Microstructure and Mechanical Properties of LaB6/Ti-6Al-4V Composites Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Powder Preparation
2.2. SLM Process
2.3. Microstructure Characterization and Analysis
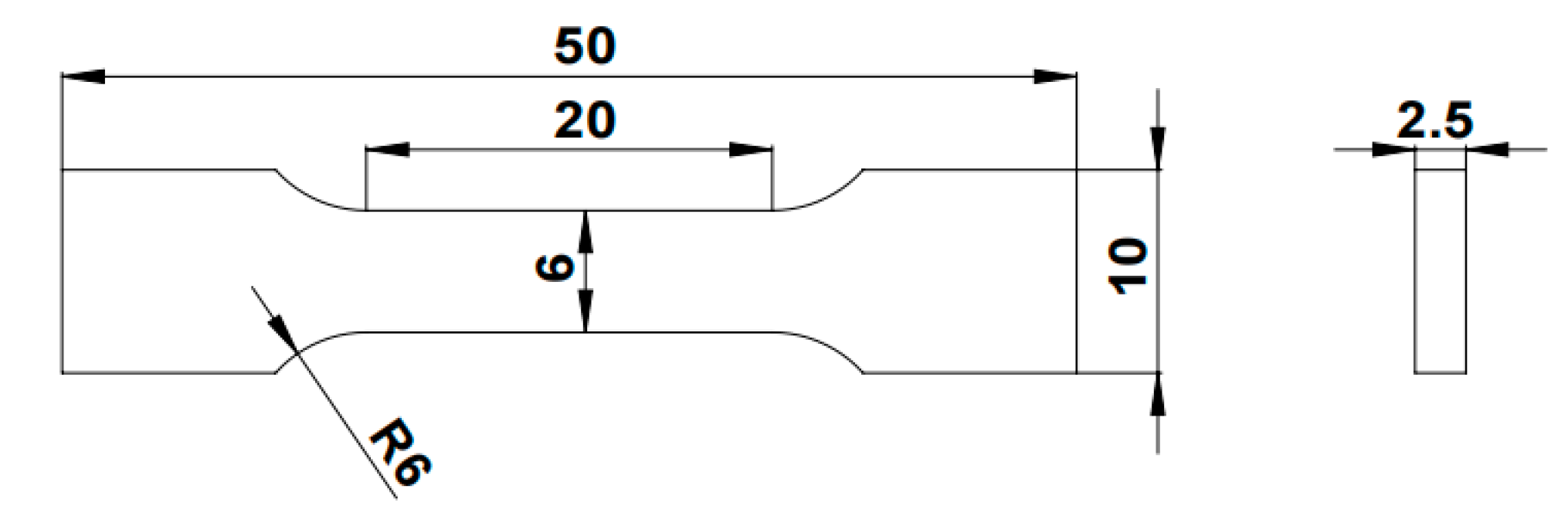
2.4. Mechanical Property Test
3. Results
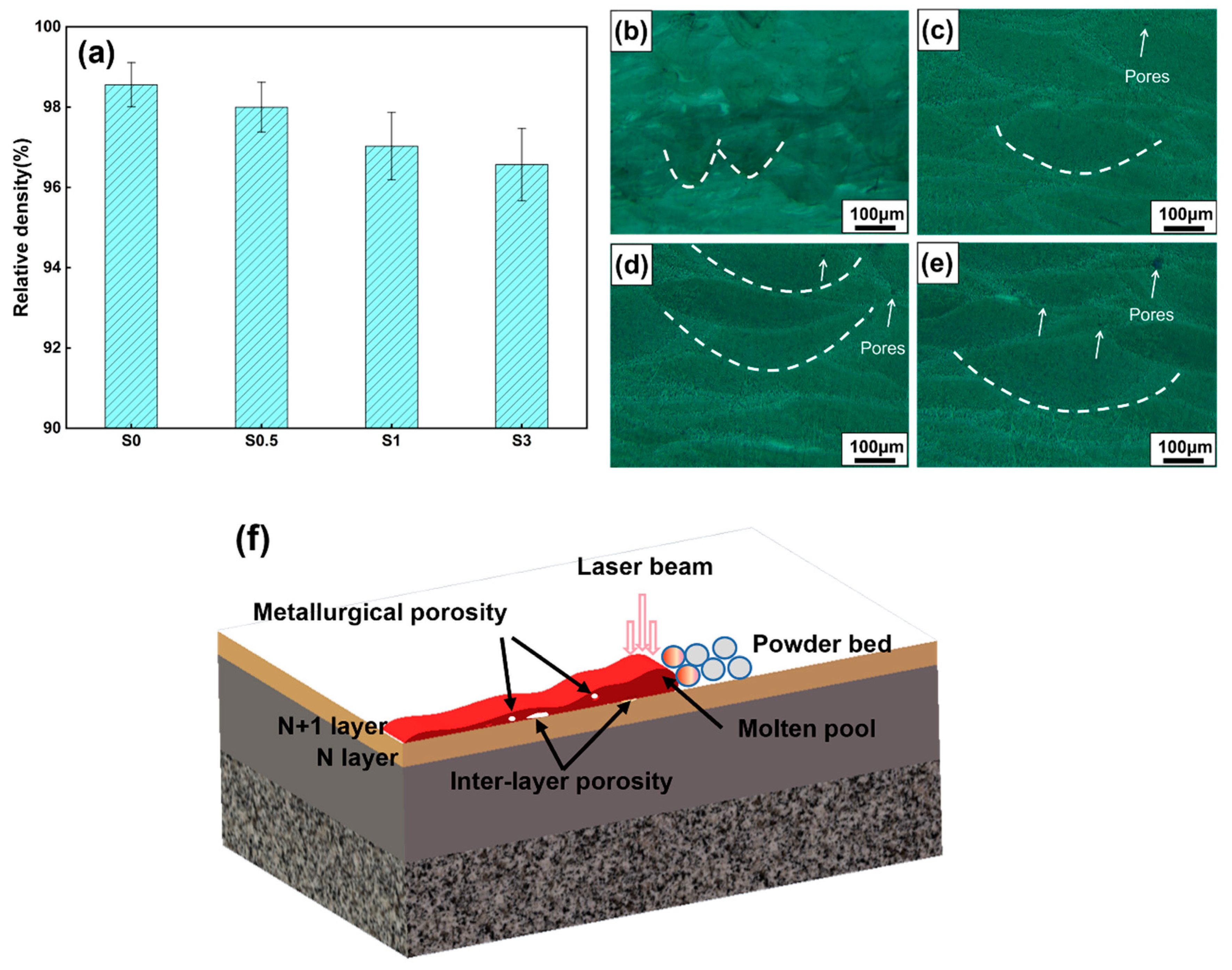
3.1. Densification Research
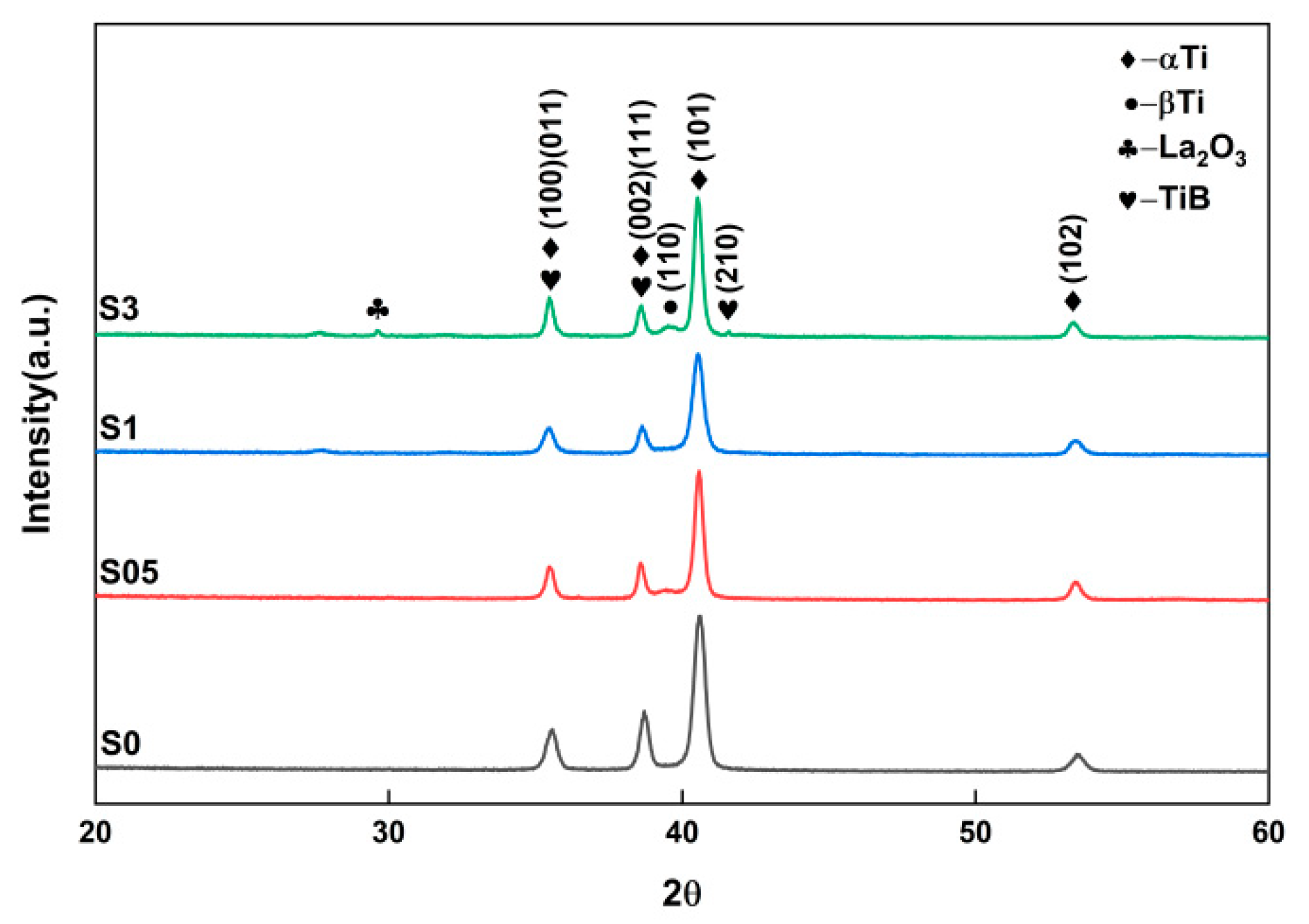
3.2. Phase Evolution and Identification
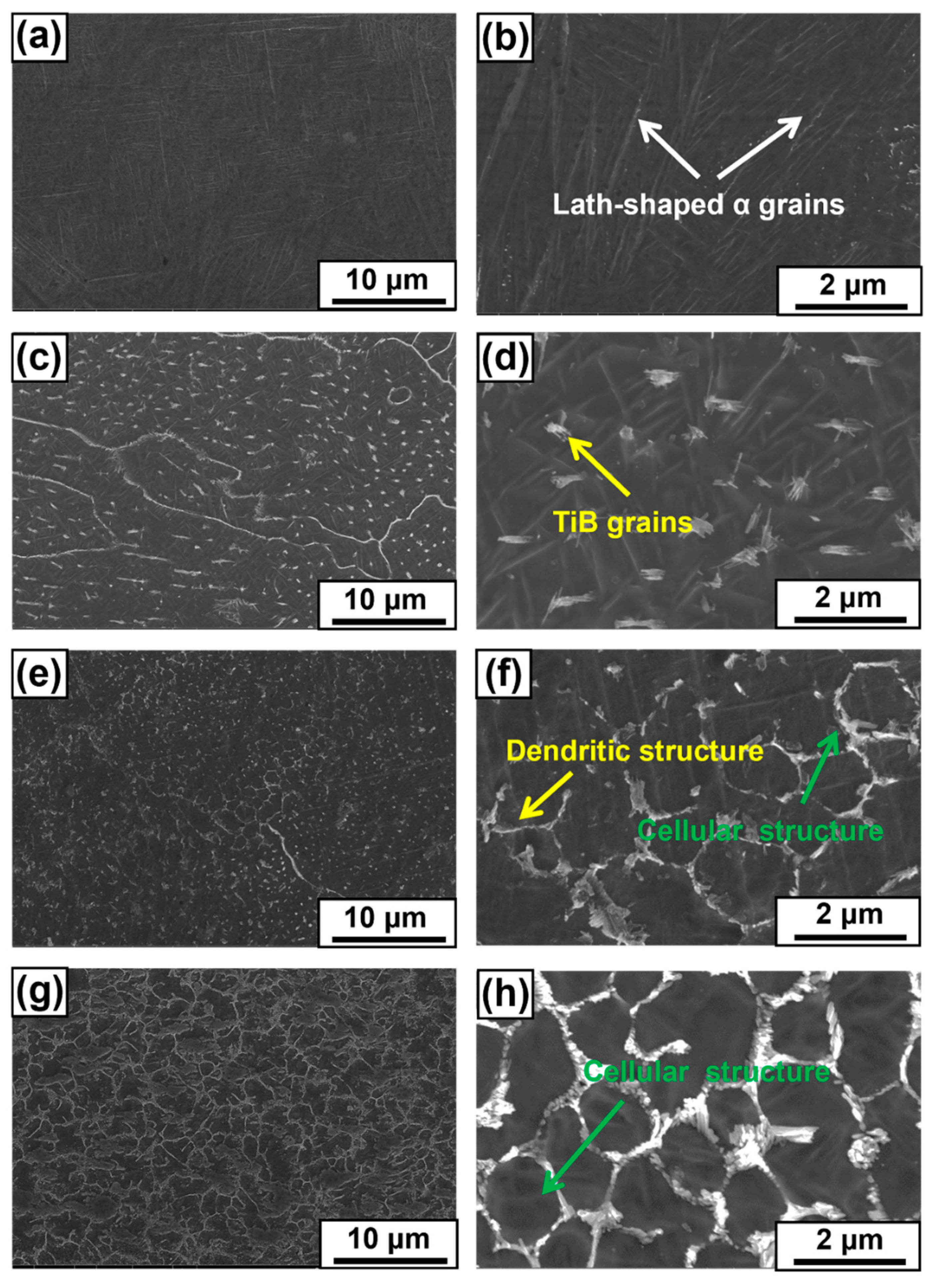
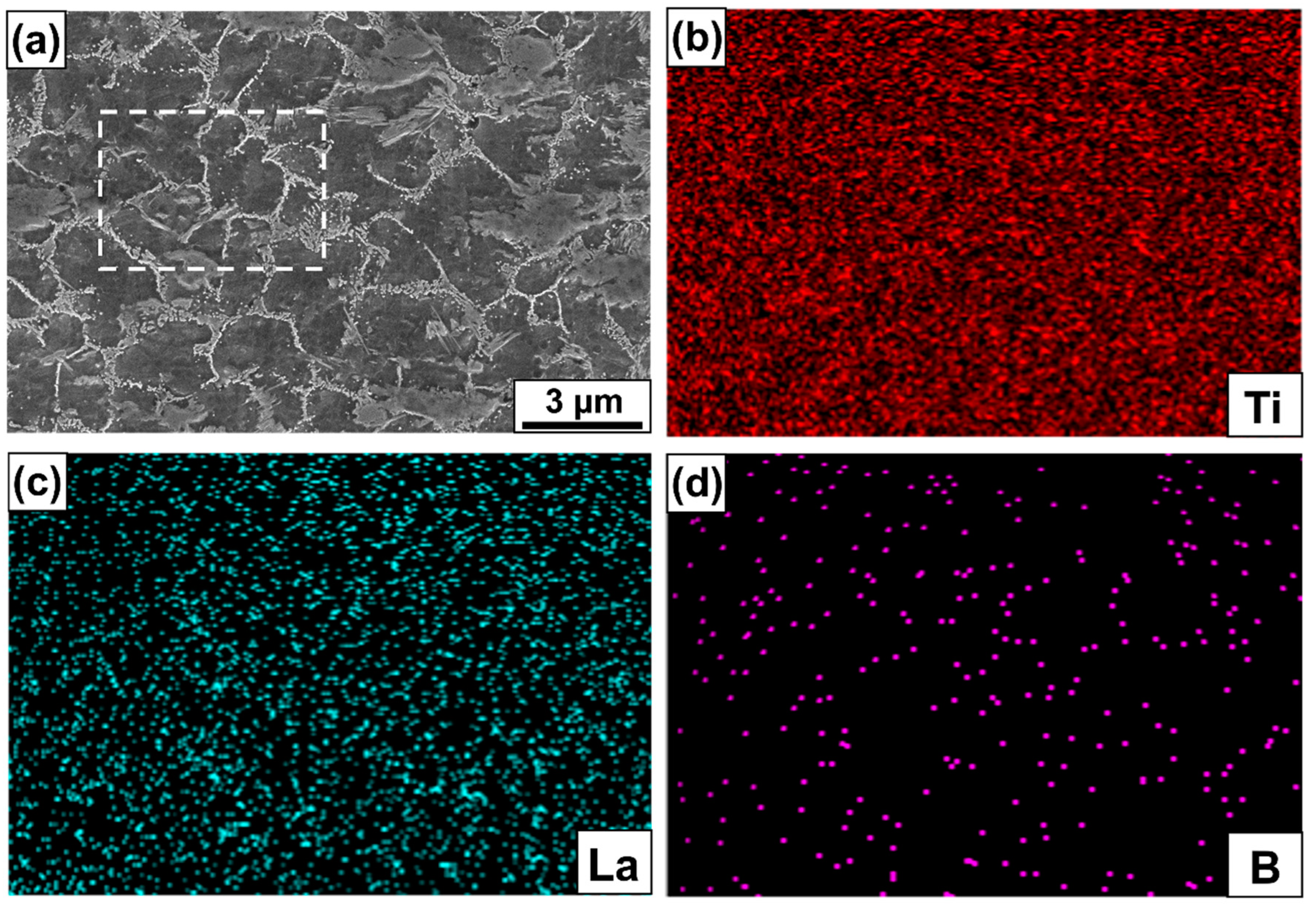
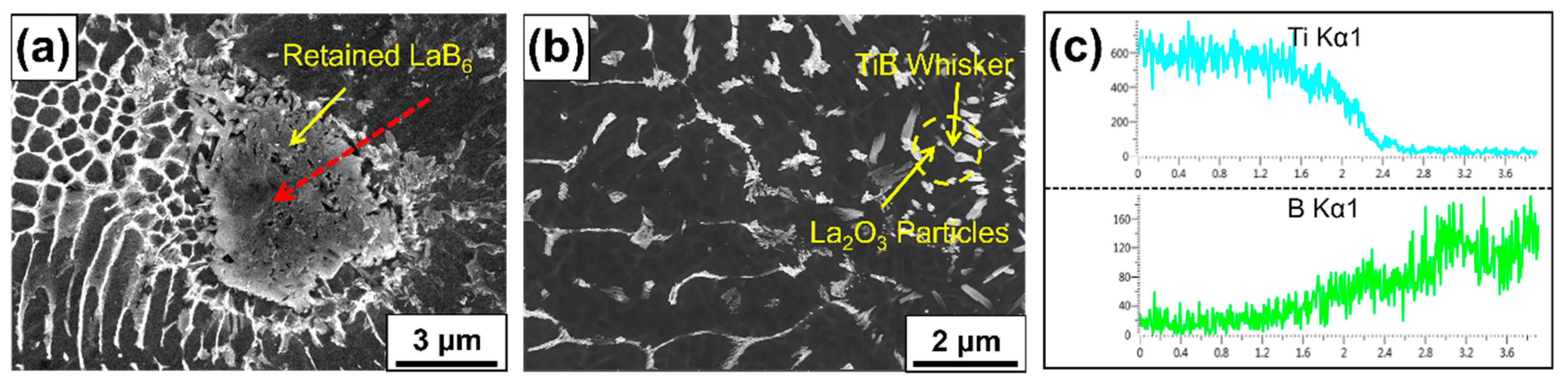
3.3. Microstructure Evolution
3.4. Mechanical Properties
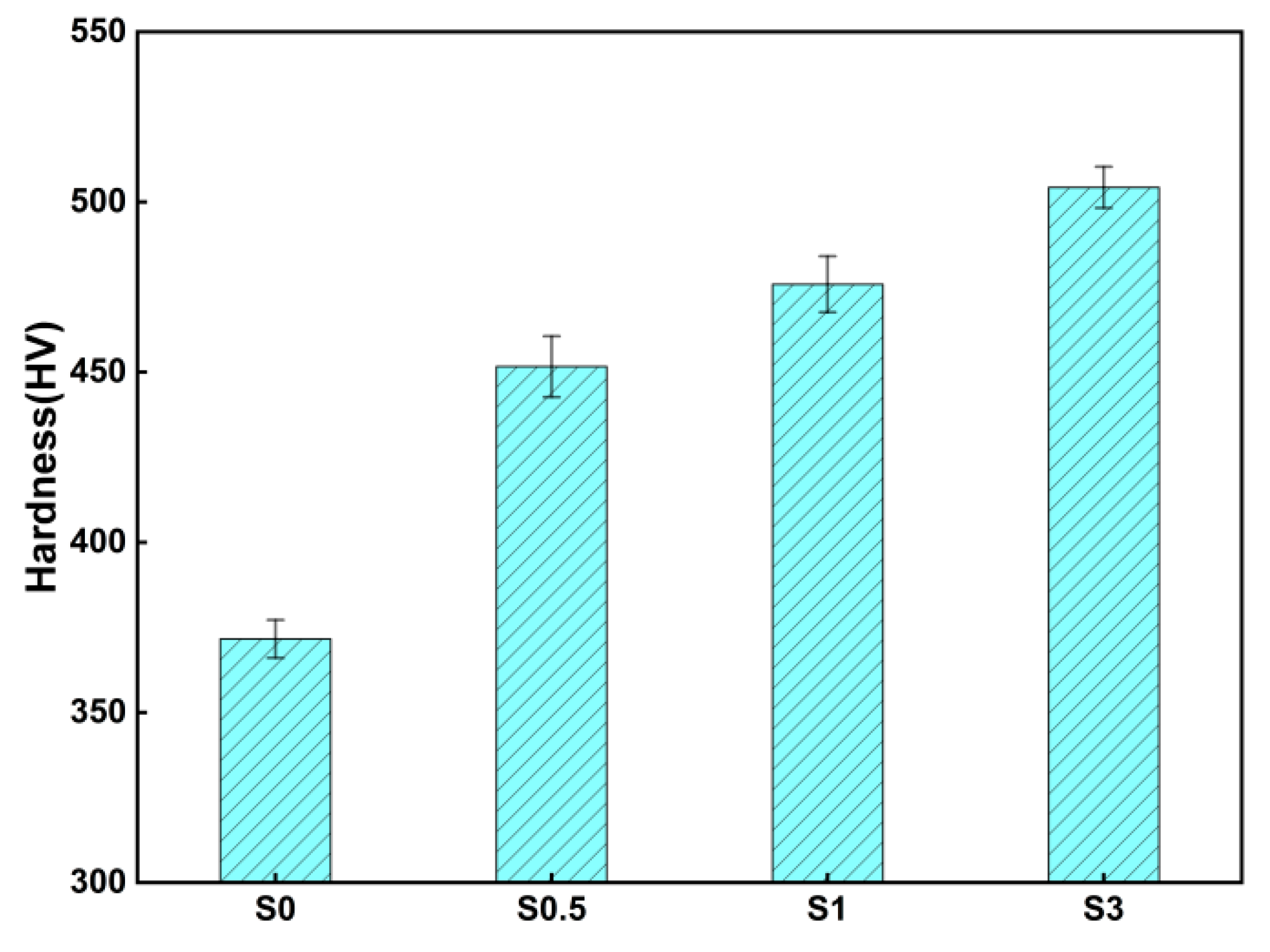
3.4.1. Hardness
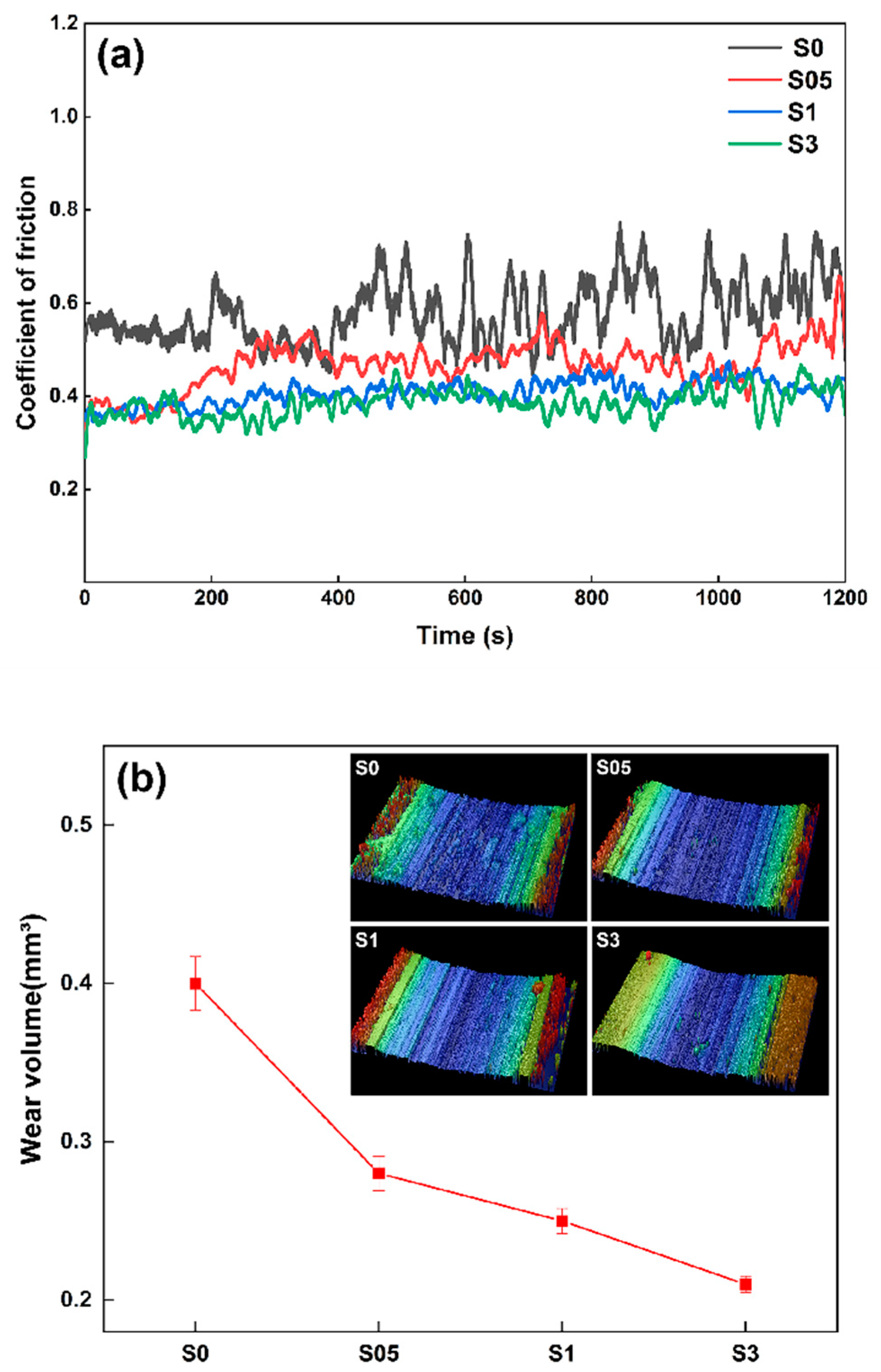
3.4.2. Wear Resistance
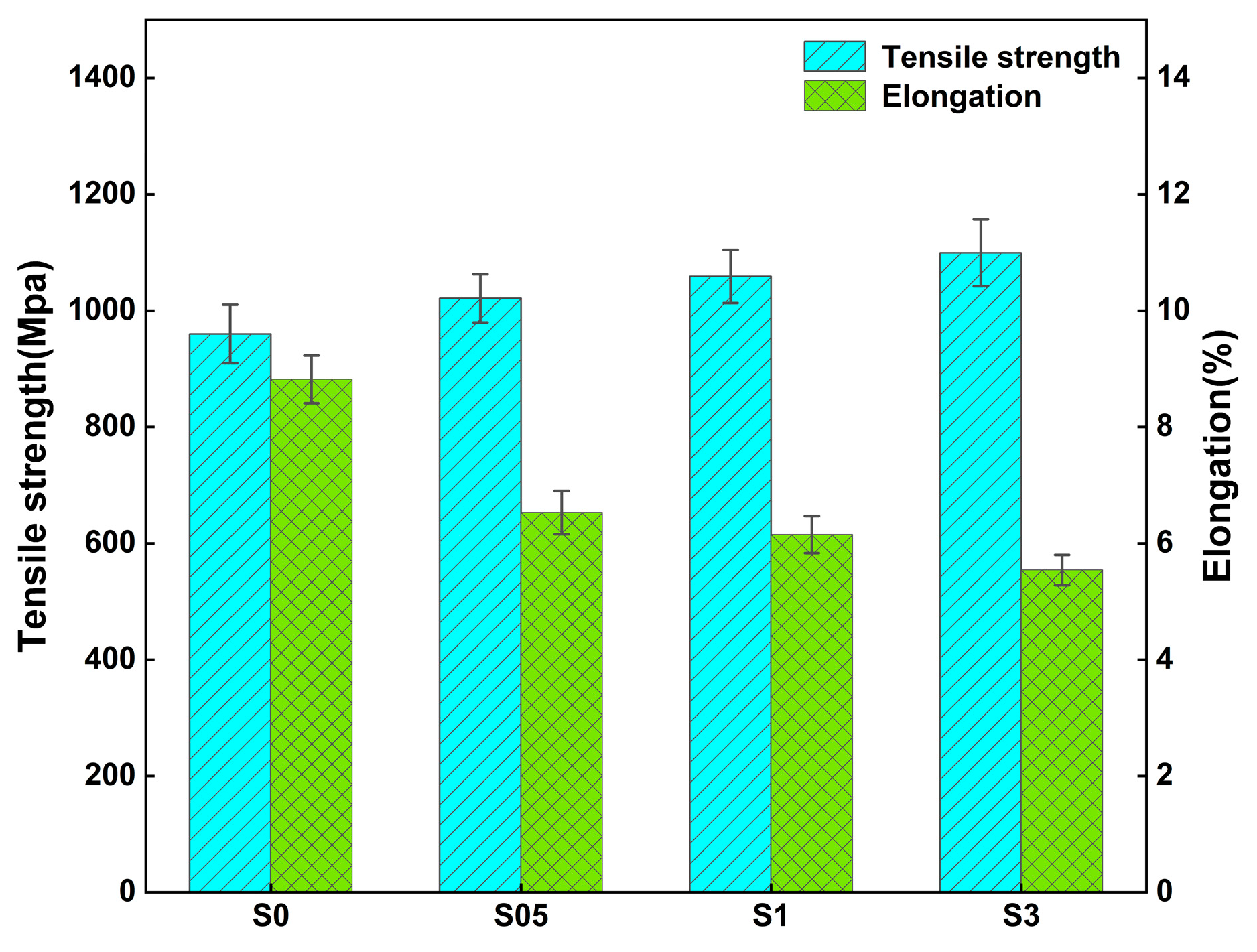
3.4.3. Room Temperature Stretching
4. Discussion
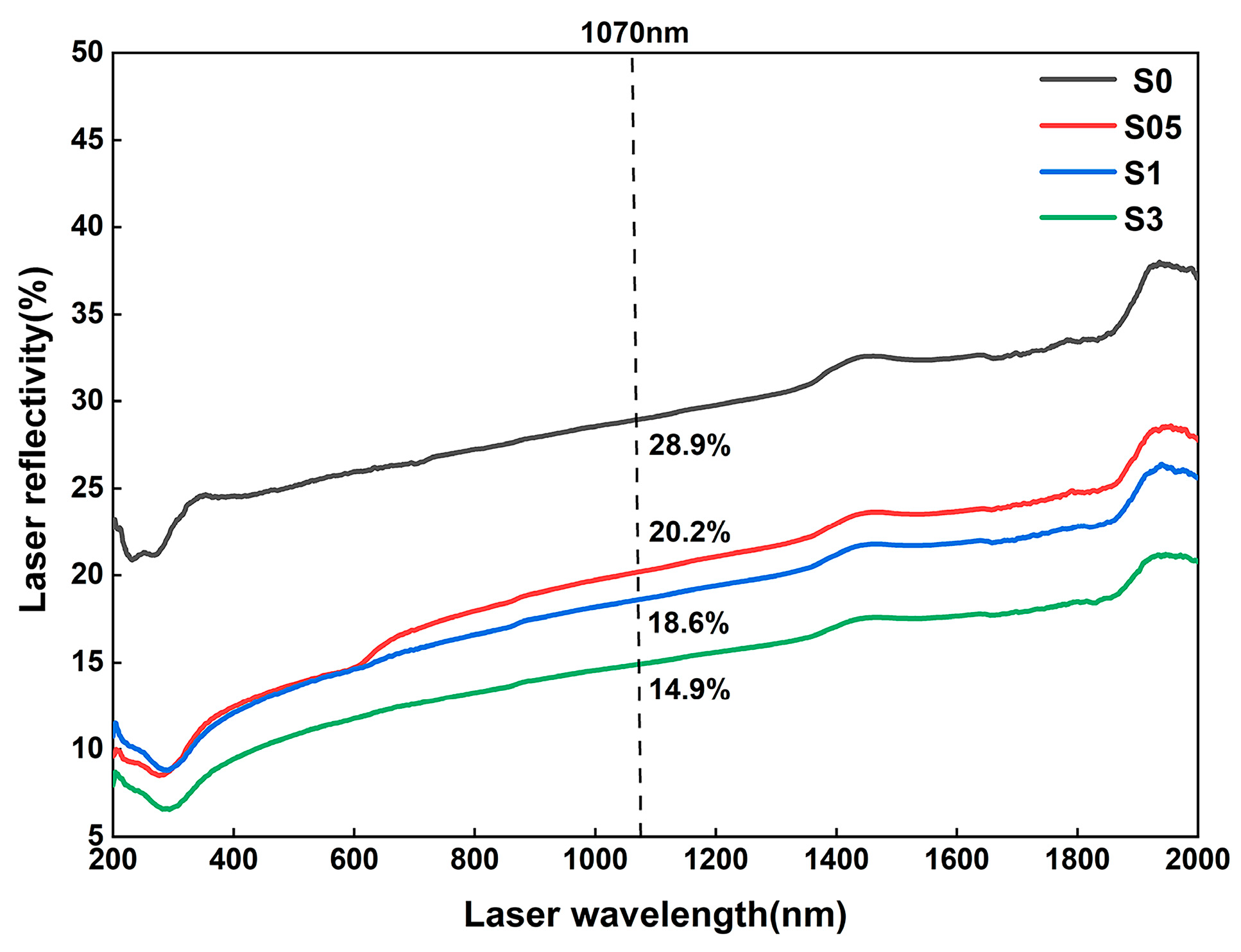
4.1. Effect of LaB6 Content on the Property of Fabrication
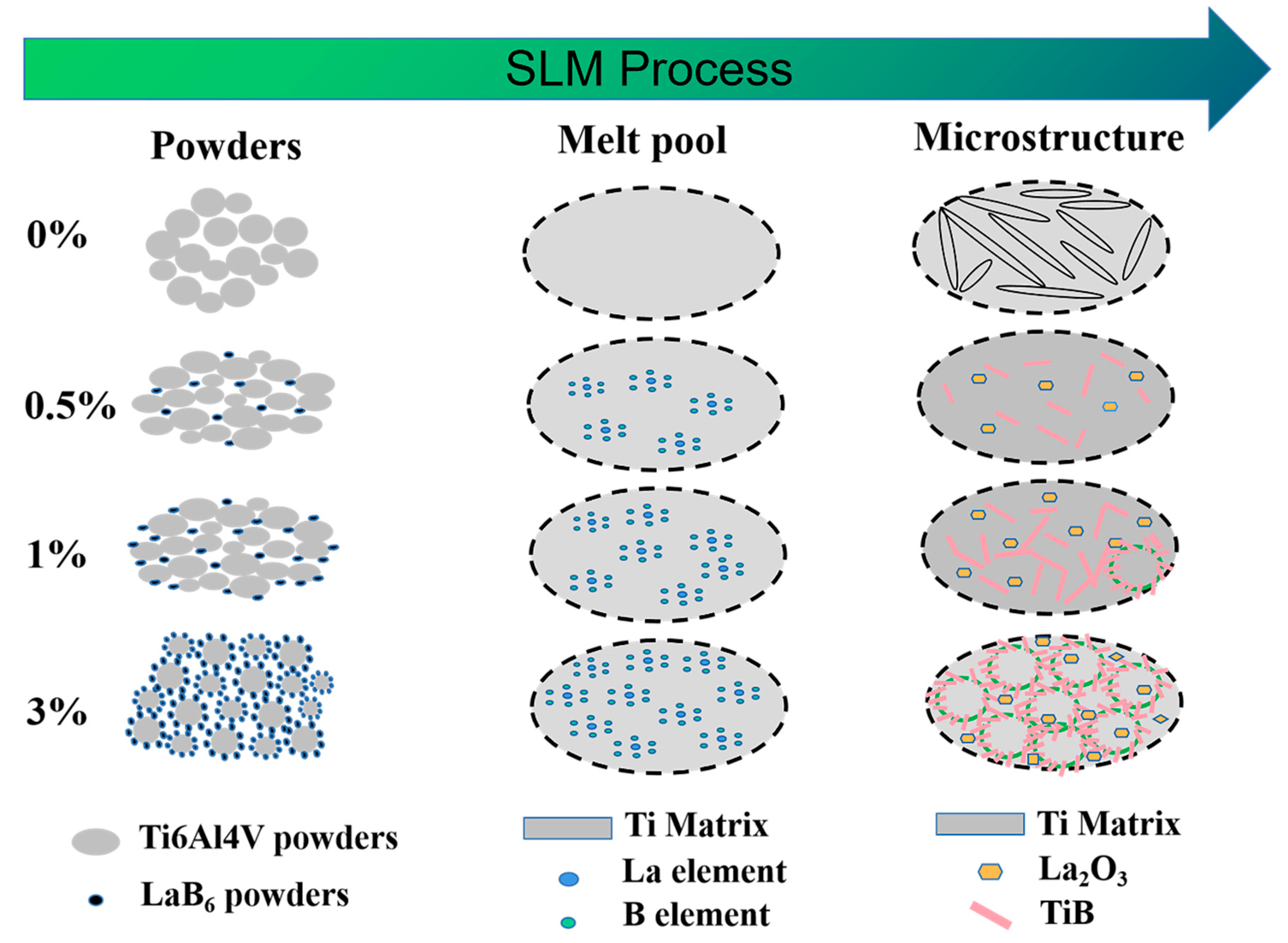
4.2. Effect of LaB6 Content on the Microstructure Evolution of Composites
4.3. Effect of LaB6 Content on the Mechanical Properties of Composites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kadirgama, K.; Harun, W.S.W.; Tarlochan, F.; Samykano, M.; Ramasamy, D.; Azir, M.Z.; Mehboob, H. Statistical and optimize of lattice structures with selective laser melting (SLM) of Ti6AL4V material. Int. J. Adv. Manuf. Technol. 2018, 97, 495–510. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Dargusch, M.S. Recent developments and opportunities in additive manufacturing of titanium-based matrix composites: A review. Int. J. Mach. Tools Manuf. 2018, 133, 85–102. [Google Scholar] [CrossRef]
- Imayev, V.M.; Gaisin, R.A.; Imayev, R.M. Microstructure and mechanical properties of near α titanium alloy based composites prepared in situ by casting and subjected to multiple hot forging. J. Alloys Compd. 2018, 762, 555–564. [Google Scholar] [CrossRef]
- Traxel, K.D.; Bandyopadhyay, A. Reactive-deposition-based additive manufacturing of Ti-Zr-BN composites. Addit. Manuf. 2018, 24, 11. [Google Scholar] [CrossRef]
- Cai, C.; He, S.; Li, L.; Teng, Q.; Song, B.; Yan, C.; Shi, Y. In-situ TiB/Ti-6Al-4V composites with a tailored architecture produced by hot isostatic pressing: Microstructure evolution, enhanced tensile properties and strengthening mechanisms. Compos. Part B Eng. 2019, 164, 546–558. [Google Scholar] [CrossRef]
- Duan, H.; Han, Y.; Lv, W.; Mao, J.; Wang, L.; Zhang, D. Effect of solid carburization on surface microstructure and hardness of Ti-6Al-4V alloy and (TiB + La2O3)/Ti-6Al-4V composite. Trans. Nonferrous Met. Soc. China 2016, 26, 1871–1877. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Qin, J.; Lv, W.; Zhang, D. Microstructure and mechanical properties of (TiB + La2O3)/Ti composites heat treated at different temperatures. Trans. Nonferrous Met. Soc. China 2014, 24, 1737–1743. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Lu, W.; Qin, J.; Zhang, D. Creep rupture property of in situ synthesized (TiB + La2O3)/Ti composite. Mater. Sci. Eng. A 2008, 488, 415–419. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Yang, D.; Mao, J. Effect of Heat Treatment on Creep Properties of in situ Synthesized (TiB + La2O3)/Ti Composite. Front. Mater. 2019, 6, 10. [Google Scholar] [CrossRef]
- Han, Y.; Duan, H.; Lu, W.; Wang, L.; Zhang, D. Fabrication and characterization of laminated Ti-(TiB + La2O3)/Ti composite. Prog. Nat. Sci. Mater. Int. 2015, 25, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Wang, H.; Sun, K.; Shen, G.; Meng, X.; Gao, Z.; Cai, W. Tailoring martensitic transformation and mechanical properties of Ti–Ni composite reinforced by network structure of in-situ TiB and La2O3 phase. Vacuum 2021, 184, 109894. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Han, Y.; Li, J.; Mao, J.; Lu, W. Compressive response and microstructural evolution of bimodal sized particulates reinforced (TiB + La2O3)/Ti composites. J. Alloys Compd. 2018, 732, 524–535. [Google Scholar] [CrossRef]
- Song, B.; Wang, Z.W.; Yan, Q.; Zhang, Y.J.; Zhang, J.L.; Cai, C.; Wei, Q.S.; Shi, Y.S. Integral method of preparation and fabrication of metal matrix composite: Selective laser melting of in-situnano/submicro-sized carbides reinforced iron matrix composites. Mater. Sci. Eng. A 2017, 707, 478–487. [Google Scholar] [CrossRef]
- Li, R.; Wang, M.; Yuan, T.; Song, B.; Chen, C.; Zhou, K.; Cao, P. elective laser melting of a novel Sc and Zr modified Al-6.2Mg alloy: Processing, microstructure, and properties. Powder Technol. 2017, 319, 117–128. [Google Scholar] [CrossRef]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective laser melting of an equiatomic CoCrFeMnNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Gu, D.; Meng, G.; Li, C.; Meiners, W.; Poprawe, R. Selective laser melting of TiC/Ti bulk nanocomposites: Influence of nanoscale reinforcement. Scr. Mater. 2021, 67, 185–188. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Yadroitsev, I. Microstructure and properties of intermetallic composite coatings fabricated by selective laser melting of Ti–SiC powder mixtures. Intermetallics 2014, 46, 147–155. [Google Scholar] [CrossRef]
- Cai, C.; Radoslaw, C.; Zhang, J.; Yan, Q.; Wen, S.; Song, B.; Shi, Y. In-situ preparation and formation of TiB/Ti-6Al-4V nanocomposite via laser additive manufacturing: Microstructure evolution and tribological behavior. Powder Technol. 2018, 342, 73–84. [Google Scholar] [CrossRef]
- Xia, M.; Liu, A.; Hou, Z.; Li, N.; Chen, Z.; Ding, H. Microstructure growth behavior and its evolution mechanism during laser additive manufacture of in-situ reinforced (TiB + TiC)/Ti composite. J. Alloys Compd. 2017, 728, 436–444. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Liu, J.; Zhou, Y.; Li, M.; Wen, S.; Wei, Q.; Yan, C.; Shi, Y. Enhanced nanohardness and new insights into texture evolution and phase transformation of TiAl/TiB2 in-situ metal matrix composites prepared via selective laser melting. Acta Mater. 2017, 136, 90–104. [Google Scholar] [CrossRef]
- Su, Y.; Luo, S.C.; Meng, L.; Gao, P.; Wang, Z.M. Selective Laser Melting of In Situ TiB/Ti6Al4V Composites: Formability, Microstructure Evolution and Mechanical Performance. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 774–788. [Google Scholar] [CrossRef]
- Han, C.; Babicheva, R.; Chua, J.D.Q.; Ramamurty, U.; Tor, S.B.; Sun, C.-N.; Zhou, K. Microstructure and mechanical properties of (TiB + TiC)/Ti composites fabricated in situ via selective laser melting of Ti and B4C powders. Addit. Manuf. 2020, 36, 101466. [Google Scholar] [CrossRef]
- Kovalev, O.B.; Gurin, A.M. Multivortex convection of metal in molten pool with dispersed impurity induced by laser radiation. Int. J. Heat Mass Transf. 2014, 68, 269–277. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, W.; Zhao, L.; Qin, J.; Zhang, D. Microstructure and mechanical property of in situ synthesized multiple-reinforced (TiB+TiC+La2O3)/Ti composites. J. Alloys Compd. 2008, 455, 210–214. [Google Scholar] [CrossRef]
- Bermingham, M.J.; McDonald, S.D.; Dargusch, M.S. Effect of trace lanthanum hexaboride and boron additions on microstructure, tensile properties and anisotropy of Ti-6Al-4V produced by additive manufacturing. Mater. Sci. Eng. A 2018, 719, 1–11. [Google Scholar] [CrossRef]
- Li, D.; Wang, K.; Yan, Z.; Cao, Y.; Misra RD, K.; Xin, R.; Liu, Q. Evolution of microstructure and tensile properties during the three-stage heat treatment of TA19 titanium alloy. Mater. Sci. Eng. A 2018, 716, 157–164. [Google Scholar] [CrossRef]
- Huang, W.; He, D.; Wang, H.; Qin, S.; Wang, L.; Xu, X. The Effect of Heat Treatment on the Anisotropy of Ti-6Al-4V by Selective Laser Melting. JOM 2022, 74, 2724–2732. [Google Scholar] [CrossRef]
- Xia, M.; Gu, D.; Yu, G.; Dai, D.; Chen, H.; Shi, Q. Porosity evolution and its thermodynamic mechanism of randomly packed powder-bed during selective laser melting of Inconel 718 alloy. Tools Manuf. 2017, 116, 96–106. [Google Scholar] [CrossRef]
- Tolochko, N.K.; Laoui, T.; Khlopkov, Y.V.; Mozzharov, S.E.; Titov, V.I.; Ignatiev, M.B. Absorptance of powder materials suitable for laser sintering. Rapid. Prototyp. J. 2000, 6, 155–160. [Google Scholar] [CrossRef]
- Tamirisakandala, S.; Bhat, R.B.; Tiley, J.S.; Miracle, D.B. Grain refinement of cast titanium alloys via trace boron addition. Scr. Mater. 2005, 53, 1421–1426. [Google Scholar] [CrossRef]
- Sulima, I.; Klimczyk, P.; Malczewski, P. Effect of TiB2 Particles on the Tribological Properties of Stainless Steel Matrix Composites. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 12–18. [Google Scholar] [CrossRef]
- Sen, I.; Tamirisakandala, S.; Miracle, D.; Ramamurty, U. Microstructural effects on the mechanical behavior of B-modified Ti–6Al–4V alloys. Acta Mater. 2007, 55, 4983–4993. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, S.; Zhao, Y.; Zhang, Q.; Wang, X.; Li, W.; Chen, D.; Zhang, L.C. Refined microstructure and enhanced wear resistance of titanium matrix composites produced by selective laser melting. Opt. Laser Technol. 2021, 34, 106644. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Chen, W.; Chen, C.; Lv, Y.; Zhang, X.; Lei, P.; Lin, Y.; Zhou, K. Effect of trace lanthanum hexaboride on the phase, grain structure, and texture of electron beam melted Ti-6Al-4V. Addit. Manuf. 2019, 30, 100873. [Google Scholar] [CrossRef]
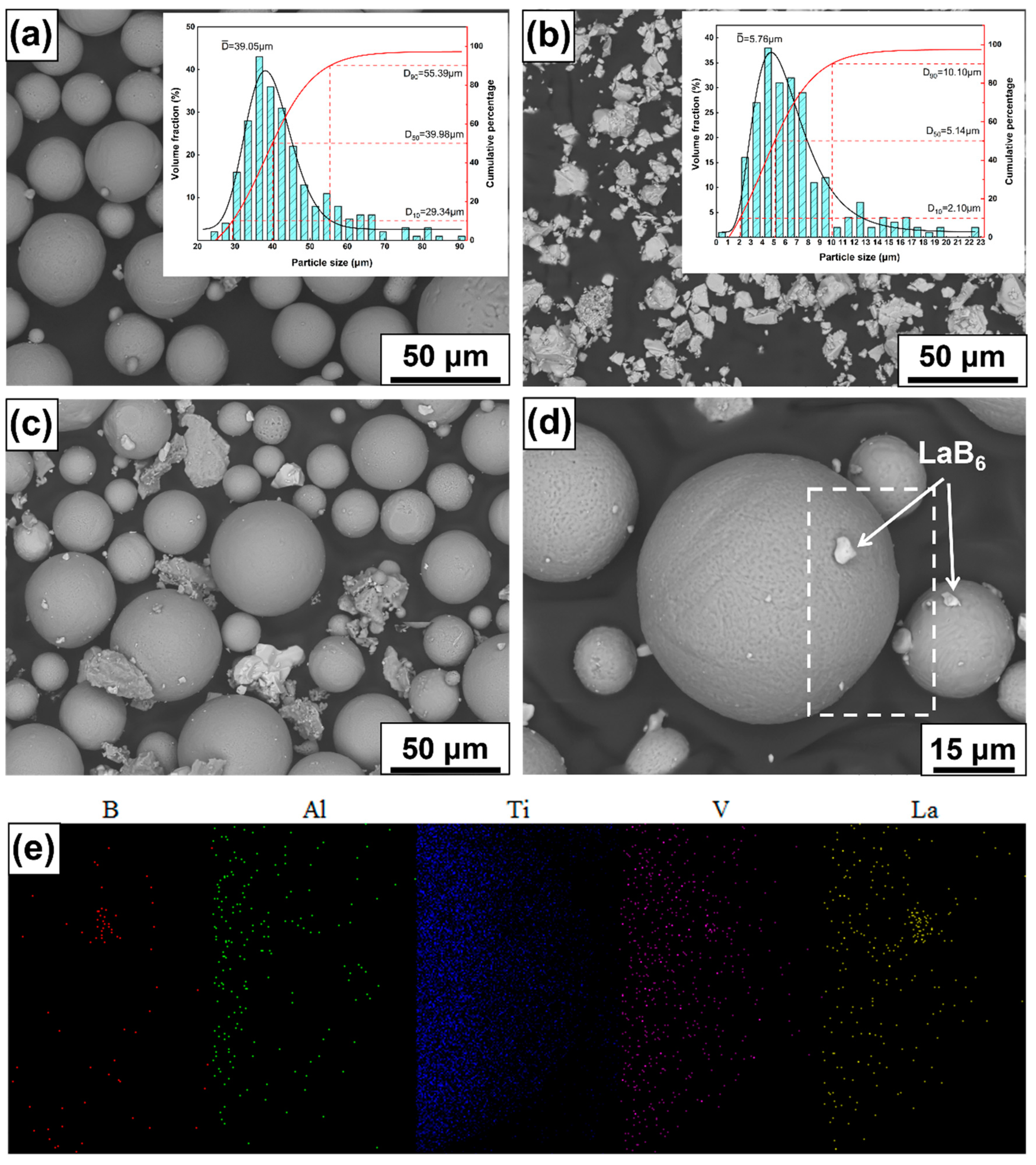




| Element | Ti | Al | V | Fe | C | N | H |
|---|---|---|---|---|---|---|---|
| Mass fraction/% | Bal. | 6.18 | 3.96 | 0.14 | 0.00078 | 0.013 | 0.003 |
| Fabricated Mode | LaB6 Content (wt%) | UTS (Mpa) | δ (%) |
|---|---|---|---|
| Wire arc additive manufacturing [25] | 0.1 | 852 | 13.2 |
| Electron beam melting [34] | 0.2 | 965.9 ± 12.8 | 10.1 ± 0.3 |
| Selective laser melting (this work) | 0.5 | 1021.1 ± 41.6 | 6.5 ± 0.4 |
| Selective laser melting (this work) | 1 | 1058.9 ± 45.8 | 6.2 ± 0.3 |
| Selective laser melting (this work) | 3 | 1099.4 ± 57.2 | 5.5 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Wang, H.; Huang, W.; Chen, X.; Lian, G.; Wang, Y. Microstructure and Mechanical Properties of LaB6/Ti-6Al-4V Composites Fabricated by Selective Laser Melting. Metals 2023, 13, 264. https://doi.org/10.3390/met13020264
He D, Wang H, Huang W, Chen X, Lian G, Wang Y. Microstructure and Mechanical Properties of LaB6/Ti-6Al-4V Composites Fabricated by Selective Laser Melting. Metals. 2023; 13(2):264. https://doi.org/10.3390/met13020264
Chicago/Turabian StyleHe, Dongdong, Hui Wang, Weidong Huang, Xinxi Chen, Guofu Lian, and Yu Wang. 2023. "Microstructure and Mechanical Properties of LaB6/Ti-6Al-4V Composites Fabricated by Selective Laser Melting" Metals 13, no. 2: 264. https://doi.org/10.3390/met13020264





