Economic and Environmental Sustainability of an Innovative Cryo-Mechano-Hydrometallurgical Process Validated at Pilot Scale for the Recycling of Li Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
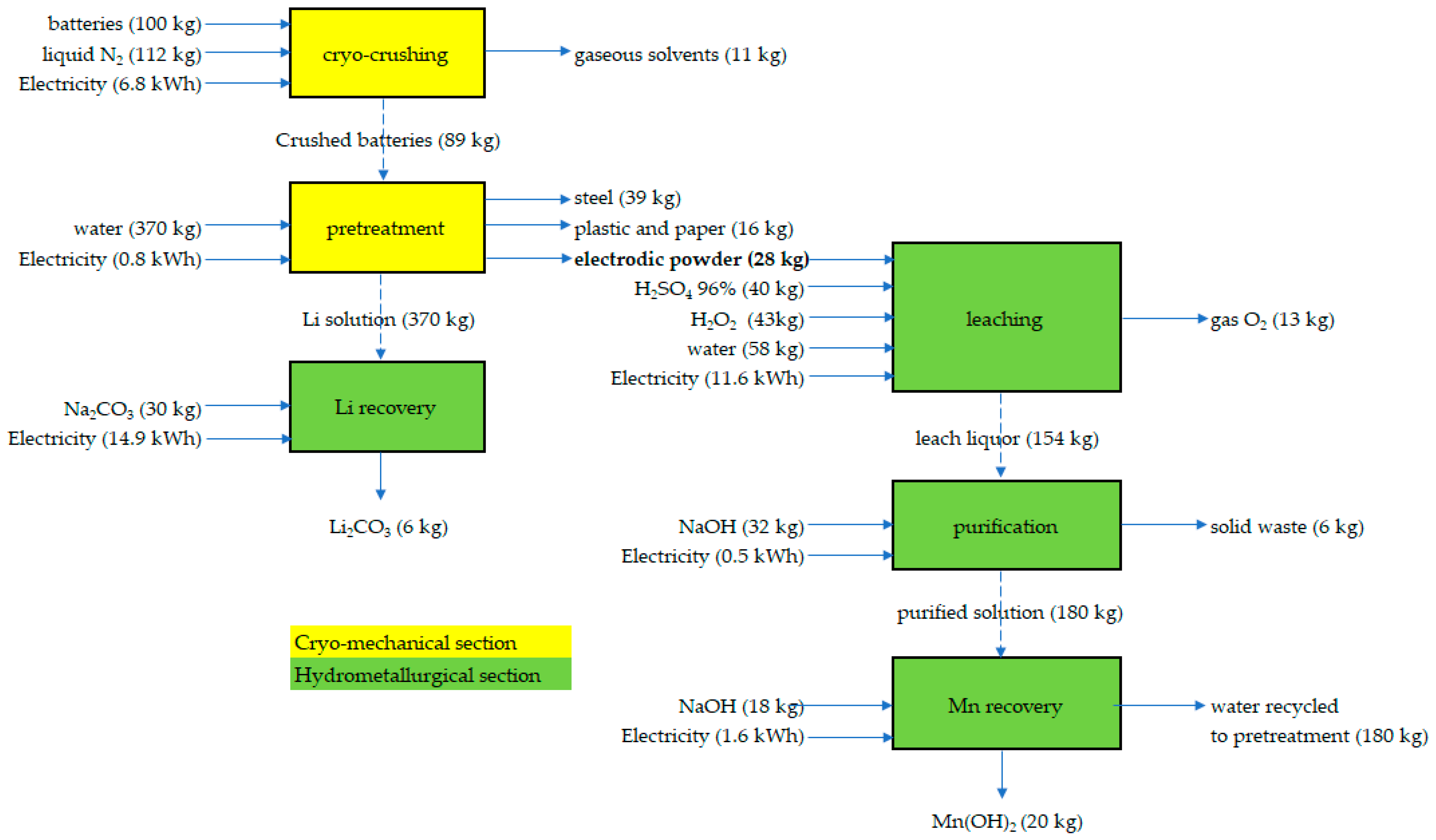
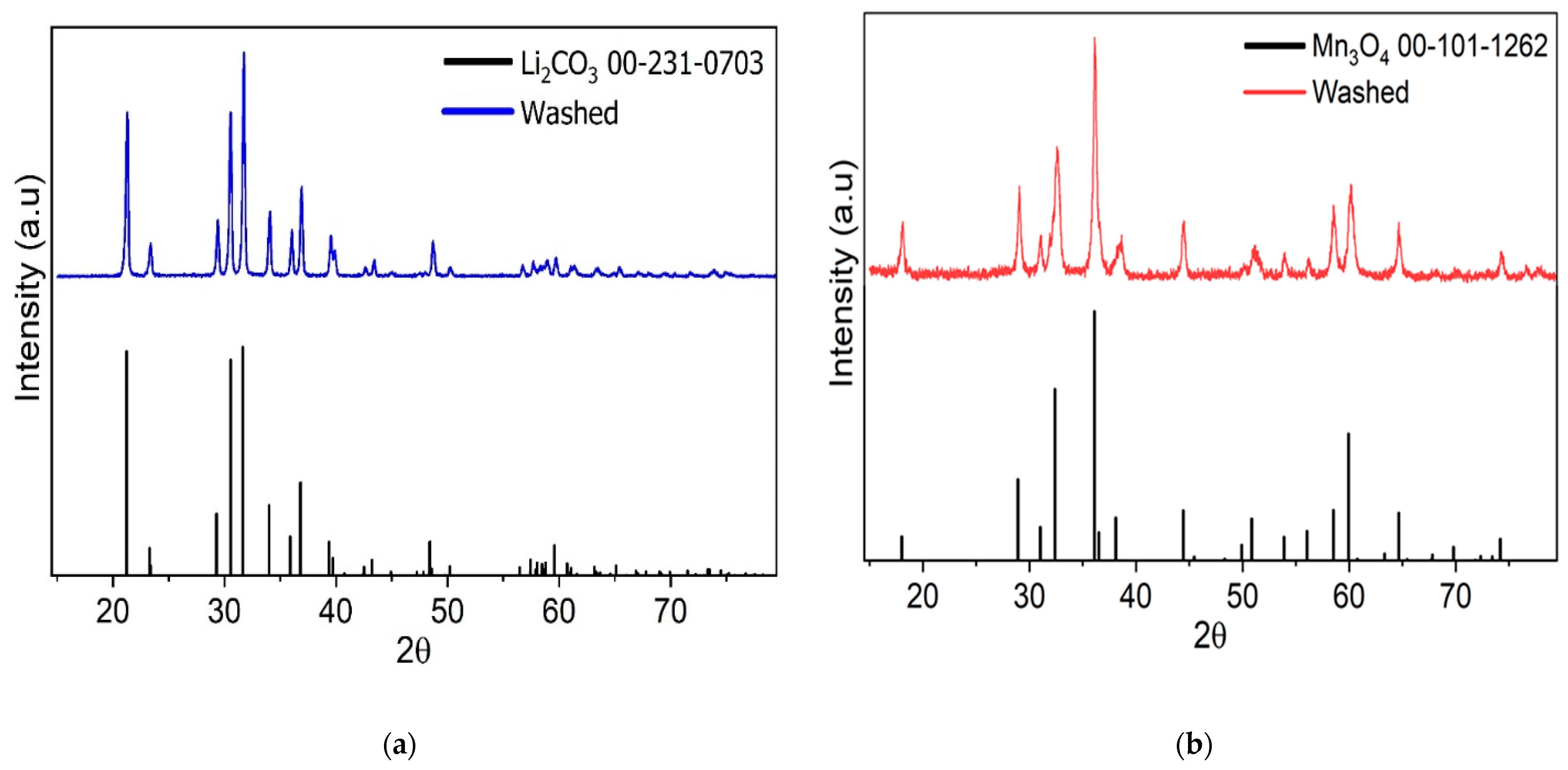
2.2. Process Demonstration and Product Characterization
- Lithium recovery from neutralization waters by precipitation as Li2CO3.
- Manganese extraction from neutralized electrode powder.
- Manganese-bearing solution purification.
- Manganese recovery by precipitation as manganese hydroxides.
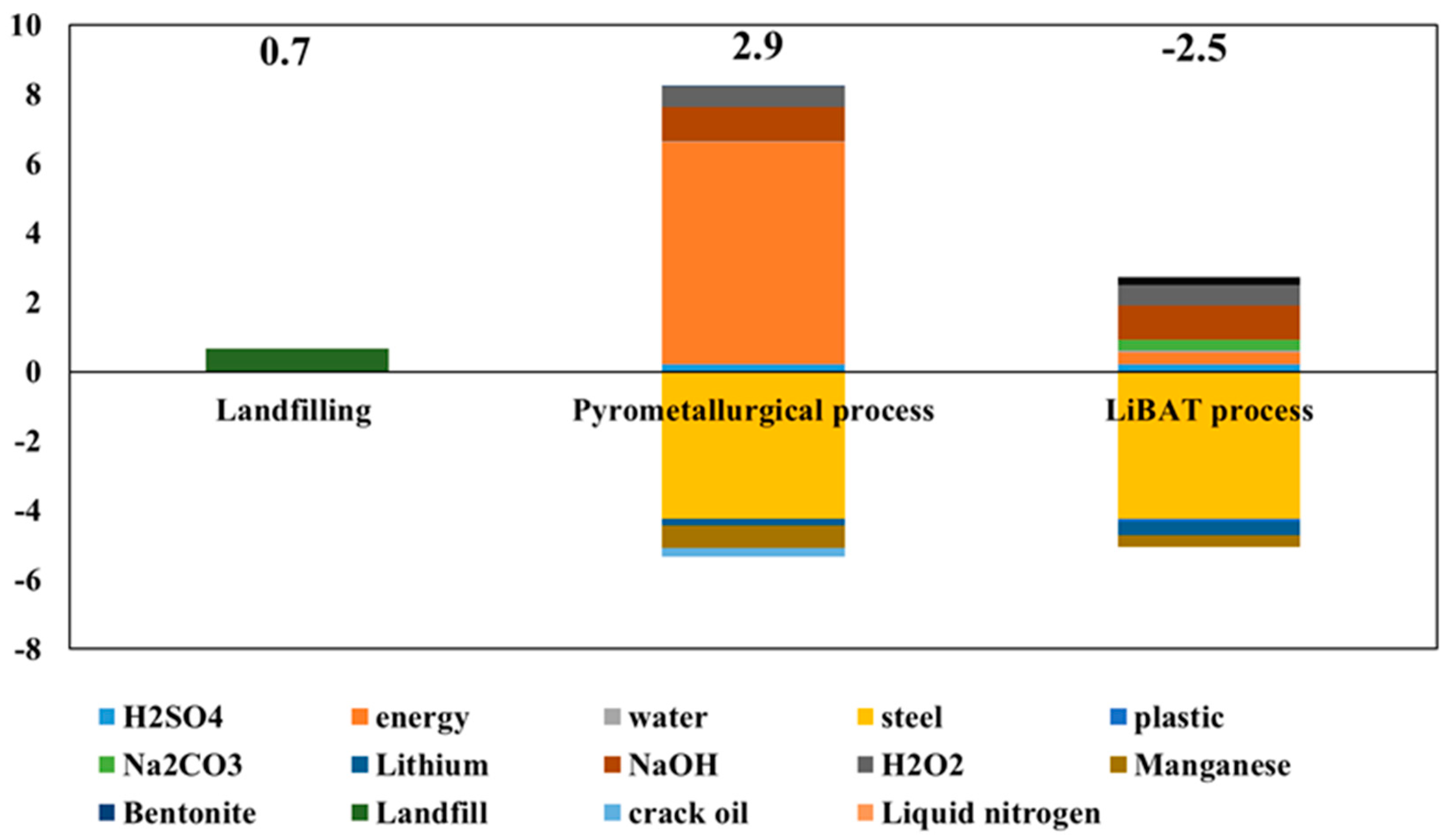
2.3. Life Cycle Assessment
2.4. Process Simulations
- Different plant potentialities in the range of 12–550 t/yr.
- With and without the “consortium fee”, the fee paid by consortia and local authorities to private recycling companies performing the end-of-life treatment of batteries.
- Different “treatment fees” paid for end-of-life battery treatment by private customers, i.e., battery collectors not having dedicated infrastructures for the treatment of Li(0) batteries.
- Different feed compositions (only Li(0), and mixtures of Li(0) and Li-ion with a 1:10 weight ratio).
3. Results
3.1. Process Demonstration and Product Characterization
3.2. Life Cycle Assessment
3.3. Process Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Input | Output |
|---|---|
| Step 1: Thermal pretreatment | |
| Batteries: 100 kg Electricity: 64.3 kWh | Crack oil: 26.8 |
| Step 2: Mechanical pretreatment | |
| Pyrolyzed batteries: 73.17 kg Electricity: 41.6 kWh | Steel: 35 kg |
| Step 3: Lithium thermal recovery | |
| Black mass: 37.9 kg Bentonite: 0.6 kg Methane: 258.5 Electricity: 158.7 kWh | Gaseous emission: 8.9 kg Lithium carbonate cake: 4 kg |
| Step 4. Leaching | |
| Electrodic powder: 28.8 kg Sulfuric acid (96%): 41 kg Water: 60 kg Electricity: 11.6 kWh Hydrogen peroxide: 45 kg | Leaching solution: 155 kg |
| Step 5. Purification | |
| Leaching solution: 155 kg Sodium hydroxide: 39 kg Electricity: 0.5 kWh | Solid waste: 7 kg |
| Step 6. Manganese recovery | |
| Purified solution: 187 kg Sodium hydroxide: 16 kg Electricity: 1.6 kWh | Wastewater: 185 kg Manganese hydroxide cake: 18 kg |
| Production Cost | ||
|---|---|---|
| Raw materials | Sodium carbonate | 0.22 |
| Hydrogen peroxide | 1.37 | |
| Sodium hydroxide | 0.25 | |
| Sulfuric acid | 0.18 | |
| Water | 0.0005 | |
| Liquid nitrogen | 0.34 | |
| Waste treatment | Solid waste treatment | 0.64 |
| Wastewater | 0.14 | |
| Utility | Electricity | 0.27 |
| Selling prices of the products | ||
| Products | Steel scraps | 0.18 |
| Mn hydroxide | 0.68 | |
| NMC precursor | 22.8 | |
| Graphite | 7 | |
| Sodium sulphate | 0.1 | |
| Copper scraps | 2.6 | |
| Aluminium scraps | 0.7 | |
| Lithium carbonate | 59 | |
References
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Wang, Y.; An, N.; Wen, L.; Wang, L.; Jiang, X.; Hou, F.; Yin, Y.; Liang, J. Recent progress on the recycling technology of Li-ion batteries. J. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling of Electronic Scrap at Umicore’s Integrated Metals Smelter and Refinery. Erzmetall 2006, 59, 152–161. [Google Scholar]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Pavón, S.; Kahl, M.; Hippmann, S.; Bertau, M. Lithium recovery from production waste by thermal pre-treatment. Sustain. Chem. Pharm. 2022, 28, 100725. [Google Scholar] [CrossRef]
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kondás, J.; Jandová, J.; Nemeckova, M. Processing of spent Li/MnO2 batteries to obtain Li2CO3. Hydrometallurgy 2006, 84, 247–249. [Google Scholar] [CrossRef]
- Friedrich, B.; Weyhe, R.; Träger, T. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar]
- Pindar, S.; Dhawan, N. Microwave processing of spent coin cells for recycling of metallic values. J. Clean. Prod. 2021, 280, 124144. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Recycling of discarded coin cells for recovery of metal values. Miner. Eng. 2020, 159, 106650. [Google Scholar] [CrossRef]
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium battery recycling process. J. Power Sources 1999, 83, 75–78. [Google Scholar] [CrossRef]
- McLaughlin, W.; Adams, T.S. Li Reclamation Process. U.S. Patent US5888463A, 30 March 1999. [Google Scholar]
- Schiavi, P.G.; Baldassari, L.; Altimari, P.; Moscardini, E.; Toro, L.; Pagnanelli, F. Process Simulation for Li-MnO2 Primary Battery Recycling: Cryo-Mechanical and Hydrometallurgical Treatments at Pilot Scale. Energies 2020, 13, 4546. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Zanoni, R.; Branchi, M.; Marcucci, C.; Zamparelli, C.; Altimari, P.; Navarra, M.A.; Pagnanelli, F. Upcycling Real Waste Mixed Lithium-Ion Batteries by Simultaneous Production of rGO and Lithium-Manganese-Rich Cathode Material. ACS Sustain. Chem. Eng. 2021, 9, 13303–13311. [Google Scholar] [CrossRef] [PubMed]
- Abo Atia, T.; Elia, G.; Hahn, R.; Altimari, P.; Pagnanelli, F. Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: Towards zero-waste process and metal recycling in advanced batteries. J. Energy Chem. 2019, 35, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Schiavi, P.G.; dos Santos Martins Padoan, F.C.; Altimari, P.; Pagnanelli, F. Cryo-Mechanical Treatment and Hydrometallurgical Process for Recycling Li-MnO2 Primary Batteries with the Direct Production of LiMnPO4 Nanoparticles. Energies 2020, 13, 4004. [Google Scholar] [CrossRef]
- Peters, M.; Timmerhaus, K. Plant Design and Economics for Chemical Engineers, 4th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Mohr, M.; Peters, J.F.; Baumann, M.; Weil, M. Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. J. Ind. Ecol. 2020, 24, 1310–1322. [Google Scholar] [CrossRef]
- Xiong, S.; Ji, J.; Ma, X. Environmental and economic evaluation of remanufacturing lithium-ion batteries from electric vehicles. Waste Manag. 2020, 102, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Furlani, G.; Moscardini, E.; Pagnanelli, F.; Ferella, F.; Vegliò, F.; Toro, L. Recovery of manganese from zinc alkaline batteries by reductive acid leaching using carbohydrates as reductant. Hydrometallurgy 2009, 99, 115–118. [Google Scholar] [CrossRef]
- Available online: https://www.scrapmetalbuyers.com (accessed on 12 January 2023).
- Available online: https://www.metal.com (accessed on 12 January 2023).
- Schiavi, P.G.; Branchi, M.; Casalese, E.; Altimari, P.; Navarra, M.A.; Pagnanelli, F. Resynthesis of NMC111 Cathodic Material from Real Waste Lithium Ion Batteries. Chem. Eng. Trans. 2021, 86, 463–468. [Google Scholar]
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric car battery: An overview on global demand, recycling and future approaches towards sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.lifedrone.eu/ (accessed on 12 January 2023).

| Fractions | [%] | Li [mg/g] | Mn [mg/g] |
|---|---|---|---|
| Electrodic Powder | 32 ± 9 | 40 ± 2 | 364 ± 6 |
| Magnetic Fraction | 39 ± 7 | 6 ± 7 | 6 ± 1 |
| Non-Magnetic Fraction | 16 ± 2 | 29 ± 7 | 288 ± 76 |
| Process | Feed | Potentiality (t/y) | Consortium Fee (EUR/kg) | Treatment Fee (EUR/kg) | CAPEX (kEUR) | OPEX (kEUR) | Gross Margin (%) | PBT (y) |
|---|---|---|---|---|---|---|---|---|
| LIBAT | Li(0) | 12 | 0.4 | 0 | 320 | 58 | −60.90 | N/A |
| LIBAT | Li(0) | 300 | 0 | 1 | 619 | 572 | 20.40 | 3.72 |
| LIBAT | Li(0) | 500 | 0 | 1 | 840 | 859 | 24.51 | 2.53 |
| Cryo-mech | Li(0) | 50 | 0.4 | 0 | 200 | 83 | −33.23 | N/A |
| Cryo-mech | Li(0) | 100 | 0.4 | 0 | 200 | 125 | −14.01 | N/A |
| Cryo-mech | Li(0) | 300 | 0 | 1 | 387 | 395 | 9.26 | 8.65 |
| Cryo-mech | Li(0) | 300 | 0 | 1.5 | 387 | 395 | 23.62 | 2.85 |
| Cryo-mech | Li(0) | 500 | 0 | 1 | 525 | 565 | 16.22 | 4.03 |
| Cryo-mech | Li(0) | 500 | 0 | 1.5 | 525 | 756 | 29.47 | 1.87 |
| LIBAT | Li-ion + Li(0) | 550 | 0.4 | 0 | 2.792 | 1.400 | 42.03 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnanelli, F.; Schiavi, P.G.; Altimari, P.; Beolchini, F.; Amato, A.; Coletta, J.; Forte, F.; Moscardini, E.; Toro, L. Economic and Environmental Sustainability of an Innovative Cryo-Mechano-Hydrometallurgical Process Validated at Pilot Scale for the Recycling of Li Batteries. Metals 2023, 13, 497. https://doi.org/10.3390/met13030497
Pagnanelli F, Schiavi PG, Altimari P, Beolchini F, Amato A, Coletta J, Forte F, Moscardini E, Toro L. Economic and Environmental Sustainability of an Innovative Cryo-Mechano-Hydrometallurgical Process Validated at Pilot Scale for the Recycling of Li Batteries. Metals. 2023; 13(3):497. https://doi.org/10.3390/met13030497
Chicago/Turabian StylePagnanelli, Francesca, Pier Giorgio Schiavi, Pietro Altimari, Francesca Beolchini, Alessia Amato, Jacopo Coletta, Flavia Forte, Emanuela Moscardini, and Luigi Toro. 2023. "Economic and Environmental Sustainability of an Innovative Cryo-Mechano-Hydrometallurgical Process Validated at Pilot Scale for the Recycling of Li Batteries" Metals 13, no. 3: 497. https://doi.org/10.3390/met13030497
APA StylePagnanelli, F., Schiavi, P. G., Altimari, P., Beolchini, F., Amato, A., Coletta, J., Forte, F., Moscardini, E., & Toro, L. (2023). Economic and Environmental Sustainability of an Innovative Cryo-Mechano-Hydrometallurgical Process Validated at Pilot Scale for the Recycling of Li Batteries. Metals, 13(3), 497. https://doi.org/10.3390/met13030497









