An Electrochemical Study of the Corrosion Behaviour of T91 Steel in Molten Nitrates
Abstract
1. Introduction
2. Experimental Procedure
2.1. Testing Material
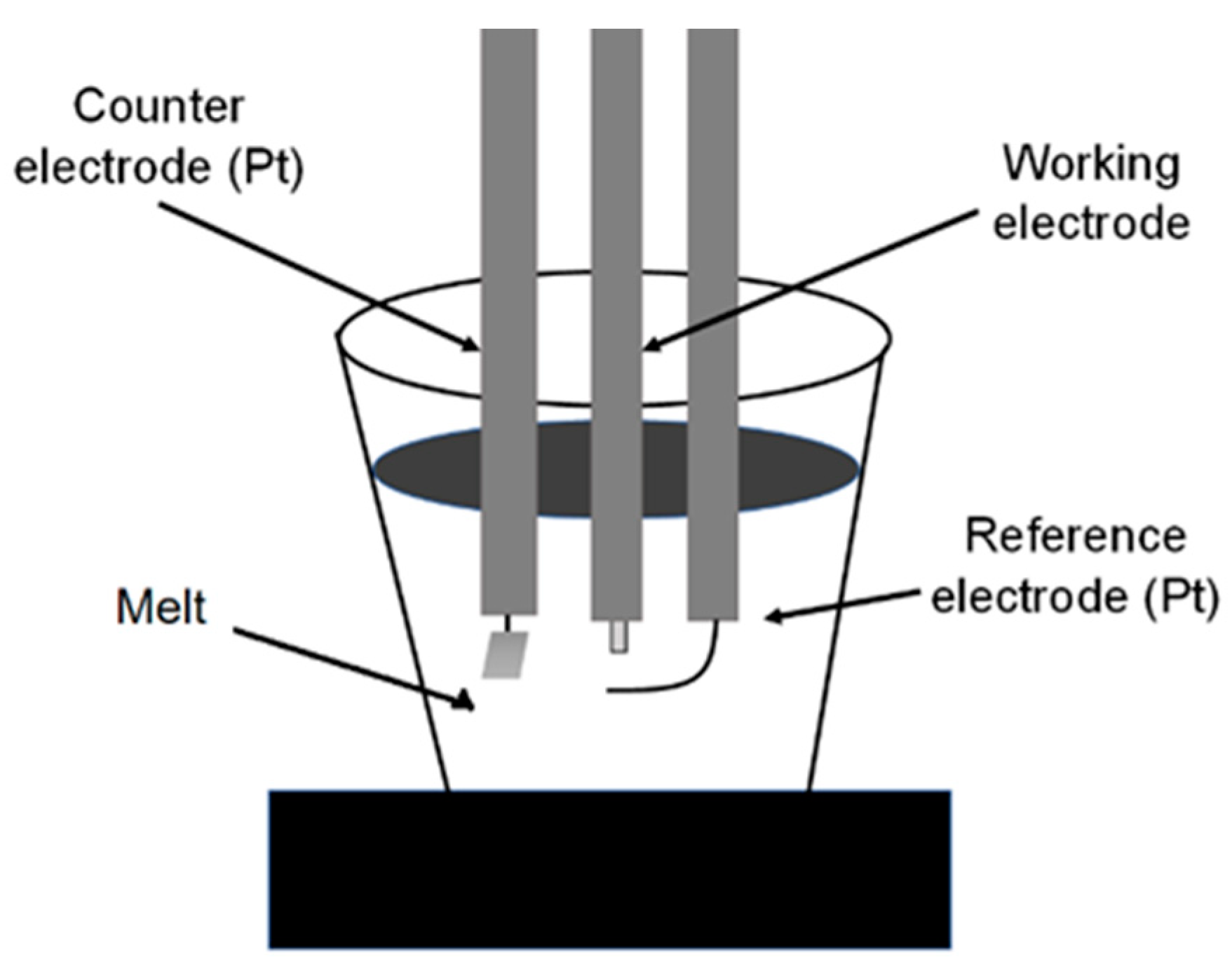
2.2. Corrosion Studies
3. Results and Discussion
3.1. Open Circuit Potential
3.2. Potentiodynamic Polarization Curves
3.3. Linear Polarization Resistance Measurements
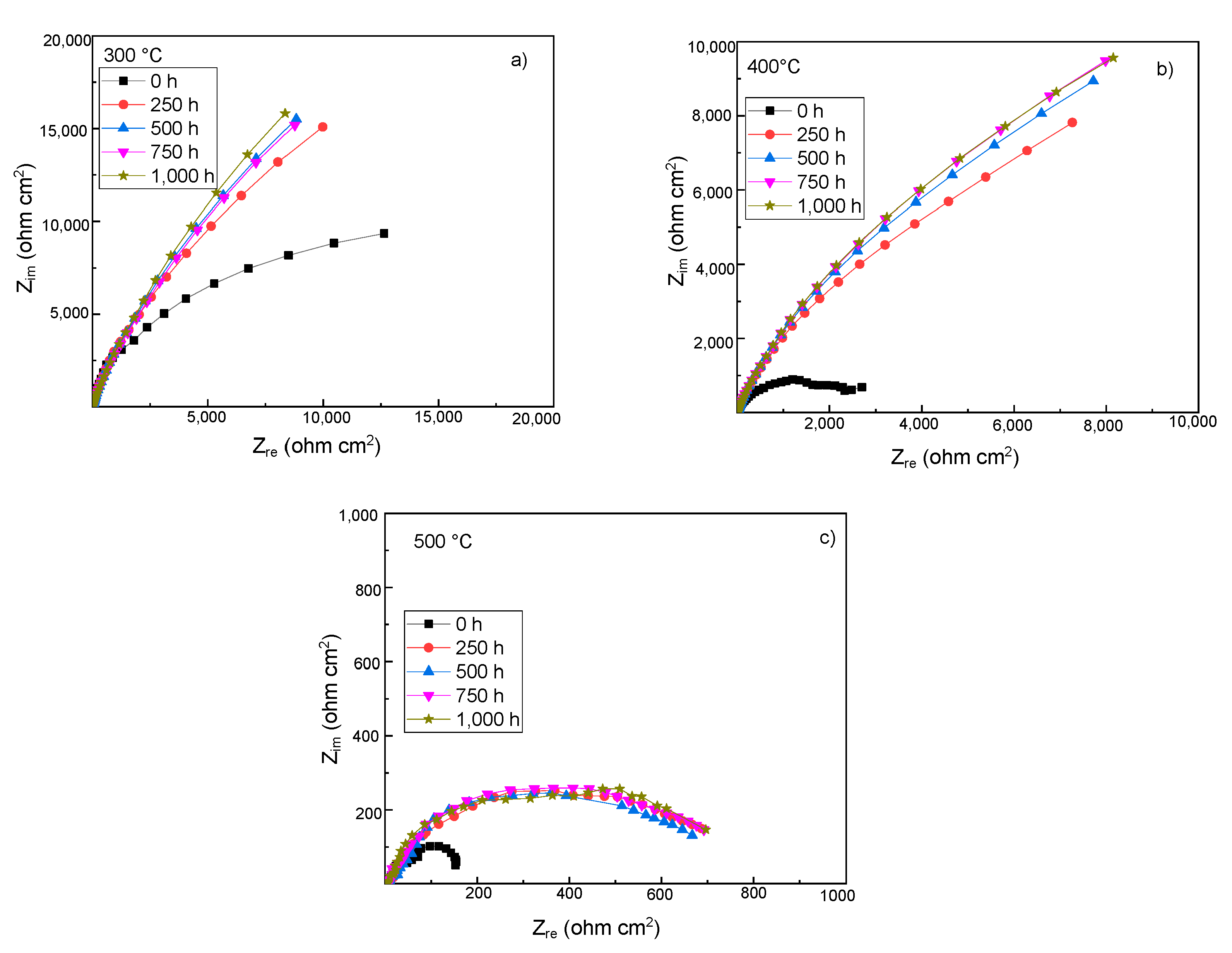
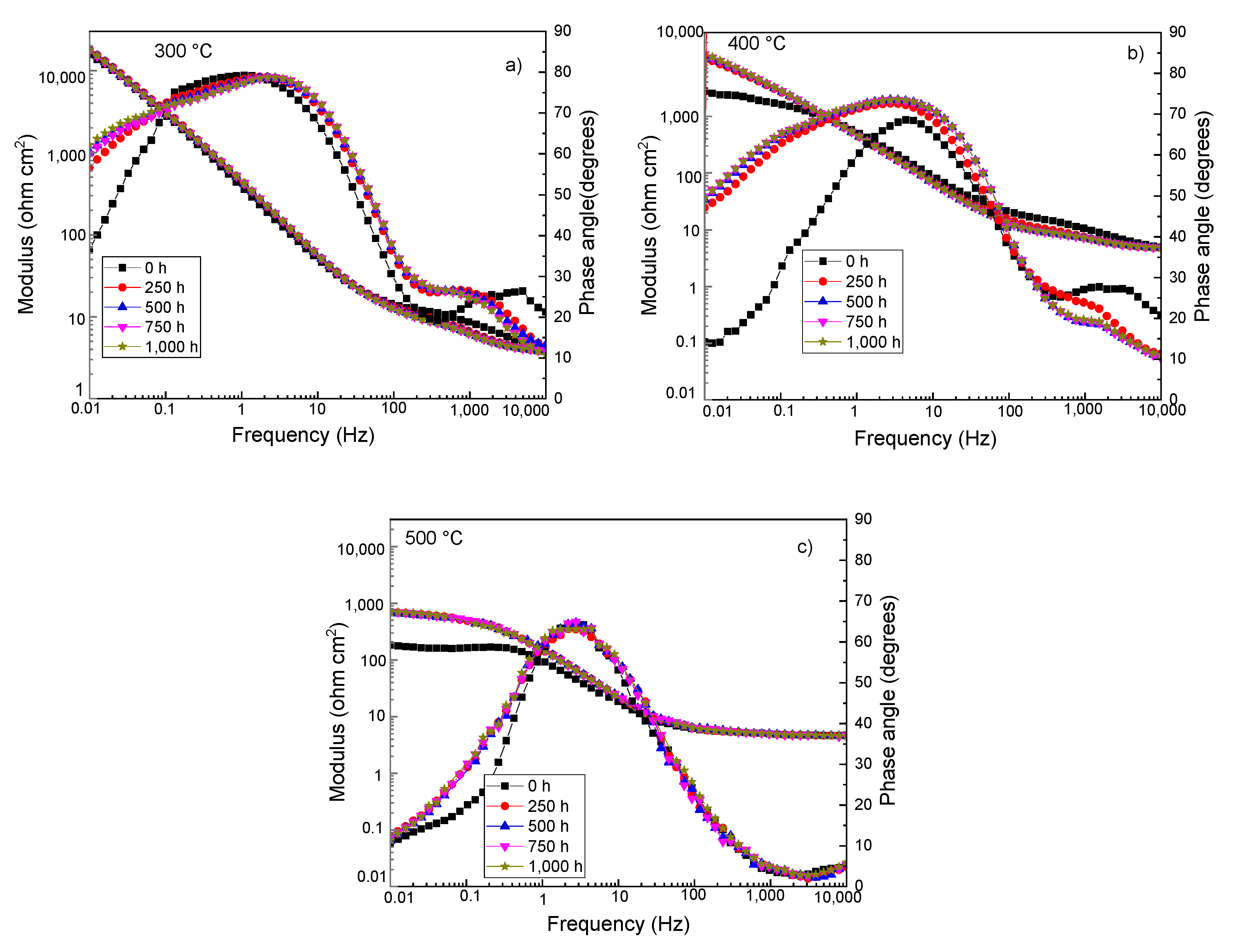
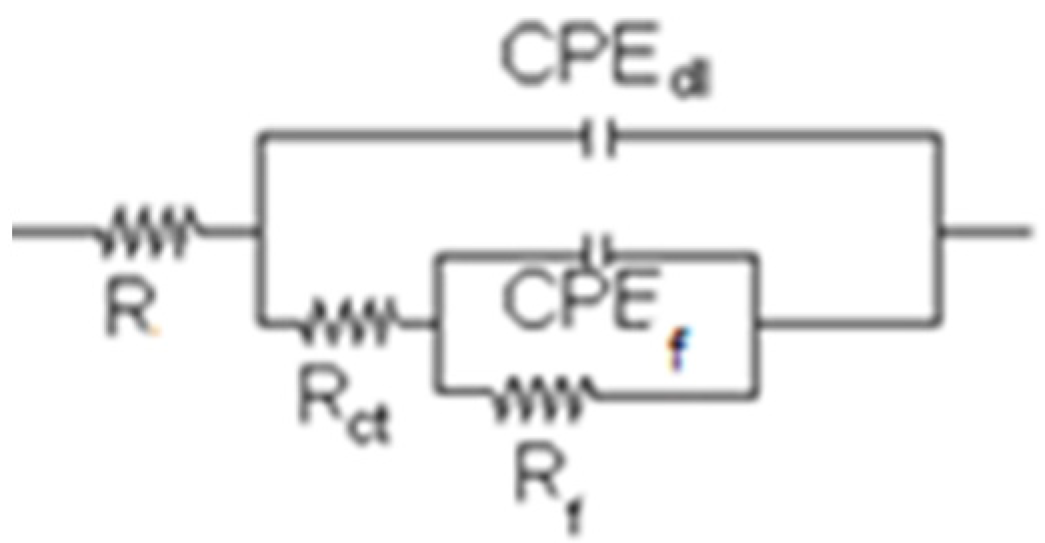
3.4. Electrochemical Impedance Spectroscopy Measurements
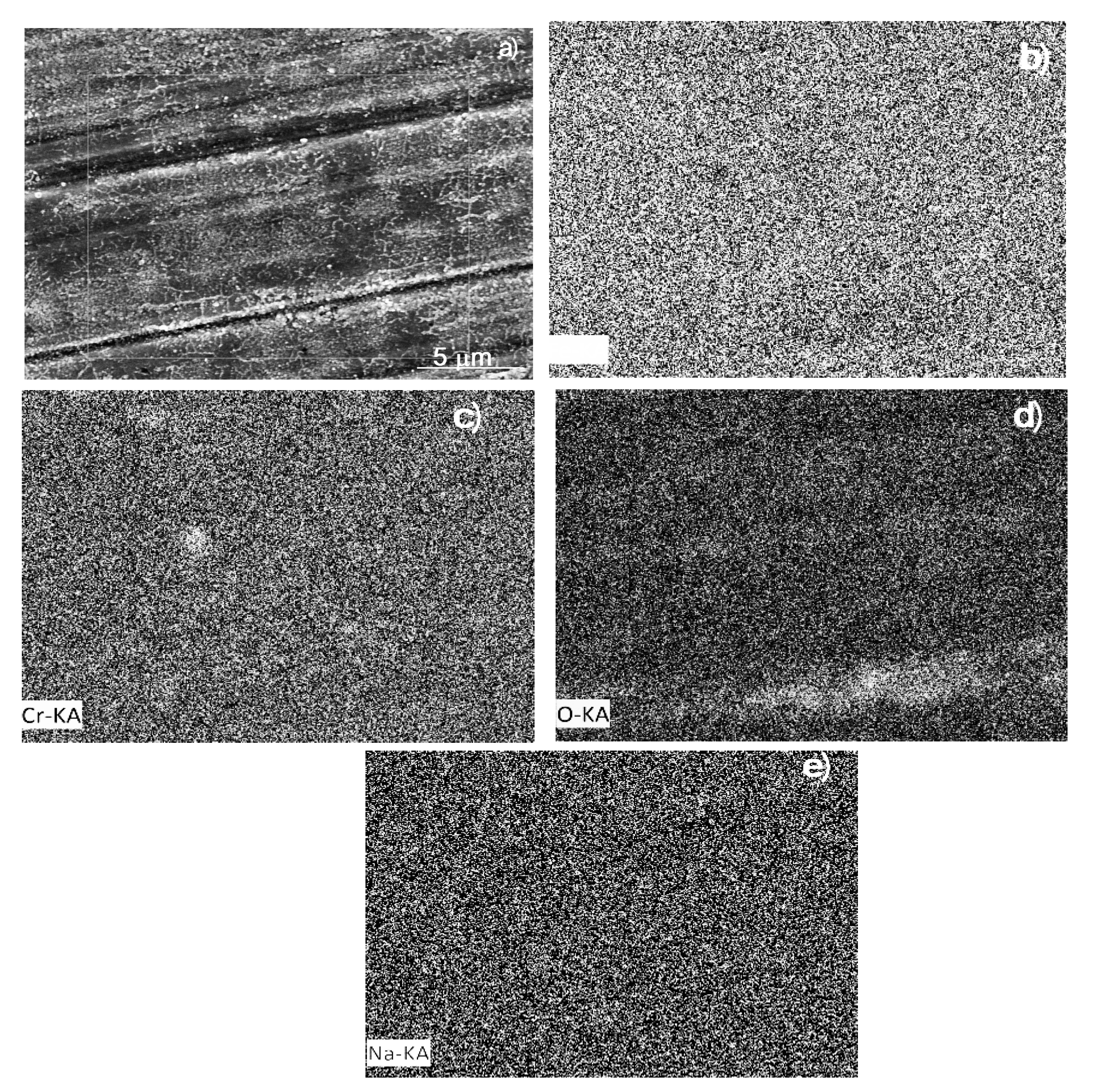
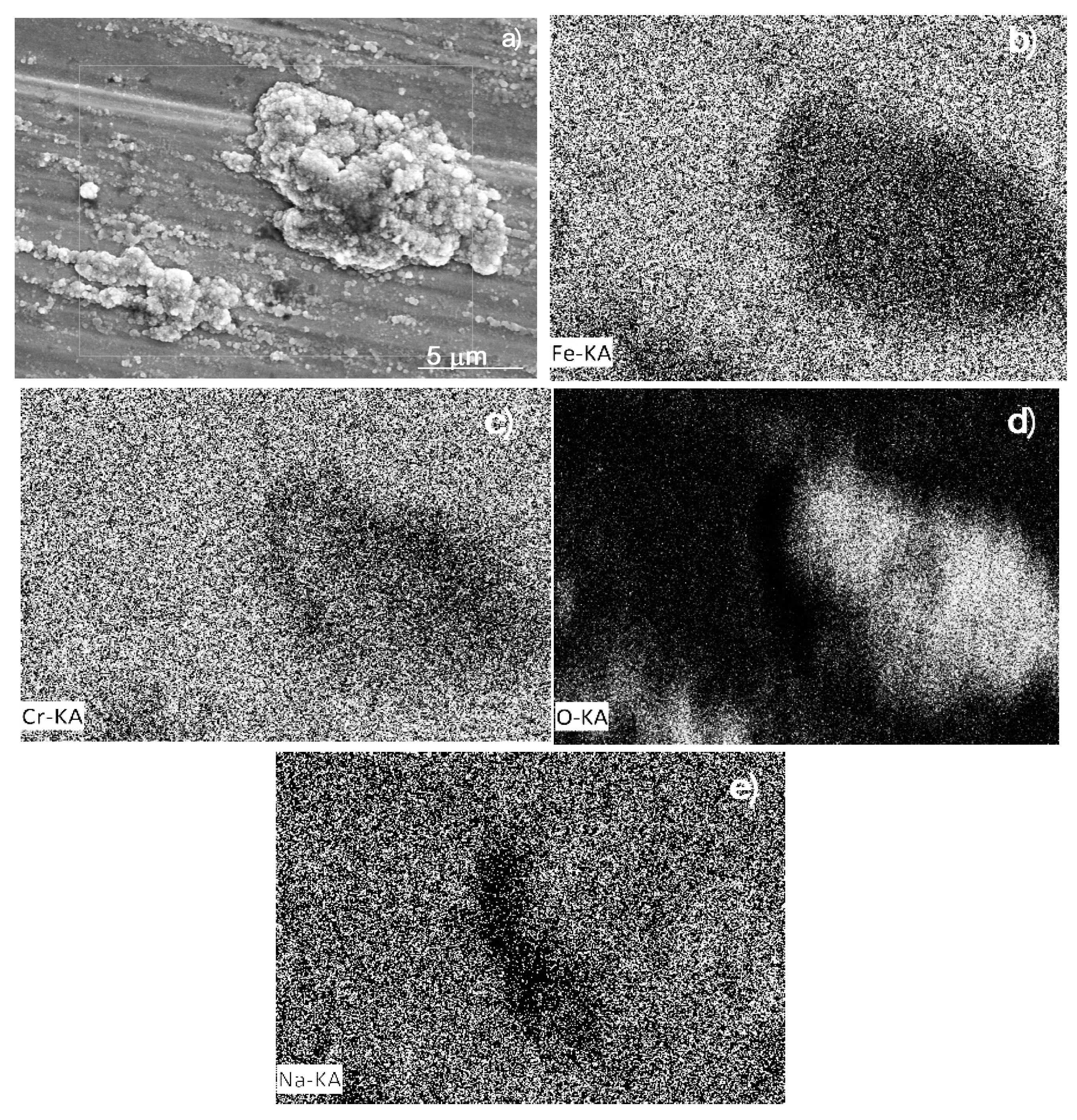
3.5. Corroded Surface Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schellekens, A.; Battaglini, G.; Lilliestam, J.; McDonnell, J.; Patt, A. 100% Renewable Electricity: A Roadmap to 2050 For Europe and North Africa; Pricewaterhouse Coopers: London, UK, 2010; pp. 152–199. [Google Scholar]
- China Energy Research Institute. High Renewable Energy Penetration Scenario and Roadmap Study, 2050; Energy Research Institute: Beijing, China, 2015; pp. 210–225. [Google Scholar]
- Aarab, F.; Kuhn, B.; Bonk, A.; Bauer, T. A New Approach to Low-Cost, Solar Salt-Resistant Structural Materials for Concentrating Solar Power (CSP) and Thermal Energy Storage (TES). Metals 2021, 11, 1970. [Google Scholar] [CrossRef]
- Vignarooban, K. Vapor pressure and corrosivity of ternary metal-chloride molten salt based heat transfer fluids for use in concentrating solar power systems. Appl. Energy 2015, 159, 206–213. [Google Scholar] [CrossRef]
- Morales, M.; Gordon, S.; Fernández-Arana, Ó.; García-Marro, F.; Mateo, A.; Llanes, L.; Fargas, G. Duplex Stainless Steels for Thermal Energy Storage: Characterization of Oxide Scales Formed in Carbonate Salts at 500 °C. Metals 2022, 12, 2156. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina-forming alloys against molten chlorides for energy production. I: Pre-oxidation treatment and isothermal corrosion tests. Sol. Energy Mater. Sol. Cells 2017, 166, 222–233. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina forming alloys against molten chlorides for energy production. II: Electrochemical impedance spectroscopy under thermal cycling conditions. Sol. Energy Mater. Sol. Cells 2017, 166, 234–245. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Yin, X.; Yang, X.; Tang, J.; Gong, J. Corrosion and electrochemical investigations for stainless steels in molten Solar Salt: The influence of chloride impurity. J. Energy Storage 2021, 39, 102675. [Google Scholar] [CrossRef]
- Mallco, A.; Fernández, A.G. Corrosion Monitoring Assessment on Lithium Nitrate Molten Salts as Thermal Energy Storage Material Applied to CSP Plants. Oxid. Met. 2020, 94, 383–396. [Google Scholar] [CrossRef]
- Spiegel, M.; Schraven, P. Corrosion of Austenitic Steels and Nickel Alloys in Molten KNO3–NaNO3 at Different Temperatures: Role of Alloying Elements. Oxid. Met. 2021, 96, 145–155. [Google Scholar] [CrossRef]
- Encinas-Sánchez, V.; Lasanta, M.I.; De Miguel, M.T.; García-Martín, G.; Pérez, F.J. Corrosion monitoring of 321H in contact with a quaternary molten salt for parabolic trough CSP plants. Corros. Sci. 2021, 178, 109070. [Google Scholar] [CrossRef]
- Vignarooban, K.; Pugazhendhi, P.; Tucker, C.; Gervasio, D.; Kannan, A.M. Corrosion resistance of Hastelloys in molten metal-chloride heat transfer fluids for concentrating solar power applications. Sol. Energy 2014, 103, 62–69. [Google Scholar] [CrossRef]
- Goods, S.H.; Bradshaw, R.W. Corrosion of stainless steels and carbon steel by molten mixtures of commercial nitrate salts. J. Mater. Eng. Perform. 2004, 13, 78–87. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, C.Z.; Li, Z.H.; Zhou, H.X.; He, J.X.; Yu, J.C. Corrosion behavior of nickel-based superalloys in thermal storage medium, of molten eutectic NaCl-MgCl2 in atmosphere. Sol. Energy Mater. Sol. Cells 2017, 164, 146–155. [Google Scholar] [CrossRef]
- Palacios, A.; Navarro, M.E.; Jiang, Z.; Avila, A.; Qiao, G.; Mura, E.; Ding, Y. High-temperature corrosion behaviour of metal alloys in commercial molten Salts. Sol. Energy 2020, 201, 437–452. [Google Scholar] [CrossRef]
- Ibrahim, A.; Peng, H.; Riaz, A.; Basit, M.A.; Rashid, U.; Basit, A. Molten salts in the light of corrosion mitigation strategies and embedded with nanoparticles to enhance the thermophysical properties for CSP plants. Sol. Energy Mater. Sol. Cells 2021, 219, 110768. [Google Scholar] [CrossRef]
- Pineda, F.; Mallco, A.; De Barbieri, F.; Carrasco, C.; Henriquez, M.; Fuentealba, E.; Fernández, Á.G. Corrosion evaluation by electrochemical real-time tracking of VM12 martensitic steel in a ternary molten salt mixture with lithium nitrates for CSP plants. Sol. Energy Mater. Sol. Cells 2021, 231, 111302. [Google Scholar] [CrossRef]
- Bendick, W.; Cipolla, L.; Gabrel, J.; Hald, J. New ECCC assessment of creep rupture strength for steel grade X10CrMoVNb9-1 (Grade 91). Int. J. Press. Vessel. Pip. 2010, 87, 304–309. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Li, L.; Gao, K.; Pang, X.; Volinsky, A.A. Mechanical properties and phases evolution in T91 steel during long-term high-temperature exposure. Eng. Fail. Anal. 2020, 111, 104451. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, D.K.; Kumaran, S. Effect of post weld heat treatment on microstructure and mechanical properties of Hot Wire GTA welded joints of SA213 T91 steel. Mater. Today Proc. 2018, 5, 8049–8056. [Google Scholar] [CrossRef]
- Singh, M.P.; Basu, B.; Chattopadhyay, K. Probing High-Temperature Electrochemical Corrosion of 316 Stainless Steel in Molten Nitrate Salt for Concentrated Solar Power Plants. J. Mater. Eng. Perform. 2022, 31, 4902–4908. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Wu, Y.; Lu, Y. Comparative review of different influence factors on molten salt corrosion characteristics for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 235, 111485. [Google Scholar] [CrossRef]
- Mallco, A.; Pineda, F.; Mendoza, M.; Henriquez, M.; Carrasco, C.; Vergara, V.; Fuentealba, E.; Fernandez, A.G. Evaluation of flow accelerated corrosion and mechanical performance of martensitic steel T91 for a ternary mixture of molten salts for CSP plants. Solar Sol. Energy Mater. Sol. Cells 2022, 238, 111623. [Google Scholar] [CrossRef]
- Ning, Z.; Zhou, Q.; Liu, Z.; Li, N.; Luo, Q.; Wen, D. Effects of imposed stresses on high temperature corrosion behaviour of T91. Corros. Sci. 2021, 189, 109595. [Google Scholar] [CrossRef]
- Sundaresan, C.; Rajasekaran, B.; Varalakshmi, S.; Santhy, K.; Rao, D.S.; Sivakumar, G. Comparative hot corrosion performance of APS and Detonation sprayed CoCrAlY, NiCoCrAlY and NiCr coatings on T91 boiler steel. Corros. Sci. 2021, 189, 109556. [Google Scholar]
- Dorcheh, A.S.; Durham, R.N.; Galetz, M.C. Corrosion behavior of stainless and low-chromium steels and IN625 in molten nitrate salts at 600 °C. Sol. Energy Mater. Sol. Cells 2016, 144, 109–116. [Google Scholar] [CrossRef]
- Fähsing, D.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion testing of diffusion-coated steel in molten salt for concentrated solar power tower systems. Surf. Coat. Technol. 2018, 354, 46–55. [Google Scholar] [CrossRef]
- Zhai, W.; Yang, B.; Li, S.; Wang, Z.; Zhang, S.; Huang, G. High Temperature Corrosion behavior of Metallic Materials in modified Hitec Molten Salts. Adv. Comp. Sci. Res. 2016, 71, 707–713. [Google Scholar]
- Baraka, A.; Rohman, A.; El Hosary, A.A. Corrosion on mild steel in molten sodium nitrate-potassium nitrate eutectic. Br. Corros. J. 1976, 11, 44–46. [Google Scholar] [CrossRef]
- De Jong, J.M.; Broers, G.H.J. A reversible oxygen electrode in an equimolar KNO3–NaNO3 melt saturated with sodium peroxide II. A voltammetric study. Electrochim. Acta 1976, 21, 893–900. [Google Scholar] [CrossRef]
- Zhu, M.; Zeng, S.; Sharif, A.; Cao, B.; Zhang, H.; Wang, M. Effects of chloride ions on electrochemical reaction of 316 stainless steel in mixtures of molten nitrate salts. Mater. Werkst. 2020, 51, 1161–1169. [Google Scholar] [CrossRef]
- Encinas-Sánchez, V.; De Miguel, M.T.; Lasanta, M.I.; García-Martín, G.; Pérez, F.J. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP Applications. Sol. Energy Mat. Sol Cells 2019, 191, 157–163. [Google Scholar] [CrossRef]
- Encinas-Sánchez, V.; Macías-García, A.; De Miguel, M.T.; Pérez, F.J.; Rodríguez-Rego, J.M. Electrochemical Impedance Analysis for Corrosion Rate Monitoring of Sol–Gel Protective Coatings in Contact with Nitrate Molten Salts for CSP Applications. Materials 2023, 16, 546. [Google Scholar] [CrossRef]
- Zhu, M.; Zeng, S.; Zhang, H.; Li, J.; Cao, B. Electrochemical study on the corrosion behaviors of 316 SS in HITEC molten salt at different temperatures. Sol. Energy Mater. Sol. Cells 2018, 186, 200–207. [Google Scholar] [CrossRef]
- Ni, C.S.; Lu, L.Y. Electrochemical impedance and modelling studies of the corrosion of three commercial stainless steels in molten carbonate. Int. J. Corros. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Dorcheh, S.; Durham, R.N.; Galetz, M.C. High temperature corrosion in molten solar salt: The role of chloride impurities. Mater. Corr. 2017, 68, 943–951. [Google Scholar] [CrossRef]
- Powder Diffraction File, International Center of Diffraction Data, 12 Campus Boulevard, Newton Square, PA, USA, 2003. Available online: https://www.icdd.com/pdfsearch/ (accessed on 20 February 2023).
- Fernández, A.G.; Galleguillos, H.; Fuentealba, E.; Pérez, F.J. Corrosion of stainless steels and low-Cr steel in molten Ca(NO3)2–NaNO3–KNO3 eutectics for direct energy storage in CSP plants. Sol. Energy Mat. Sol. Cells 2015, 141, 7–13. [Google Scholar] [CrossRef]
- Fernández, A.G.; Lasanta, M.I.; Pérez, F.J. Molten salt corrosion of stainless steels and low-Cr steel in CSP plants. Oxid. Met. 2012, 78, 329–348. [Google Scholar] [CrossRef]

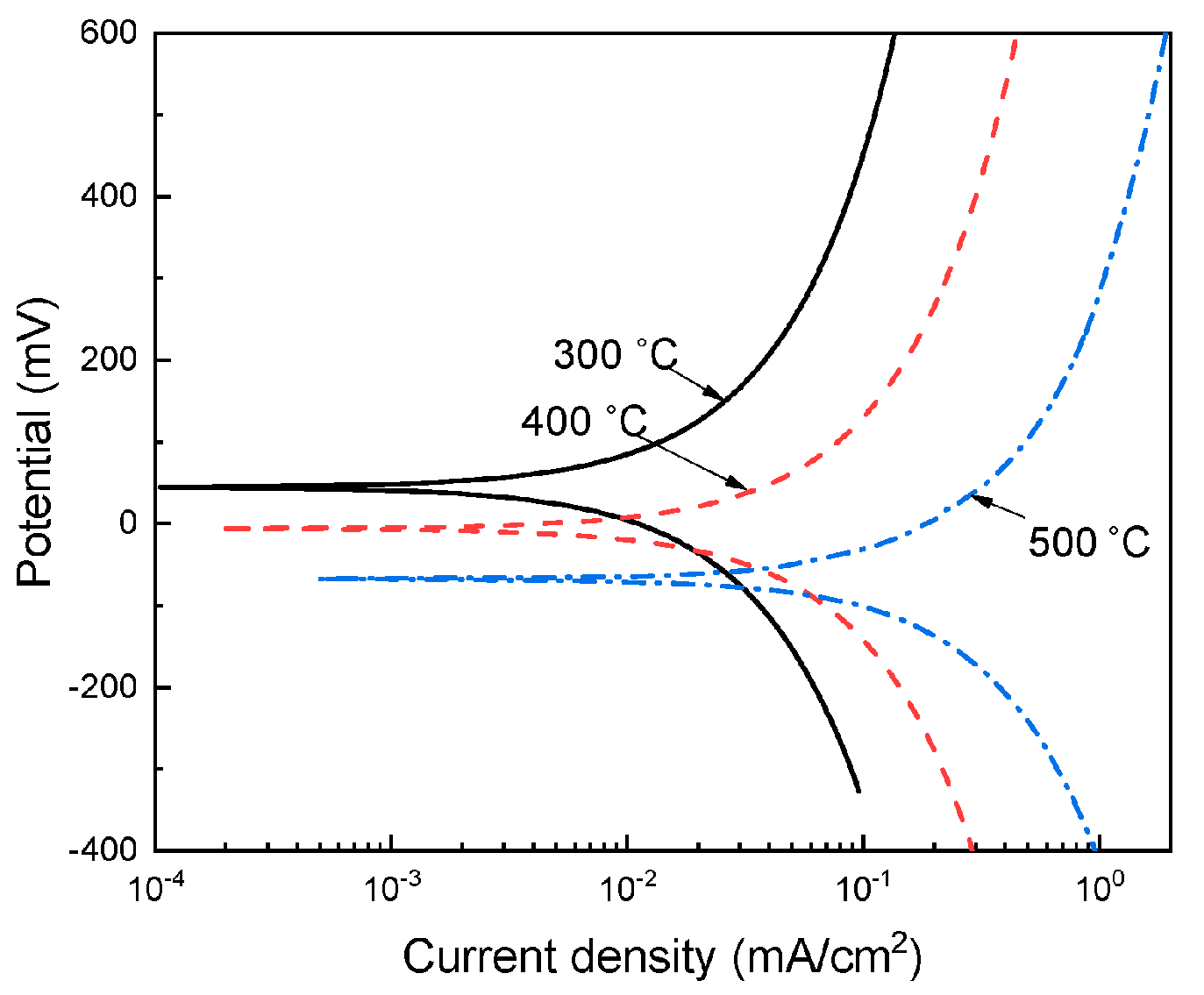




| Testing Temperature (°C) | Ecorr (mV) | Icorr (mA/cm2) | βa (mV/dec) | βc (mV/dec) |
|---|---|---|---|---|
| 300 | 60 | 0.01 | 295 | 420 |
| 400 | −10 | 0.05 | 360 | 490 |
| 500 | −70 | 0.41 | 420 | 570 |
| Time (h) | Re (ohm cm2) | CPEdl (F/cm2) | ndl | Rct (ohm cm2) | CPEf (F/cm2) | nf | Rf (ohm cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 3.2 | 8.14 | 0.91 | 10.32 | 4.12 | 0.92 | 12,840 |
| 250 | 3.4 | 1.78 | 0.90 | 14.59 | 2.65 | 0.94 | 15,797 |
| 500 | 3.4 | 2.10 | 0.90 | 14.28 | 2.44 | 0.95 | 17,869 |
| 750 | 3.5 | 2.36 | 0.89 | 13.69 | 2.14 | 0.96 | 18,050 |
| 1000 | 3.6 | 2.37 | 0.89 | 13.56 | 2.15 | 0.96 | 18,306 |
| Time (h) | Re (ohm cm2) | CPEdl (F/cm2) | ndl | Rdl (ohm cm2) | CPEf (F/cm2) | nf | Rf (ohm cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 3.2 | 3.72 | 0.9 | 12.66 | 1.21 | 0.9 | 2808 |
| 250 | 4.3 | 2.56 | 0.8 | 9.94 | 2.20 | 0.9 | 10,348 |
| 500 | 4.2 | 2.72 | 0.8 | 7.36 | 2.03 | 0.9 | 11,659 |
| 750 | 4.3 | 2.75 | 0.8 | 7.65 | 1.93 | 0.9 | 12,476 |
| 1000 | 4.2 | 2.74 | 0.7 | 7.91 | 1.93 | 0.9 | 12,717 |
| Time (h) | Re (ohm cm2) | CPEdl (F/cm2) | ndl | Rdl (ohm cm2) | CPEf (F/cm2) | nf | Rf (ohm cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 4.7 | 1.88 | 0.8 | 6.0 | 1.55 | 0.8 | 196 |
| 250 | 6.3 | 2.09 | 0.7 | 3.8 | 1.05 | 0.7 | 694 |
| 500 | 4.6 | 3.29 | 0.7 | 3.1 | 2.05 | 0.7 | 665 |
| 750 | 4.7 | 5.72 | 0.7 | 2.5 | 2.32 | 0.7 | 655 |
| 1000 | 4.0 | 1.28 | 0.7 | 5.4 | 8.99 | 0.7 | 750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Dominguez, D.; Gomez-Guzman, N.B.; Porcayo-Calderón, J.; Lopez-Sesenes, R.; Larios-Galvez, A.K.; Sarmiento-Bustos, E.; Rodriguez-Clemente, E.; Gonzalez-Rodriguez, J.G. An Electrochemical Study of the Corrosion Behaviour of T91 Steel in Molten Nitrates. Metals 2023, 13, 502. https://doi.org/10.3390/met13030502
Lopez-Dominguez D, Gomez-Guzman NB, Porcayo-Calderón J, Lopez-Sesenes R, Larios-Galvez AK, Sarmiento-Bustos E, Rodriguez-Clemente E, Gonzalez-Rodriguez JG. An Electrochemical Study of the Corrosion Behaviour of T91 Steel in Molten Nitrates. Metals. 2023; 13(3):502. https://doi.org/10.3390/met13030502
Chicago/Turabian StyleLopez-Dominguez, D., N. B. Gomez-Guzman, J. Porcayo-Calderón, R. Lopez-Sesenes, A. K. Larios-Galvez, E. Sarmiento-Bustos, E. Rodriguez-Clemente, and J. G. Gonzalez-Rodriguez. 2023. "An Electrochemical Study of the Corrosion Behaviour of T91 Steel in Molten Nitrates" Metals 13, no. 3: 502. https://doi.org/10.3390/met13030502
APA StyleLopez-Dominguez, D., Gomez-Guzman, N. B., Porcayo-Calderón, J., Lopez-Sesenes, R., Larios-Galvez, A. K., Sarmiento-Bustos, E., Rodriguez-Clemente, E., & Gonzalez-Rodriguez, J. G. (2023). An Electrochemical Study of the Corrosion Behaviour of T91 Steel in Molten Nitrates. Metals, 13(3), 502. https://doi.org/10.3390/met13030502






