6Zr (1830 MPa), which also exhibited better corrosion resistance (0.164 × 10−3 mm/y) and high cytocompatibility (99% cell viability value). | [9] |
| Homogenization | |||
| Solution treatment | |||
| Cold rolled | |||
| Ti-5Mo-Fe-3Sn (at. %) Ti-9Mo-Fe-7Sn (wt.%) | Arc melting | The yield strength of Ti-5Mo-Fe-3Sn was 740 MPa, comparable with Ti6Al4V (850 MPa) but with high elongation (twice that of Ti6Al4V). | [152] |
| Homogenization | |||
| Hot rolled | |||
| Cold rolled | |||
| Annealed | |||
| Water quenched | |||
| Ti-7Cu-xSn (x = 0–5 wt.%) | Arc melting | The potential corrosion value of Ti-7Cu-5Sn was 141.8 nA/cm2 the lowest compared to Ti-7Cu and Ti-7Cu-(1 and 2.5) Sn. It indicated that Ti-7Cu-5Sn showed the highest corrosion resistance and electrochemical properties compared to Ti-7Cu. | [153] |
| Solution treated | |||
| Ti-4.3Fe-7.1Cr | As forged | The tensile strength of solution-treated Ti-Fe-Cr and Ti-Fe-Cr-Al were 900 and 1100 MPa, respectively, which is much higher than CP-Ti. The fatigue strength of these alloys is also much better than Ti6Al4V. | [154] |
| Ti-4.3Fe-7.1Cr -3.0Al | Solution treated | ||
| Ti-34Nb-7.6Zr-0.9Fe-0.16O | Levitation melting | The mechanical properties of Ti-Nb-Zr-Fe-O alloy can reach 1140 MPa (UTS) and 770 (YS) with a low elastic modulus (48.6 GPa). | [155] |
| Cold rolling | |||
| Homogenization | |||
| Severe plastic deformation | |||
| Solution treated | |||
| Ti-13.8Nb-14Zr (TNZ) | Solution treatment | The yield strength of TNZ after CCR is about 1000 MPa, compared to solution treated (600 MPa) and aged (800 MPa) samples, while the elastic modulus is constant at 50 GPa. | [151] |
| Aging | |||
| Cold caliber rolling (CCR) | |||
| Ti-13Mn | Injection molding | The solution treated Ti-13Mn show comparable tensile strength (888 MPa) compared to Ti6Al4V (850–950 MPa), but with a lower elastic modulus (96 GPa). | [82] |
| Sintering | |||
| Solution treatment | |||
| Ti-24Nb-4Zr-8Sn | Selective Laser Melting (SLM) | The tensile strength (665 MPa) and elastic modulus (53 GPa) of Ti-24Nb-4Zr-8Sn is comparable with the same composition produced by other route processes (Hot Rolled and Cold Rolled). | [156] |
| Ti-5Fe | SLM | The tensile strength of the as-printed Ti-5Fe was far from desirable (below 700 MPa). The sample failed within the elastic regime exhibiting severe brittle behaviour. The annealing process and Yttria (Y) addition were introduced to improve the tensile strength. After the addition of 0.2 wt.% Y and annealing at different temperatures, the tensile strength improved to 830–945 MPa. | [149] |
| Ti-5Fe-0.2Y | |||
| Ti-Mo-xFe (x = 1,5,9 wt.%) | Powder Metallurgy | The compressive strength of Ti-Mo-Fe was higher than CP-Ti. The elastic modulus of porous Ti-Mo-Fe was closer to that of the human bone. | [150] |
| Ti-7Mn-xNb (x = 0, 3, 7 and 10 wt.%) | Powder Metallurgy | The compressive strength of sintered Ti-7Mn-xNb was much higher (1800–2100 MPa) compared to as-sintered CP-Ti (1650 MPa). | [157] |
| Ti-4.5Fe-7Cr | Investment casting | The compressive strength of Ti-4.5Fe-7Cr after aging (1877–2118 MPa) was higher than solution-treated samples (1870–1913 MPa). | [134] |
| Solution and aging | |||
| Heat treatment | |||
| Ti-1Al-8V-5Fe (Ti185) | SLM | The compressive strength and strain value of as-built Ti185 were 1890 MPa and 11%, respectively. After the heat treatment process, the compressive strength and strain value increased to 2380 MPa and 26%, respectively. | [158] |
| Heat treatment | |||
| Ti-xMn (x = 1, 5, and 10 wt.%) | Powder Metallurgy | The tensile strength of Ti-5Mn was found to exhibit the optimum value of 1200 MPa with sufficient elongation (5%). The tensile strength of Ti-5Cu alloy was 1100 MPa; however, the elongation was only 2%. The decreasing elongation of Ti-5Cu is due to the formation of the Ti2Cu intermetallic phase. | [159] |
| Ti-xCu (x = 0.5, 2.5, and 5 wt.%) | Forging | ||
| Ti–5Al–3Mo–2Fe | Powder Metallurgy | The as-sintered Ti-5Al-3Mo-2Fe alloy had a tensile strength of 904 MPa, yield strength of 829 MPa and elongation of 4.8%. After 80% reduction via hot rolling, followed by annealing, the tensile, yield strength, and elongation increased to 1422 MPa, 1303 MPa, and 8.5%, respectively. | [160] |
| Hot rolling | |||
| Annealing | |||
| Pure Ti | SLM | The as-printed HDH-Ti had a tensile strength of 1057.05 ± 29.14 MPa, yield strength of 784.59 ± 33.25 MPa, and elongation of 24.09 ± 1.81%. This value was higher compared to standard Ti6Al4V which values are 895 MPa, 828 MPa and 10%, respectively. | [161] |
| Hydrogenated-dehydrogenated (HDH) | |||
| Gas atomized | |||
| Ti-6Al-2V-1.5Mo-0.5Zr-0.3Si | Laser Additive Manufacture | The microhardness of the as-deposited Ti-6Al-2V-1.5Mo-0.5Zr-0.3Si was higher (370 HV) than Ti6Al4V (358 HV) with similar processes and parameters. | [162] |
| Ti-5Al-5Mo-5V-3Cr (Ti-5553) | FAST-forge process | The Ti-5553, after the FAST-forge process, has a microhardness value (410–417 HV). This value was higher compared to Ti-5553 through the conventional forging process (385–405 HV). | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senopati, G.; Rahman Rashid, R.A.; Kartika, I.; Palanisamy, S. Recent Development of Low-Cost β-Ti Alloys for Biomedical Applications: A Review. Metals 2023, 13, 194. https://doi.org/10.3390/met13020194
Senopati G, Rahman Rashid RA, Kartika I, Palanisamy S. Recent Development of Low-Cost β-Ti Alloys for Biomedical Applications: A Review. Metals. 2023; 13(2):194. https://doi.org/10.3390/met13020194
Chicago/Turabian StyleSenopati, Galih, Rizwan Abdul Rahman Rashid, Ika Kartika, and Suresh Palanisamy. 2023. "Recent Development of Low-Cost β-Ti Alloys for Biomedical Applications: A Review" Metals 13, no. 2: 194. https://doi.org/10.3390/met13020194
APA StyleSenopati, G., Rahman Rashid, R. A., Kartika, I., & Palanisamy, S. (2023). Recent Development of Low-Cost β-Ti Alloys for Biomedical Applications: A Review. Metals, 13(2), 194. https://doi.org/10.3390/met13020194







