Optical Recrystallization of Nanocrystalline Silicon Ribbons
Abstract
:1. Introduction
2. Materials and Methods
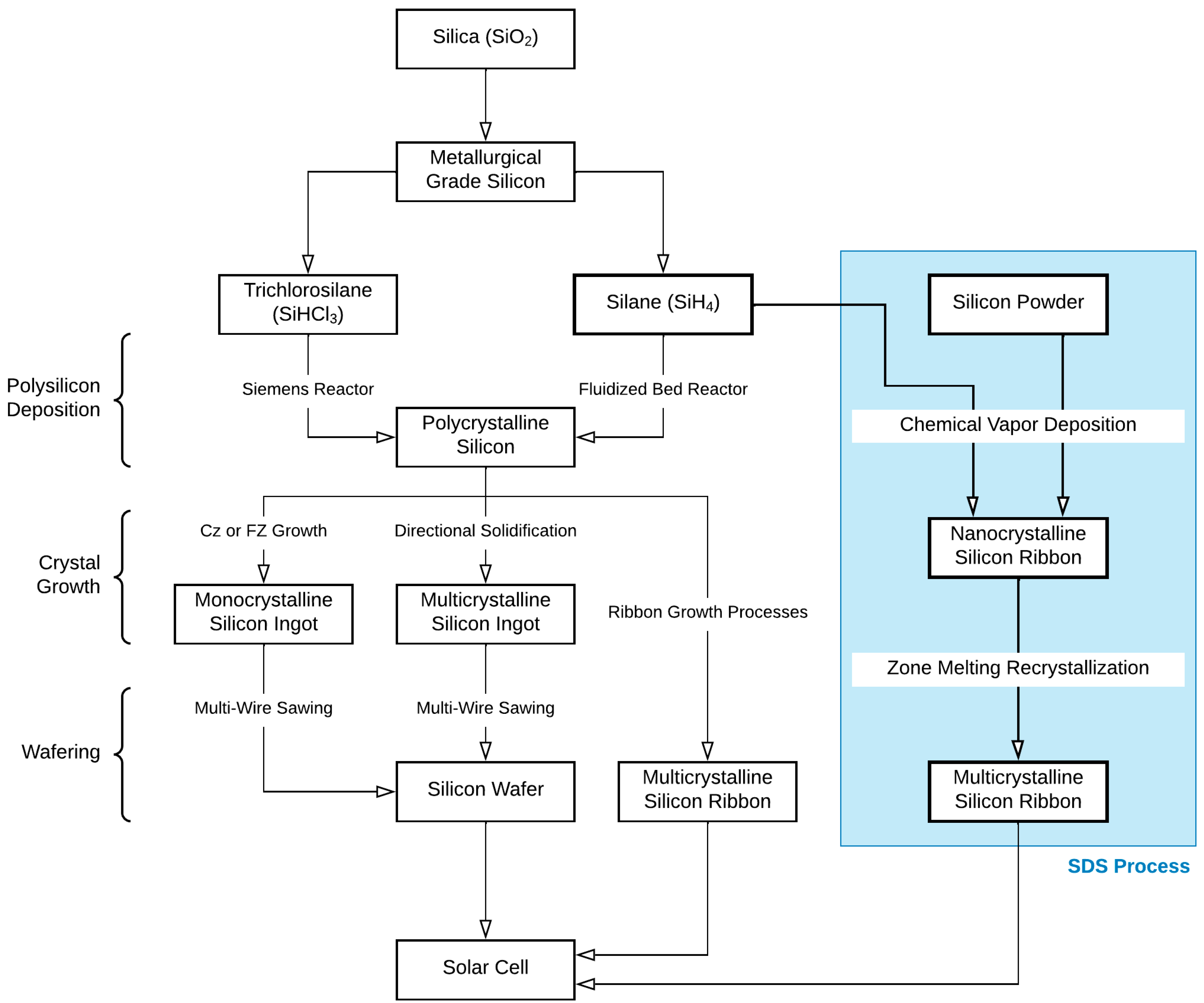
2.1. SDS Approach
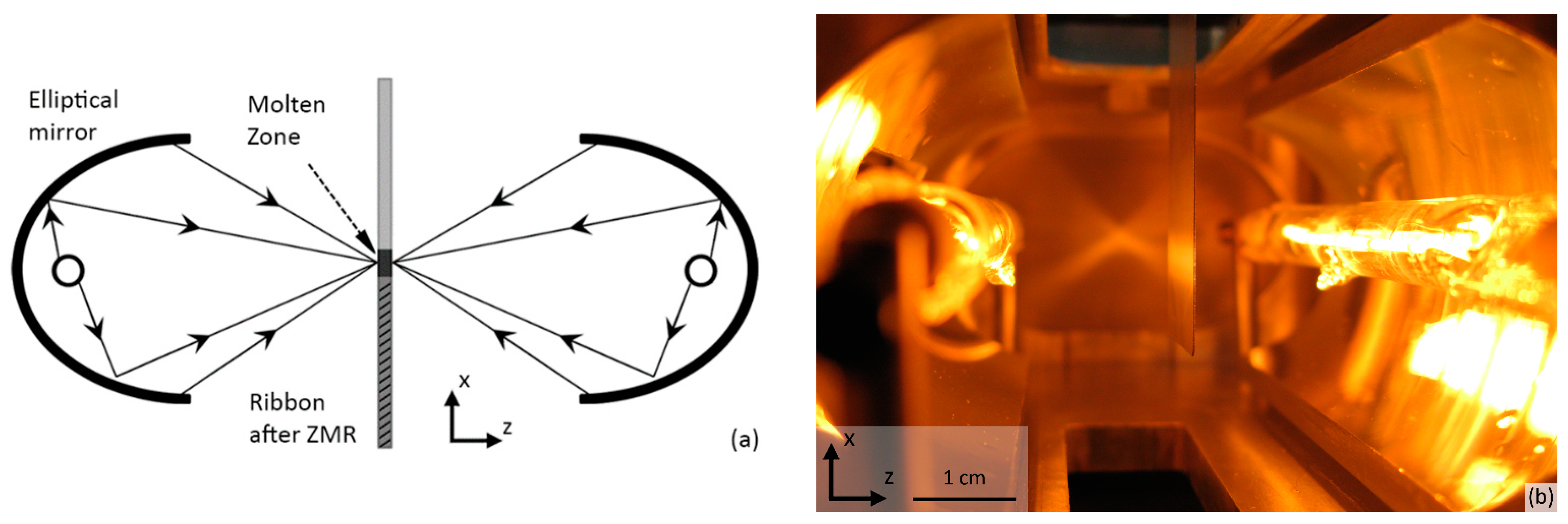
2.2. Experimental Setup
2.3. Characterization
3. Results
3.1. Pre-Ribbon Recrystallization
3.2. SDS Pre-Ribbon Recrystallization and the Role of the Powder Grain Size
3.3. Characterization of SDS Ribbons
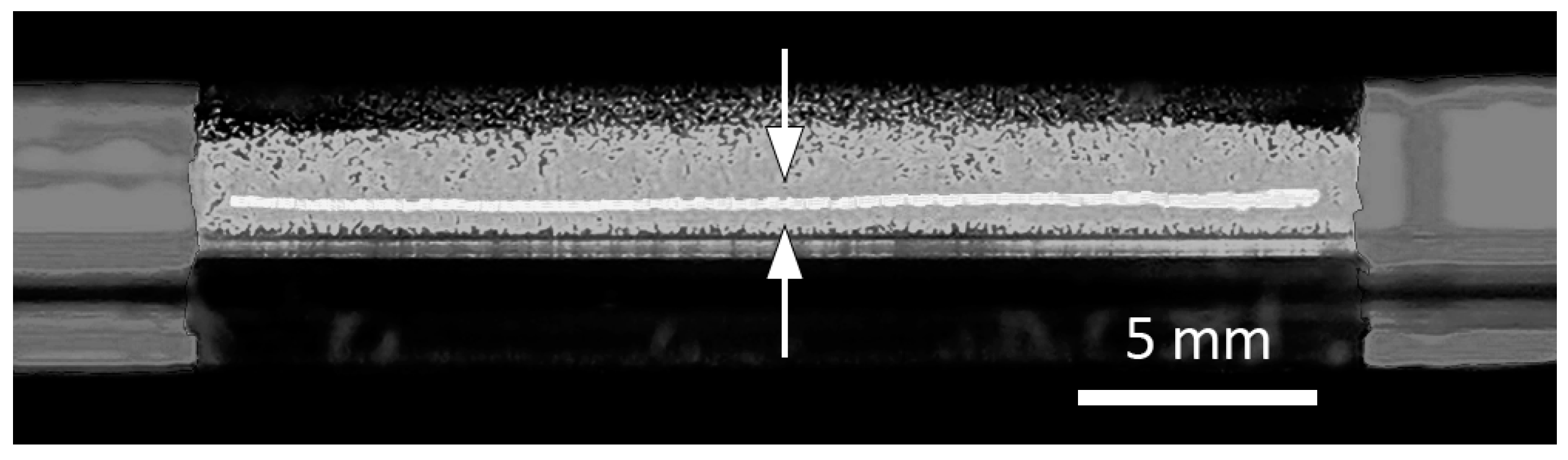
3.3.1. SDS Ribbon Thickness
3.3.2. Resistivity and Minority Carrier Lifetime Measurements
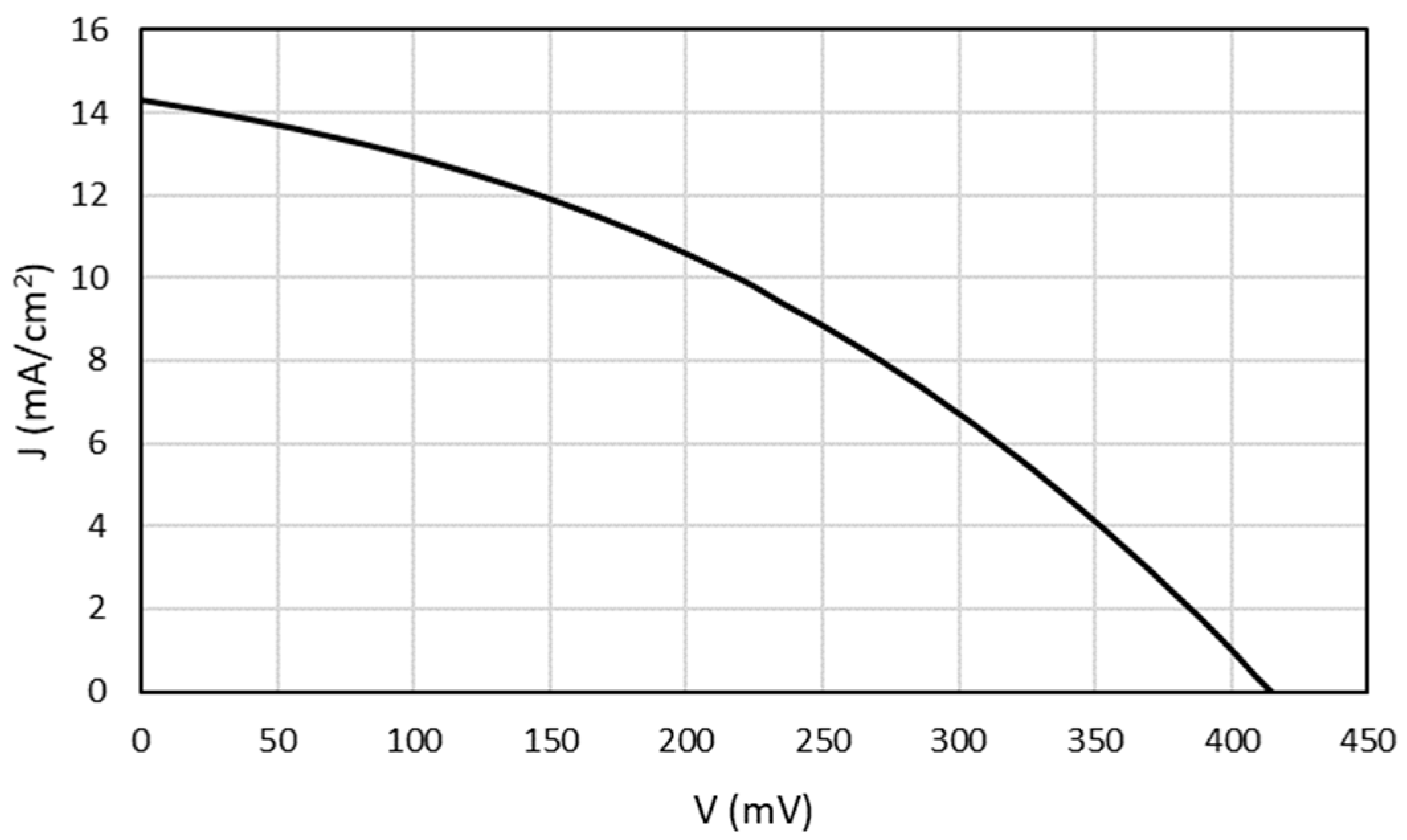
3.3.3. Solar Cell Manufacture and Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ITRPV. International Technology Roadmap for Photovoltaic. 2022. Available online: https://www.vdma.org/international-technology-roadmap-photovoltaic (accessed on 15 December 2022).
- Kost, C.; Shammugam, S.; Fluri, V.; Peper, D.; Memar, A.D.; Schlegl, T. Levelized Cost of Electricity Renewable Energy Technologies. 2021. Available online: https://www.ise.fraunhofer.de/content/dam/ise/en/documents/publications/studies/EN2021_Fraunhofer-ISE_LCOE_Renewable_Energy_Technologies.pdf (accessed on 15 December 2022).
- International Energy Agency. World Energy Outlook 2019; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Philipps, S.; Warmuth, W. Fraunhofer ISE: Photovoltaics Report; Fraunhofer ISE: Freiburg, Germany, 2022. [Google Scholar]
- Pfann, W.G. Techniques of zone melting and crystal growing. Solid State Phys. 1957, 4, 423–521. [Google Scholar]
- Pfann, W.G.; Theuerer, H.C. Applications of zone melting to analytical chemistry. Anal. Chem. 1960, 32, 1574–1578. [Google Scholar] [CrossRef]
- Ishihara, T.; Arimoto, S.; Morikawa, H.; Nishimoto, Y.; Kawama, Y.; Takami, A.; Hamamoto, S.; Naomoto, H.; Namba, K. Development of High Efficiency Thin Film Polycrystalline Silicon Solar Cells Using VEST Process. MRS Online Proc. Libr. 1997, 485, 3–11. [Google Scholar] [CrossRef]
- Kieliba, T.; Pohl, J.; Eyer, A.; Schmiga, C. Optimization of c-Si Films Formed by Zone-Melting Recrystallization for Thin-Film Solar Cells. In Proceedings of the 3rd World Conference on Photovoltaic Solar Energy Conversion, Osaka, Japan, 11–18 May 2003; p. 1170. [Google Scholar]
- Reber, S.; Eyer, A.; Haas, F. High-throughput zone-melting recrystallization for crystalline silicon thin-film solar cells. J. Cryst. Growth 2006, 287, 391–396. [Google Scholar] [CrossRef]
- Radhakrishnan, H.S.; Cho, J.; Bearda, T.; Roeth, J.; Depauw, V.; Van Nieuwenhuysen, K.; Gordon, I.; Szlufcik, J.; Poortmans, J. Freestanding and supported processing of sub-70 μm kerfless epitaxial Si and thinned Cz/FZ Si foils into solar cells: An overview of recent progress and challenges. Sol. Energy Mater. Sol. Cells 2019, 203, 110108. [Google Scholar] [CrossRef]
- Contreras-Lisperguer, R.; Muñoz-Cerón, E.; Aguilera, J.; de la Casa, J. A set of principles for applying Circular Economy to the PV industry: Modeling a closed-loop material cycle system for crystalline photovoltaic panels. Sustain. Prod. Consum. 2021, 28, 164–179. [Google Scholar] [CrossRef]
- Powell, D.M. Crystalline silicon photovoltaics: A cost analysis framework for determining technology pathways to reach baseload electricity costs. Energy Environ. Sci. 2012, 5, 5874–5883. [Google Scholar] [CrossRef]
- Augusto, A. Study of a Process to Grow Silicon Ribbons by Fast CVD. Ph.D. Thesis, Faculty of Sciences, University of Lisbon, Lisboa, Portugal, 2012. [Google Scholar]
- Serra, F.; Amar, E.; Pera, D.R.; Silva, J.A.; Serra, J.M. Zone melting recrystallization of microcrystalline silicon ribbons obtained by chemical vapor deposition. AIP Conf. Proc. 2019, 2147, 140008. [Google Scholar]
- Serra, F. Multicrystalline Silicon Ribbons Grown over a Dust Substrate. Ph.D. Thesis, Faculty of Sciences, University of Lisbon, Lisboa, Portugal, 2021. [Google Scholar]
- Kalejs, J.P. Silicon ribbons and foils—State of the art. Sol. Energy Mater. Sol. Cells 2002, 72, 139–153. [Google Scholar] [CrossRef]
- Hahn, G.; Schönecker, A. New crystalline silicon ribbon materials for photovoltaics. J. Phys. Condens. Matter 2004, 16, R1615–R1648. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A. Thin-film solar cells: Review of materials, technologies and commercial status. J. Mater. Sci. Mater. Electron. 2007, 18, 15–19. [Google Scholar] [CrossRef]
- Jäger-Waldau, A. Status of thin film solar cells in research, production and the market. Sol. Energy 2004, 77, 667–678. [Google Scholar] [CrossRef]
- Schmich, E.; Schillinger, N.; Reber, S. Silicon CVD deposition for low-cost applications in photovoltaics. Surf. Coat. Technol. 2007, 201, 9325–9329. [Google Scholar] [CrossRef]
- Henley, F.J. Kerf-Free Wafering: Technology Overview and Challenges for Thin PV Manufacturing. In Proceedings of the 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA, 20–25 June 2010; pp. 001184–001192. [Google Scholar]
- Niepelt, R.; Hensen, J.; Steckenreiter, V.; Brendel, R.; Kajari-Schöder, S. Kerfless exfoliated thin crystalline Si wafers with Al metallization layers for solar cells. J. Mater. Res. 2015, 30, 3227–3240. [Google Scholar] [CrossRef] [Green Version]
- Bellanger, P.; Slaoui, A.; Minj, A.; Martini, R.; Debucquoy, M.; Serra, J.M. First Solar Cells on Exfoliated Silicon Foils Obtained at Room Temperature by the SLIM-Cut Technique Using an Epoxy Layer. IEEE J. Photovolt. 2016, 6, 1115–1122. [Google Scholar] [CrossRef]
- Kumar, A.; Melkote, S.N. Diamond Wire Sawing of Solar Silicon Wafers: A Sustainable Manufacturing Alternative to Loose Abrasive Slurry Sawing. Procedia Manuf. 2018, 21, 549–566. [Google Scholar] [CrossRef]
- Tomono, K.; Miyamoto, S.; Ogawa, T.; Furuya, H.; Okamura, Y.; Yoshimoto, M.; Komatsu, R.; Nakayama, M. Recycling of kerf loss silicon derived from diamond-wire saw cutting process by chemical approach. Sep. Purif. Technol. 2013, 120, 304–309. [Google Scholar] [CrossRef]
- Serra, J.M. Estudo de um Processo de Preparação de Fitas de Silício para Aplicação Fotovoltaica. Ph.D. Thesis, Faculty of Sciences, University of Lisbon, Lisboa, Portugal, 1995. [Google Scholar]
- Bellanger, P.; Sow, A.; Grau, M.; Augusto, A.; Serra, J.M.; Kaminski, A.; Dubois, S.; Straboni, A. New method of fabricating silicon wafer for the photovoltaic application based on sintering and recrystallization steps. J. Cryst. Growth 2012, 359, 92–98. [Google Scholar] [CrossRef]
- Silva, J.A.; Brito, M.C.; Costa, I.; Alves, J.M.; Serra, J.M.; Vallêra, A.M. Sprayed boric acid as a dopant source for silicon ribbons. Sol. Energy Mater. Sol. Cells 2007, 91, 1948–1953. [Google Scholar] [CrossRef]
- Silva, J.A.; Platte, B.; Brito, M.C.; Serra, J.M. New doping method to obtain n-type silicon ribbons. J. Cryst. Growth 2015, 428, 29–34. [Google Scholar]
- Dubois, S.; Palais, O.; Ribeyron, P.J.; Enjalbert, N.; Pasquinelli, M.; Martinuzzi, S. Effect of intentional bulk contamination with iron on multicrystalline silicon solar cell properties. J. Appl. Phys. 2007, 102, 083525. [Google Scholar] [CrossRef]



| Resistivity (Ω·cm) | ND (cm−3) | Lifetime (μs) | |
|---|---|---|---|
| SDS ribbon | 0.70 ± 0.05 | 2.1 × 1016 | 0.3 ± 0.1 |
| Voc (mV) | Jsc (mA/cm2) | FF (%) | Eff (%) | |
|---|---|---|---|---|
| SDS | 415 | 14.3 | 37.4 | 2.2 |
| Control | 581 | 19.3 | 44.6 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, F.; Costa, I.; Silva, J.A.; Serra, J.M. Optical Recrystallization of Nanocrystalline Silicon Ribbons. Metals 2023, 13, 452. https://doi.org/10.3390/met13030452
Serra F, Costa I, Silva JA, Serra JM. Optical Recrystallization of Nanocrystalline Silicon Ribbons. Metals. 2023; 13(3):452. https://doi.org/10.3390/met13030452
Chicago/Turabian StyleSerra, Filipe, Ivo Costa, José A. Silva, and João M. Serra. 2023. "Optical Recrystallization of Nanocrystalline Silicon Ribbons" Metals 13, no. 3: 452. https://doi.org/10.3390/met13030452
APA StyleSerra, F., Costa, I., Silva, J. A., & Serra, J. M. (2023). Optical Recrystallization of Nanocrystalline Silicon Ribbons. Metals, 13(3), 452. https://doi.org/10.3390/met13030452






