Linear Thermal Expansion and Specific Heat Capacity of Cu-Fe System Laser-Deposited Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Experiment, Materials, and Set of Specimens
- The first type—50 wt.% SS 316L + 50 wt.% bronze;
- The second type—25 wt.% SS 316L + 75 wt.% bronze;
- The third type—a multilayer gradient steel–bronze structure.
- Stainless steel (SS) 316L–5520 (fraction 50–150 μm) manufactured by Höganäs Belgium SA;
- Aluminum bronze, similar to UNS C61800 (fraction 45–125 μm), manufactured by Polema JSC (Tula, Russia);
- Tin bronze, similar to the copper-tin alloy CuSn10-B/CB480K, (fraction 100–140 μm) manufactured by Polema JSC (Tula, Russia);
- Chromium bronze, similar to UNS C18400 chromium copper, (fraction 63–125 μm) manufactured by Polema JSC (Tula, Russia).
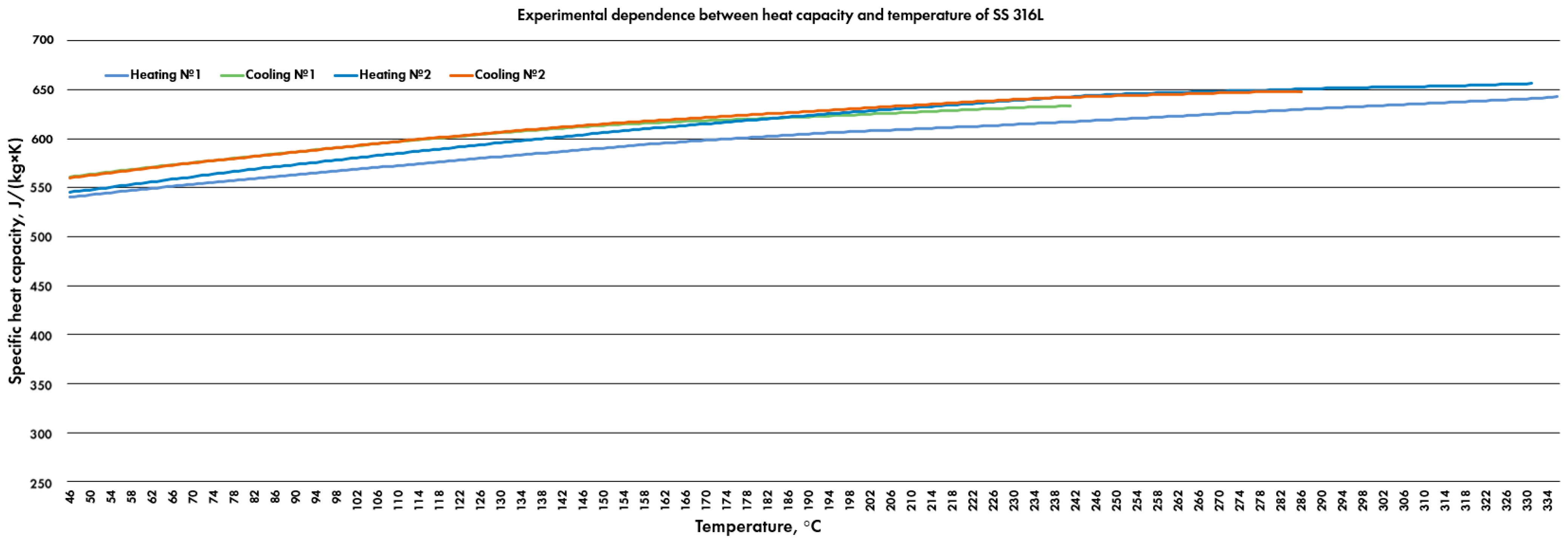
- Therefore, 8 groups of parts were fabricated in total (the alloy of alternating layers of tin bronze and SS 316L was excluded from consideration because it showed poor laser manufacturability and did not obtain an appropriate shape during the fabrication because of low heat consumption and poor adhesion of Sn bronze and steel). The study [23] approved this methodology and tested this set of groups (Table 2). It discussed the applicability of these alloys as transitional zones of Cu-Fe FGMs fabricated via the gradient path method and the alternating layers technique [23]. Additionally, commercially pure SS 316L was studied to validate the model and results in the current research.
2.2. Operation Conditions of DED
- The MX-1000 (InssTek, Daejeon, Republic of Korea) technological installation conducted DED in direct tooling mode [24]. In this mode, the laser power varied from low to high depending on the distance between a laser head and a melt pool. A 1 kW ytterbium fiber laser was the source of laser radiation. The scanning speed of all groups was 0.85 m/min. Gas flow rates amounted to: coaxial gas—0.85 L/min; powder gas—2.0 L/min; shield gas—10.0 L/min. Other fabrication conditions could be found in [23]. The geometrical parameters of the deposited powder beds were: bed height—300 μm; layer thickness—250 μm; bed width—800 μm; hatch spacing—300 μm.
2.3. Experimental Measurement of CLTE
2.4. Analytical Estimation of CLTE
2.5. Numerical Analysis of the Dependence between Thermal Expansion, Temperature, and Laser Treatment Parameters
2.6. Measurement of Specific Heat Capacity
2.7. Study of the Dependence between CLTE and Heat Capacity
3. Results
3.1. As-Built Parts
3.2. CLTE
3.2.1. Experimentally Measured CLTE
3.2.2. Theoretically Calculated CLTE
3.2.3. Calculation of Thermal Expansion Based on the Regression Models
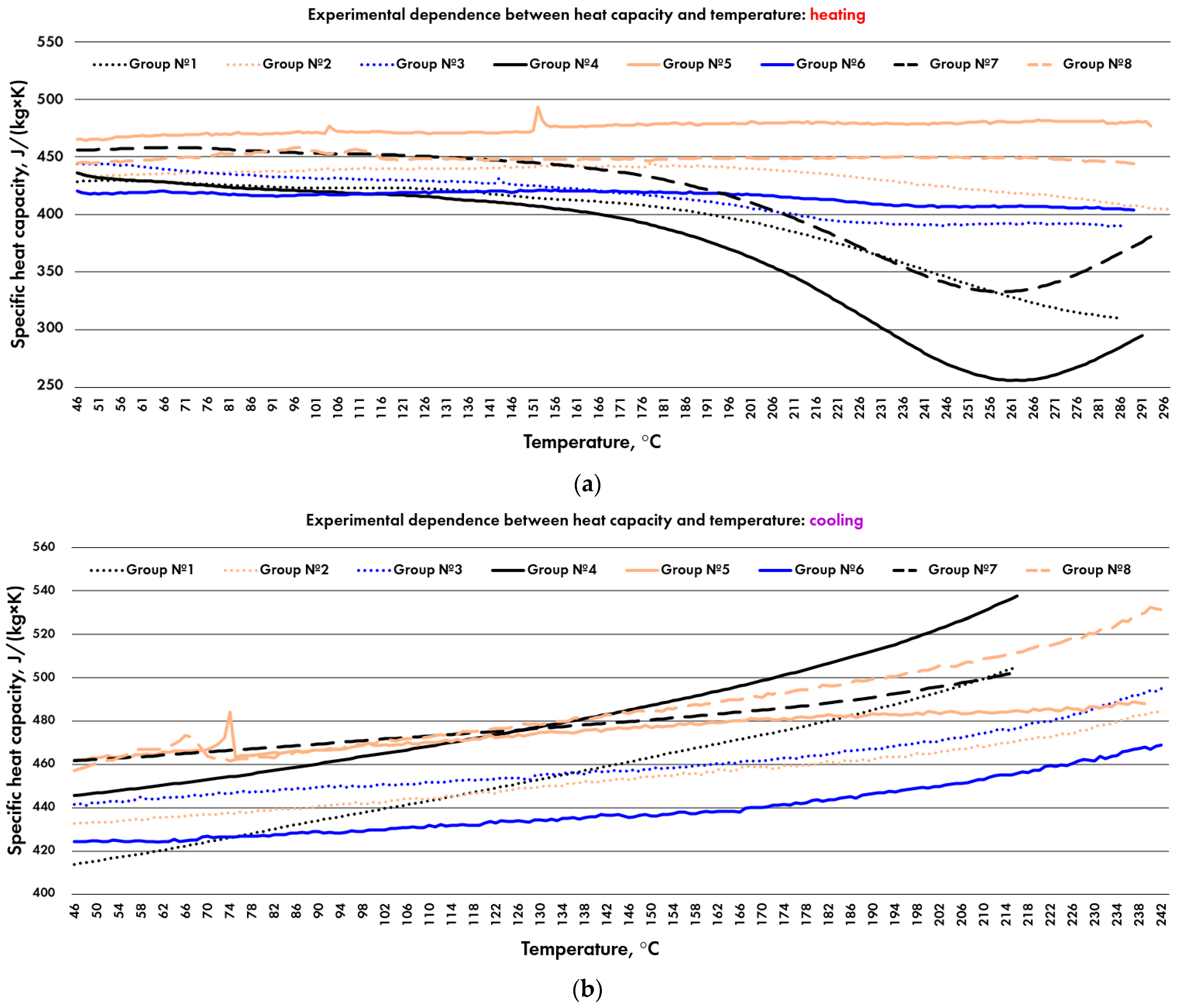
3.3. Specific Heat Capacity
3.4. Dependence between CLTE and Heat Capacity
4. Discussion
4.1. Discussion of As-Fabricated Parts
4.2. Discussion of CLTE
4.3. Discussion of Specific Heat Capacity
4.4. Dependence between CLTE and Specific Heat Capacity
5. Conclusions
- The analysis of the specific heat capacity of the Cu-Fe materials in a wider temperature range (up to ~900 °C);
- The study of the phase transitions of the Cu-Fe-Cr system in the low-temperature area;
- The fabrication and the tests of the real functionally graded parts using the results of the conducted experiments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Svetlizky, D.; Das, M.; Zheng, B.; Vyatskikh, A.L.; Bose, S.; Bandyopadhyay, A.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed energy deposition (DED) additive manufacturing: Physical characteristics, defects, challenges and applications. Mater. Today 2021, 49, 271–295. [Google Scholar] [CrossRef]
- Ghosal, P.; Chandra Majumder, M.; Chattopadhyay, A. Study on direct laser metal deposition. Mater. Today Proc. 2018, 5, 12509–12518. [Google Scholar] [CrossRef]
- Ahn, D.-G. Directed Energy Deposition (DED) Process: State of the Art. Int. J. Precis. Eng. Manuf. Green Technol. 2021, 8, 703–742. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Roberts, S.; Otis, R.; Kolodziejska, J.; Dillon, R.P.; Suh, J.-o.; Shapiro, A.A.; Liu, K.-Z.; Borgonia, J.-P. Developing gradient metal alloys through radial deposition additive manufacturing. Sci. Rep. 2014, 4, 5357. [Google Scholar] [CrossRef]
- Feenstra, D.R.; Banerjee, R.; Fraser, H.L.; Huang, A.; Molotnikov, A.; Birbilis, N. Critical review of the state of the art in multi-material fabrication via directed energy deposition. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100924. [Google Scholar] [CrossRef]
- Ansari, M.; Jabari, E.; Toyserkani, E. Opportunities and challenges in additive manufacturing of functionally graded metallic materials via powder-fed laser directed energy deposition: A review. J. Mater. Process. Technol. 2021, 294, 117117. [Google Scholar] [CrossRef]
- Farzaneh, A.; Khorasani, M.; Farabi, E.; Gibson, I.; Leary, M.; Ghasemi, A.; Rolfe, B. Sandwich structure printing of Ti-Ni-Ti by directed energy deposition. Virtual Phys. Prototyp. 2022, 17, 1006–1030. [Google Scholar] [CrossRef]
- Chen, B.; Wang, T.; Xi, X.; Tan, C.; Song, X. Additive manufacturing of Ti-Al functionally graded material by laser based directed energy deposition. Rapid Prototyp. J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Tudu, N.; Baruah, M.; Prasad, S.B. Comparison of properties at the interface of deposited IN625 and mixture of IN625 SS304L by laser directed energy deposition and SS304L substrate. Rapid Prototyp. J. 2022. [Google Scholar] [CrossRef]
- Karnati, S.; Sparks, T.E.; Liou, F.; Newkirk, J.W.; Taminger, K.M.B.; Seufzer, W.J. Laser metal deposition of functionally gradient materials from elemental copper and nickel powders. In Proceedings of the 26th Solid Freeform Fabrication Symposium, Austin, TX, USA, 10–12 August 2015. [Google Scholar]
- Jamroziak, K.; Roik, T.A.; Gavrysh, O.A.; Vitsiuk, I.; Lesiuk, G.; Correia, J.A.F.O.; De Jesus, A.M.P. Improved manufacturing performance of a new antifriction composite parts based on copper. Eng. Fail. Anal. 2018, 91, 225–233. [Google Scholar] [CrossRef]
- Makarenko, K.; Dubinin, O.; Shishkovsky, I. Analytical evaluation of the dendritic structure parameters and crystallization rate of laser-deposited Cu-Fe functionally graded materials. Materials 2020, 13, 5665. [Google Scholar] [CrossRef] [PubMed]
- Moharana, B.R.; Sahu, S.K.; Maiti, A.; Sahoo, S.K.; Moharana, T.K. An experimental study on joining of AISI 304 SS to Cu by Nd-YAG laser welding process. Mater. Today Proc. 2020, 33, 5262–5268. [Google Scholar] [CrossRef]
- Makarenko, K.I.; Shishkovsky, I.V. Direct Energy Deposition of Cu-Fe System Functionally Graded Structures. IOP Conf. Ser. Mater. Sci. Eng. 2020, 969, 012104. [Google Scholar] [CrossRef]
- Pichler, P.; Simonds, B.J.; Sowards, J.W.; Pottlacher, G. Measurements of thermophysical properties of solid and liquid NIST SRM 316L stainless steel. J. Mater. Sci. 2020, 55, 4081–4093. [Google Scholar] [CrossRef] [PubMed]
- Hidnert, P. Thermal expansion of some bronzes. J. Res. Natl. Bur. Stand. 1943, 30, 75–88. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Pan, T.; Chen, Y.; Zhang, Y.; Li, W.; Liou, F. Additive manufacturing of copper-tool steel dissimilar joining: Experimental characterization and thermal modeling. Mater. Charact. 2020, 170, 110692. [Google Scholar] [CrossRef]
- Gao, N.; Starink, M.J.; Langdon, T.G. Using differential scanning calorimetry as an analytical tool for ultrafine grained metals processed by severe plastic deformation. Mater. Sci. Technol. 2013, 25, 687–698. [Google Scholar] [CrossRef]
- Makarenko, K.; Dubinin, O.; Shishkovsky, I. Direct Energy Deposition of Cu-Fe System Functionally Graded Materials: Miscibility Aspects, Cracking Sources, and Methods of Assisted Manufacturing. In Advanced Additive Manufacturing; Shishkovsky, I.V., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Chen, N.; Ali Khan, H.; Wan, Z.; Lippert, J.; Sun, H.; Shang, S.-L.; Liu, Z.-K.; Li, J. Microstructural characteristics and crack formation in additively manufactured bimetal material of 316L stainless steel and Inconel 625. Addit. Manuf. 2020, 32, 101037. [Google Scholar] [CrossRef]
- Koga, N.; Tomono, S.; Umezawa, O. Low-temperature tensile properties of Cu-Fe laminated sheets with various number of layers. Mater. Sci. Eng. A 2021, 811, 141066. [Google Scholar] [CrossRef]
- Koga, N.; Zhang, W.; Umezawa, O.; Tschan, V.; Sas, J.; Weiss, K.P. Temperature dependence on tensile properties of Cu-40mass%Fe dual phase alloy. IOP Conf. Ser. Mater. Sci. Eng. 2017, 279, 012004. [Google Scholar] [CrossRef]
- Makarenko, K.I.; Konev, S.D.; Dubinin, O.N.; Shishkovsky, I.V. Mechanical characteristics of laser-deposited sandwich structures and quasi-homogeneous alloys of Fe-Cu system. Mater. Des. 2022, 224, 111313. [Google Scholar] [CrossRef]
- Makarenko, K.; Dubinin, O.; Shornikov, P.; Shishkovsky, I. Specific aspects of the transitional layer forming in the aluminium bronze–stainless steel functionally graded structures after laser metal deposition. Procedia CIRP 2020, 94, 346–351. [Google Scholar] [CrossRef]
- Yakout, M.; Elbestawi, M.A.; Veldhuis, S.C. A study of thermal expansion coefficients and microstructure during selective laser melting of Invar 36 and stainless steel 316L. Addit. Manuf. 2018, 24, 405–418. [Google Scholar] [CrossRef]
- Pavlovic, A.S.; Suresh Babu, V.; Seehra, M.S. High-temperature thermal expansion of binary alloys of Ni with Cr, Mo and Re: A comparison with molecular dynamics simulations. J. Phys. Condens. Matter 1996, 8, 3139–3149. [Google Scholar] [CrossRef]
- Ferreira, A.J.M.; Batra, R.C.; Roque, C.M.C.; Qian, L.F.; Martins, P.A.L.S. Static analysis of functionally graded plates using third-order shear deformation theory and a meshless method. Compos. Struct. 2005, 69, 449–457. [Google Scholar] [CrossRef]
- Large-Area Picosecond Photo-Detectors Project Database. Chapter 17. Material Expansion Coefficients. Linear Thermal Expansion Coefficients of Metals and Alloys//The University of Chicago, Argonne, Fermilab and Berkeley. Available online: https://psec.uchicago.edu/thermal_coefficients/cte_metals_05517-0143.pdf (accessed on 14 March 2022).
- AZo Materials. High-Leaded Tin Bronze UNS C93200. Available online: http://www.azom.com/article.aspx?ArticleID=6552 (accessed on 26 May 2022).
- Dubenko, I.S.; Gaidukova, I.Y.; Granovsky, S.A.; Gratz, E.; Gurjazkas, D.; Markosyan, A.S.; Müller, H. Strongly Enhanced Thermal Expansion in Binary Y-Mn Intermetallic Compounds. Solid State Commun. 1997, 103, 495–499. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Chang, H.-X. Mechanical Behavior of Rectangular Plates with Functionally Graded Coefficient of Thermal Expansion Subjected to Thermal Loading. J. Therm. Stress. 2008, 31, 368–388. [Google Scholar] [CrossRef]
- Drebushchak, V.A.; Turkin, A.I. Relationship between heat capacity and thermal expansion derived from the Lennard-Jones potential. J. Therm. Anal. Calorim. 2001, 65, 745–753. [Google Scholar] [CrossRef]
- Oxtoby, D.W.; Gillis, H.P.; Butler, L.J. Principles of Modern Chemistry, 8th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Liu, M.; Kumar, A.; Bukkapatnam, S.; Kuttolamadom, M. A review of the anomalies in directed energy deposition (DED) processes & potential solutions-part quality & defects. Proc. Manuf. 2021, 53, 507–518. [Google Scholar] [CrossRef]
- Liu, Z.; Kim, H.; Liu, W.; Cong, W.; Jiang, Q.; Zhang, H. Influence of energy density on macro/micro structures and mechanical properties of as-deposited Inconel 718 parts fabricated by laser engineered net shaping. J. Manuf. Process. 2019, 42, 96–105. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Keicher, D.M.; Miller, W.D. LENSTM moves beyond RP to direct fabrication. J. Met. Powder Rep. 1998, 12, 26–28. [Google Scholar] [CrossRef]
- Wang, W.; Takata, N.; Suzuki, A.; Kobashi, M.; Kato, M. Formation of multiple intermetallic phases in a hypereutectic Al-Fe binary alloy additively manufactured by laser powder bed fusion. Intermetallics 2020, 125, 106892. [Google Scholar] [CrossRef]
- Dubinin, O.N.; Chernodubov, D.A.; Kuzminova, Y.O.; Shaysultanov, D.G.; Akhatov, I.S.; Stepanov, N.D.; Evlashin, S.A. Gradient soft magnetic materials produced by additive manufacturing from non-magnetic powders. J. Mater. Process. Technol. 2022, 300, 117393. [Google Scholar] [CrossRef]
- Cui, S.; Jung, I.-H. Thermodynamic modeling of the Cu-Fe-Cr and Cu-Fe-Mn systems. Calphad 2017, 56, 241–259. [Google Scholar] [CrossRef]
- Raghavan, V. Cr-Cu-Fe (Chromium-Copper-Iron). J. Phase Equilibria 2002, 23, 257. [Google Scholar] [CrossRef]
- Fernee, H.; Nairn, J.; Atrens, A. Cu-rich corner of the Cu-Fe-Cr phase diagram. J. Mater. Sci. Lett. 2011, 20, 2213–2215. [Google Scholar] [CrossRef]
- Dreval, L.A.; Turchanin, M.A.; Abdulov, A.R.; Bondar, A.A. Thermodynamic assessment of the Cu–Fe–Cr phase diagram. Chem. Met. Alloy. 2010, 3, 132–139. [Google Scholar] [CrossRef]
- Sato, K. Polymorphic transformations in crystal growth. J. Phys. D Appl. Phys. 1993, 26, B77–B84. [Google Scholar] [CrossRef]
- Tsymburskaya, A.T. Polymorphic transformations of metals. Izv. Vyss. Uchebnykh Zaved. Fiz. 1971, 9, 141–144. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Kraposhin, V.S.; Talis, A.L.; Simich-Lafitskii, N.D. Transfer of diagonals in a rhombus: Elementary act of polymorphic transformation. Analysis of the energy threshold of transformation in metals. Metalloved. Termicheskaya Obrab. Metalloved. 2020, 2, 8–17. [Google Scholar] [CrossRef]
- Wilthan, B.; Reschab, H.; Tanzer, R.; Schuetzenhoefer, W.; Pottlacher, G. Thermophysical properties of a chromium–nickel–molybdenum steel in the solid and liquid phases. Int. J. Thermophys. 2008, 29, 434–444. [Google Scholar] [CrossRef]
- Kaschnitz, E.; Kaschnitz, H.; Schleutker, T.; Guelhan, A.; Bonvoisin, B. Electrical resistivity measured by millisecond pulse-heating in comparison to thermal conductivity of the stainless steel AISI 316L at elevated temperature. High Temp. High Press. 2017, 40, 27. [Google Scholar] [CrossRef]
- Fetni, S.; Enrici, T.M.; Niccolini, T.; Tran, H.S.; Dedry, O.; Duchêne, L.; Mertens, A.; Habraken, A.M. Thermal model for the directed energy deposition of composite coatings of 316L stainless steel enriched with tungsten carbides. Mater. Des. 2021, 204, 109661. [Google Scholar] [CrossRef]
- Kumar, K.S. Numerical modeling and simulation of a butt joint welding of AISI 316L stainless steels using a pulsed laser beam. Mater. Today Proceed. 2015, 2, 2256–2266. [Google Scholar] [CrossRef]
- Sayman, O.; Sen, F.; Celik, E.; Arman, Y. Thermal stress analysis of WC-co/Cr-Ni multilayer coatings on 316L steel substrate during cooling process. Mater. Des. 2009, 30, 770–774. [Google Scholar] [CrossRef]


| Element | Al Bronze | Sn Bronze | Cr Bronze |
|---|---|---|---|
| Cu | Base | Base | Base |
| Al | 9.50 | 0.05 | – |
| Cr | – | – | 0.76 |
| Fe | 1.00 | 0.10 | 0.05 |
| Ni | – | 0.10 | 0.05 |
| O2 | – | – | 0.05 |
| P | – | – | 0.02 |
| Pb | 0.02 | 0.05 | – |
| S | – | – | 0.01 |
| Sb | 0.05 | 0.05 | – |
| Si | 0.10 | 0.05 | – |
| Sn | 0.05 | 9.96 | – |
| Zn | 0.05 | 0.11 | – |
| Group No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Description | C61800 + SS (1:1) | C18400 + SS (1:1) | CB480K + SS (1:1) | C61800 + SS (3:1) | C18400 + SS (3:1) | CB480K + SS (3:1) | C61800 + SS(alt.) | C18400 + SS (alt.) |
| Group No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| CLTE, K−1 10−5 | 1.640 | 1.625 | 1.700 | 1.660 | 1.638 | 1.750 | 1.640 1 | 1.625 1 |
| Cycle | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 |
|---|---|---|---|---|---|---|---|---|
| Heating | 409 10.2 | 436 10.9 | 421 10.5 | 386 9.7 | 474 11.9 | 415 10.4 | 428 10.7 | 450 11.3 |
| Cooling | 445 11.1 | 446 11.2 | 452 11.3 | 473 11.8 | 472 11.8 | 433 10.8 | 474 11.9 | 476 11.9 |
| Cycle | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 |
|---|---|---|---|---|---|---|---|---|
| Heating | 24.40.6 | 26.00.7 | 25.10.6 | 23.80.6 | 29.20.7 | 25.60.6 | 25.60.6 | 26.90.7 |
| Cooling | 26.60.7 | 26.60.7 | 27.00.7 | 29.10.7 | 29.10.7 | 26.70.7 | 28.30.7 | 28.40.7 |
| Cycle | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 |
|---|---|---|---|---|---|---|---|---|
| Heating | 0.73 | 0.75 | 1.16 | 0.85 | 0.83 | 0.52 | 0.57 | |
| Cooling | 9.350.79 | 0.76 | 0.80 | 1.41 | 0.85 | 0.87 | 0.57 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarenko, K.I.; Dubinin, O.N.; Shishkovsky, I.V. Linear Thermal Expansion and Specific Heat Capacity of Cu-Fe System Laser-Deposited Materials. Metals 2023, 13, 451. https://doi.org/10.3390/met13030451
Makarenko KI, Dubinin ON, Shishkovsky IV. Linear Thermal Expansion and Specific Heat Capacity of Cu-Fe System Laser-Deposited Materials. Metals. 2023; 13(3):451. https://doi.org/10.3390/met13030451
Chicago/Turabian StyleMakarenko, Konstantin I., Oleg N. Dubinin, and Igor V. Shishkovsky. 2023. "Linear Thermal Expansion and Specific Heat Capacity of Cu-Fe System Laser-Deposited Materials" Metals 13, no. 3: 451. https://doi.org/10.3390/met13030451
APA StyleMakarenko, K. I., Dubinin, O. N., & Shishkovsky, I. V. (2023). Linear Thermal Expansion and Specific Heat Capacity of Cu-Fe System Laser-Deposited Materials. Metals, 13(3), 451. https://doi.org/10.3390/met13030451








