Research on Grain Refinement of Sc and Zr Addition in an Al-Mg-Zn Alloy from Experiments and First-Principles Calculations
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Procedures
2.2. DFT Calculation Details
3. Results and Discussions
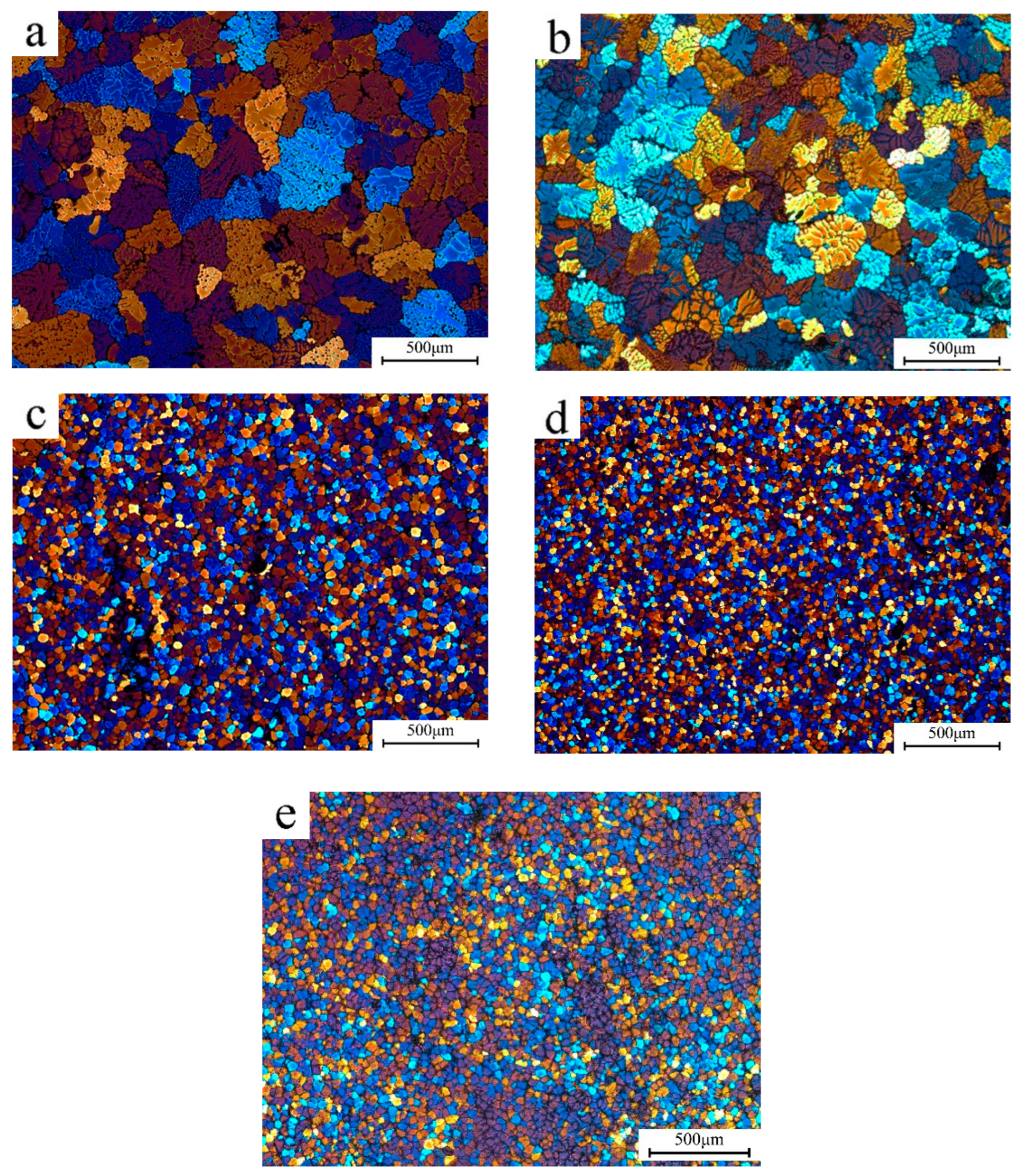
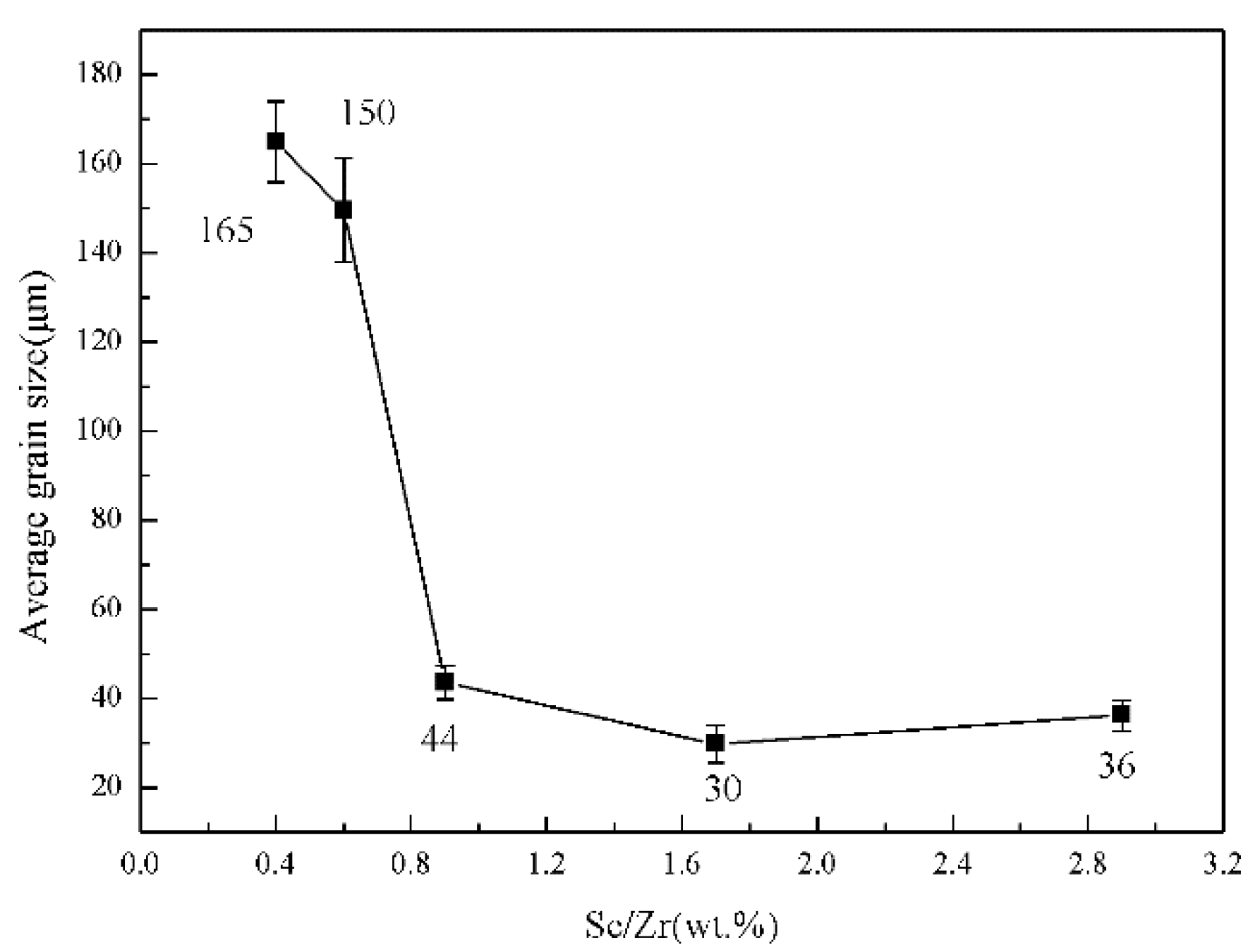
3.1. Grain Refinement of Sc and Zr Addition in Al-Mg-Zn Alloys
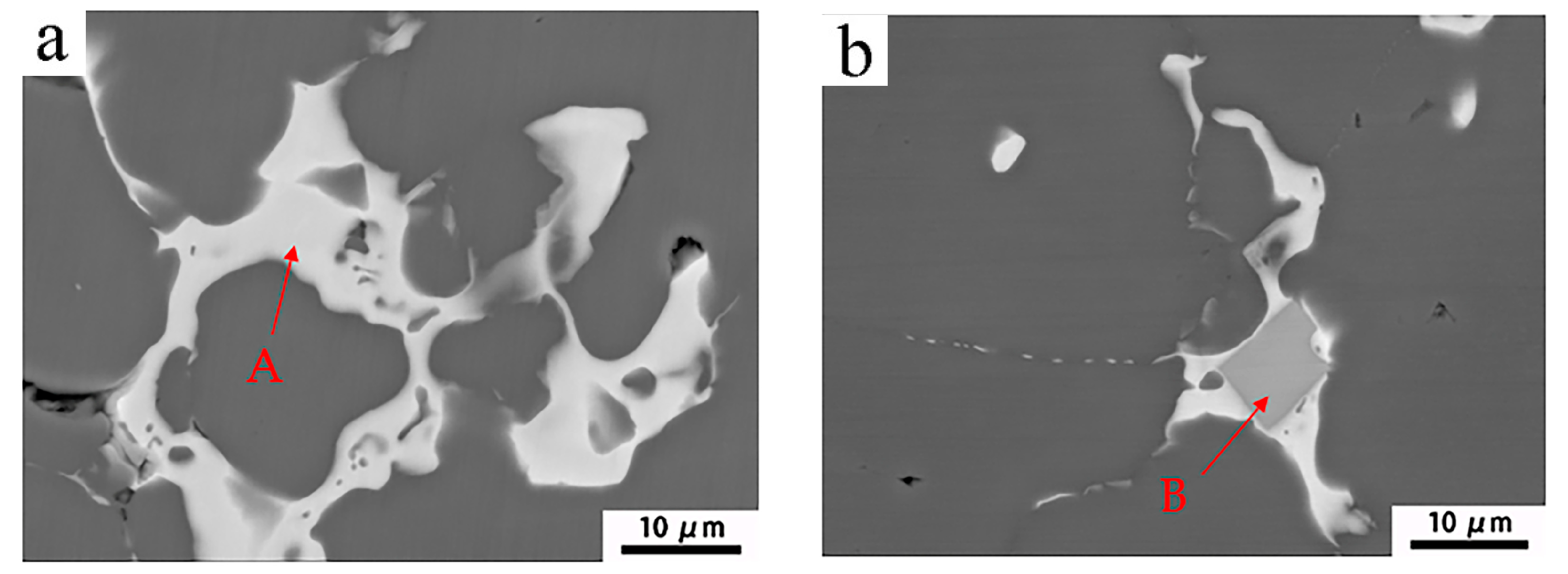
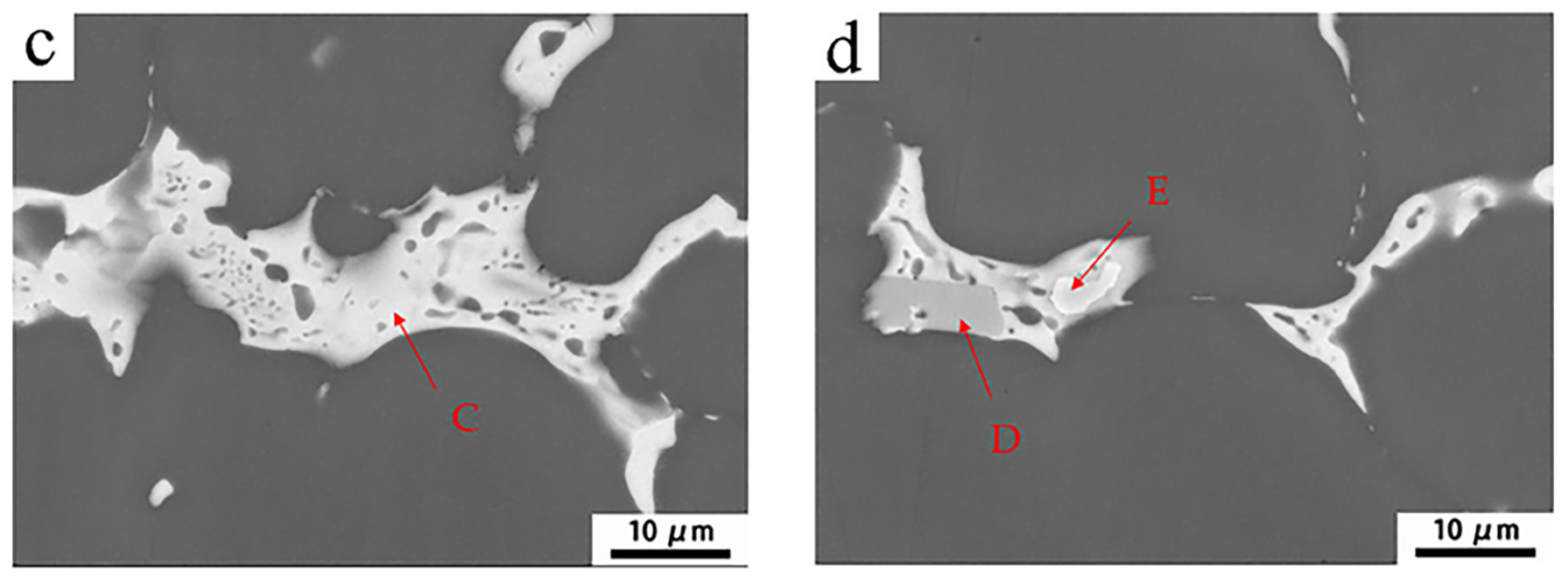
3.2. Intermetallics of as-Cast Al-Mg-Zn Alloys
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, C.; Zhang, D.; Cui, H.; Zhuang, L.; Zhang, J. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al–Mg alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Hou, S.; Liu, P.; Zhang, D.; Zhang, J.; Zhuang, L. Precipitation hardening behavior and microstructure evolution of Al-5.1Mg-0.15Cu alloy with 3.0Zn (wt%) addition. J. Mater. Sci. 2018, 53, 3846–3861. [Google Scholar]
- Takata, N.; Ishihara, M.; Suzuki, A.; Kobashi, M. Microstructure and strength of a novel heat-resistant aluminum alloy strengthened by T-Al6Mg11Zn11 phase at elevated temperatures. Mater. Sci. Eng. A 2018, 739, 62–70. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Zhuang, L.; Zhang, J. Correlations between stress corrosion cracking, grain boundary precipitates and Zn content of Al-Mg-Zn alloys. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid-State Chem. Phys. 2016, 655, 178–187. [Google Scholar] [CrossRef]
- Liu, T.W.; Wang, Q.D.; Tang, H.P.; Li, Z.Y.; Chuan, L.E.I.; Ebrahimi, M.; Jiang, H.Y.; Ding, W.J. Microstructure and mechanical properties of squeeze-cast Al−5.0 Mg−3.0 Zn−1.0 Cu alloys in solution-treated and aged conditions. Trans. Nonferrous Met. Soc. China 2020, 30, 2326–2338. [Google Scholar] [CrossRef]
- El-Danaf, E.; Soliman, M.; Almajid, A.; El-Rayes, M. Enhancement of mechanical properties and grain size refinement of commercial purity aluminum 1050 processed by ECAP. Mater. Sci. Eng. A 2007, 458, 226–234. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Attarilar, S.; Gode, C.; Djavanroodi, F. Damage prediction of 7025 aluminum alloy during equal-channel angular pressing. Int. J. Miner. Met. Mater. 2014, 21, 990–998. [Google Scholar] [CrossRef]
- Jung, S.S.; Son, Y.G.; Park, Y.H.; Lee, Y.C. A Study on the Grain Refining Mechanisms and Melt Superheat Treatment of Aluminum-Bearing Mg Alloys. Metals 2022, 12, 464. [Google Scholar] [CrossRef]
- Clouet, E.; Nastar, M.; Sigli, C. Nucleation of Al3Zr and Al3Sc in aluminum alloys: From kinetic Monte Carlo simulations to classical theory. Phys. Rev. B 2004, 69, 064109. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, M.S.; Datta, S.; Roychowdhury, A.; Banerjee, M.K. Effect of scandium on the microstructure and ageing behaviour of cast Al-6Mg alloy. Mater. Charact. 2008, 59, 1661–1666. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Barkov, R.Y.; Mikhaylovskaya, A.V.; Loginova, I.S.; Yakovtseva, O.A.; Pozdniakov, A.V. Structure and Properties of Al–4.5 Mg–0.15 Zr Compo-sitions Alloyed with Er, Y, and Yb. Phys. Met. Metallogr. 2022, 123, 466–473. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Calderwood, F.W. The Al−Er (Aluminum-Erbium) system. Bull. Alloy. Phase Diagr. 1988, 9, 676–678. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Calderwood, F.W. The Al-Yb (aluminum-ytterbium) system. Bull. Alloy. Phase Diagr. 1989, 10, 47–49. [Google Scholar] [CrossRef]
- Murray, J.L.; McAlister, A.J.; Kahan, D.J. The Al-Hf (aluminum-hafnium) system. J. Phase Equilibria Diffus. 1998, 19, 376. [Google Scholar] [CrossRef]
- Fuller, C.B.; Murray, J.L.; Seidman, D.N. Temporal evolution of the nanostructure of Al3(Sc, Zr) alloys: Part I–Chemical compositions of Al3(Sc1−xZrx) precipitates. Acta Mater. 2005, 53, 5401–5413. [Google Scholar] [CrossRef]
- Fuller, C.B.; Seidman, D.N. Temporal evolution of the nanostructure of Al3(Sc, Zr) alloys: Part II-coarsening of Al3 (Sc1−x Zrx) precipitates. Acta Mater. 2005, 53, 5415–5428. [Google Scholar] [CrossRef]
- Sun, F.; Nash, G.L.; Li, Q.; Liu, E.; He, C.; Shi, C.; Zhao, N. Effect of Sc and Zr additions on microstructures and corrosion behavior of Al-Cu-Mg-Sc-Zr alloys. J. Mater. Sci. Technol. 2017, 33, 1015–1022. [Google Scholar] [CrossRef]
- Norman, A.; Prangnell, P.; McEwen, R. The solidification behaviour of dilute aluminium–scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]
- Pan, D.; Zhou, S.; Zhang, Z.; Li, M.; Wu, Y. Effects of Sc(Zr) on the microstructure and mechanical properties of as-cast Al–Mg alloys. Mater. Sci. Technol. 2017, 33, 751–757. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Li, M.; Pan, D.; Su, H.; Du, X.; Li, P.; Wu, Y. Correlative characterization of primary particles formed in as-cast Al-Mg alloy containing a high level of Sc. Mater. Charact. 2016, 118, 85–91. [Google Scholar] [CrossRef]
- Wang, Z.G.; Xu, J.; Li, B.; Zhang, Z.F. Study on Refinement Mechanism of Sc and Zr as-cast Al-7.2 Zn-2.2 Mg-1.8 Cu alloys. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013. [Google Scholar]
- Zhou, S.; Zhang, Z.; Li, M.; Pan, D.; Su, H.; Du, X.; Li, P.; Wu, Y. Effect of Sc on microstructure and mechanical properties of as-cast Al–Mg alloys. Mater. Des. 2015, 90, 1077–1084. [Google Scholar] [CrossRef]
- Li, J.; Wiessner, M.; Albu, M.; Wurster, S.; Sartory, B.; Hofer, F.; Schumacher, P. Correlative characterization of primary Al3(Sc, Zr) phase in an Al–Zn–Mg based alloy. Mater. Charact. 2015, 102, 62–70. [Google Scholar] [CrossRef]
- Li, J.H.; Oberdorfer, B.; Wurster, S.; Schumacher, P. Impurity effects on the nucleation and growth of primary Al3(Sc,Zr) phase in Al alloys. J. Mater. Sci. 2014, 49, 5961–5977. [Google Scholar] [CrossRef]
- Cong, X.U.; Rou, D.U.; Wang, X.J.; Hanada, S.; Yamagata, H.; Wang, W.H. Effect of cooling rate on morphology of primary particles in Al-Sc-Zr master alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2420–2426. [Google Scholar]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Wang, H.; Jiang, H.; Lei, W.; Jiang, Y.; Yi, D. First-principles investigations on the electronic properties and stabilities of low-index surfaces of L12–Al3Sc intermetallic. Appl. Surf. Sci. 2013, 288, 609–618. [Google Scholar] [CrossRef]
- Yang, T.; Han, X.; Ding, Z.; Wang, Y.; Li, J. Electronic and structural properties of low-index L12–Al3Zr surfaces by first-principle calculations. Calphad 2019, 66, 101645. [Google Scholar] [CrossRef]
- Wen, S.P.; Xing, Z.B.; Huang, H.; Li, B.L.; Wang, W.; Nie, Z.R. The effect of erbium on the microstructure and mechanical properties of Al–Mg–Mn–Zr alloy. Mater. Sci. Eng. A 2009, 516, 42–49. [Google Scholar] [CrossRef]
- Forbord, B.; Lefebvre, W.; Danoix, F.; Hallem, H.; Marthinsen, K. Three dimensional atom probe investigation on the formation of Al3(Sc, Zr)-dispersoids in aluminium alloys. Scr. Mater. 2004, 51, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Knipling, K.E.; Karnesky, R.A.; Lee, C.P.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al–0.1 Sc, Al–0.1 Zr and Al–0.1 Sc–0.1 Zr (at.%) alloys during isochronal aging. Acta Mater. 2010, 58, 5184–5195. [Google Scholar] [CrossRef]
- Li, S.-S.; Li, L.; Han, J.; Wang, C.-T.; Xiao, Y.-Q.; Jian, X.-D.; Qian, P.; Su, Y.-J. First-Principles study on the nucleation of precipitates in ternary Al alloys doped with Sc, Li, Zr, and Ti elements. Appl. Surf. Sci. 2020, 526, 146455. [Google Scholar] [CrossRef]
- Chunmei, L.; Xianquan, J.; Nanpu, C.; Jianfeng, T.; Zhiqian, C. First-principles Investigation on Formation Energy, Elasticity and Interfacial Properties of Refining Phase Al-3 (Zr, Sc) in Al Alloys. Rare Met. Mater. Eng. 2020, 49, 2557–2566. [Google Scholar]
- Zhang, C.; Jiang, Y.; Cao, F.; Hu, T.; Wang, Y.; Yin, D. Formation of coherent, core-shelled nano-particles in dilute Al-Sc-Zr alloys from the first-principles. J. Mater. Sci. Technol. 2019, 35, 930–938. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.-C.; Zhang, X.-Y.; Huang, Y.-C. Understanding grain refinement of Sc addition in a Zr containing Al-Zn-Mg-Cu aluminum alloy from experiments and first-principles. Intermetallics 2020, 123, 106823. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, D.; Jiang, Y.; Wang, Y. Precipitation of L12-phase nano-particles in dilute Al-Er-Zr alloys from the first-principles. Comput. Mater. Sci. 2019, 162, 171–177. [Google Scholar] [CrossRef]
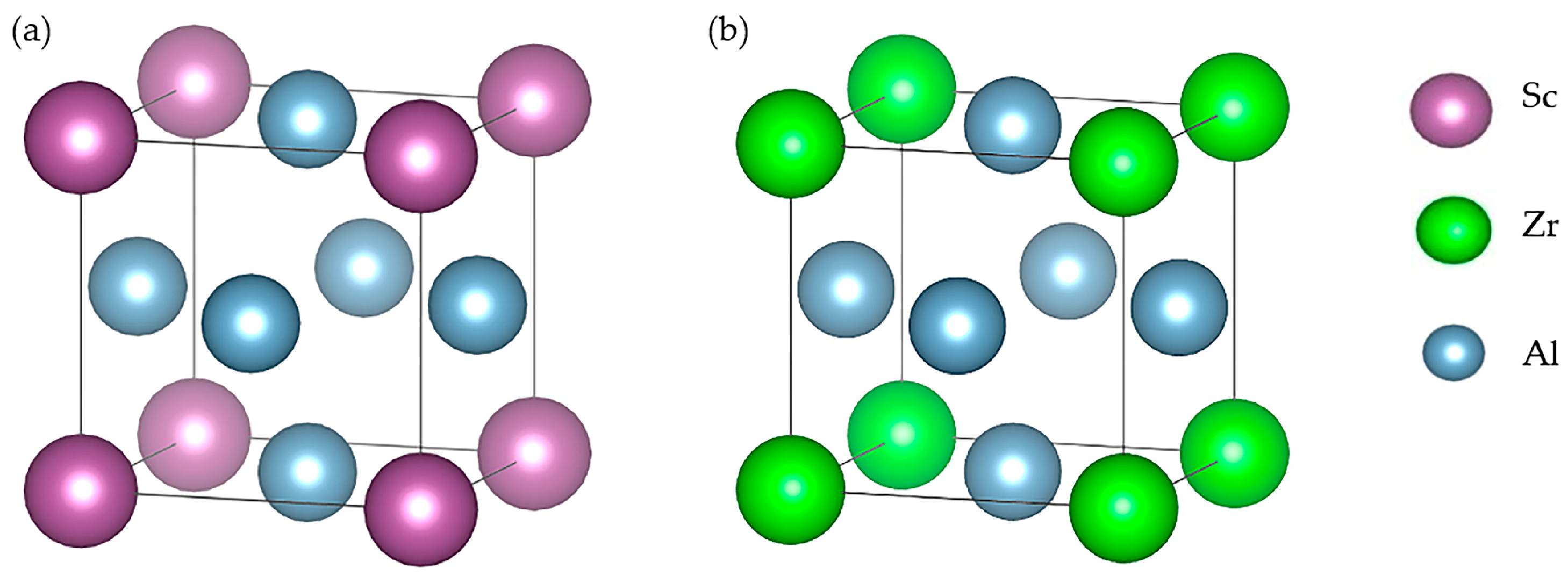
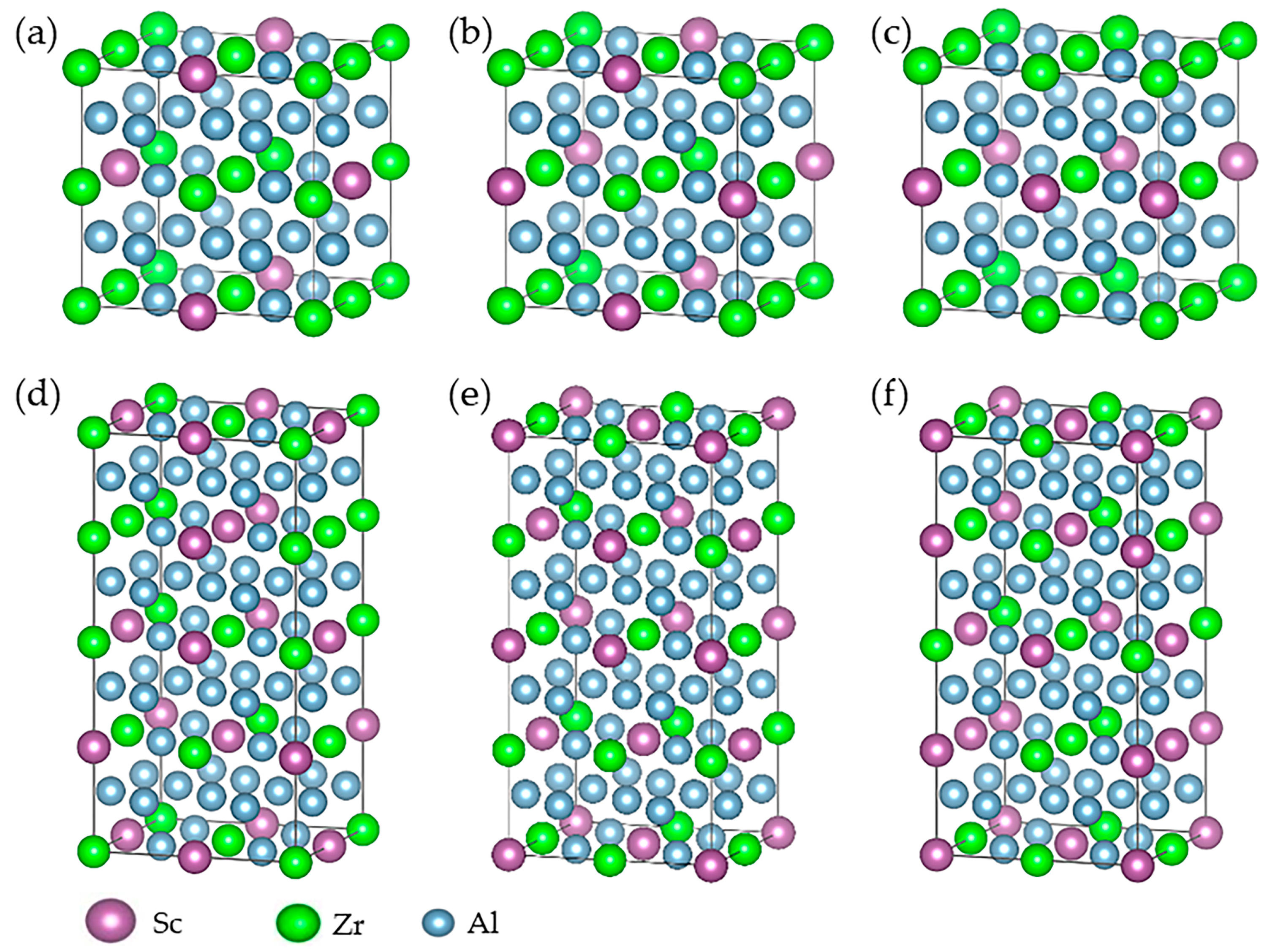


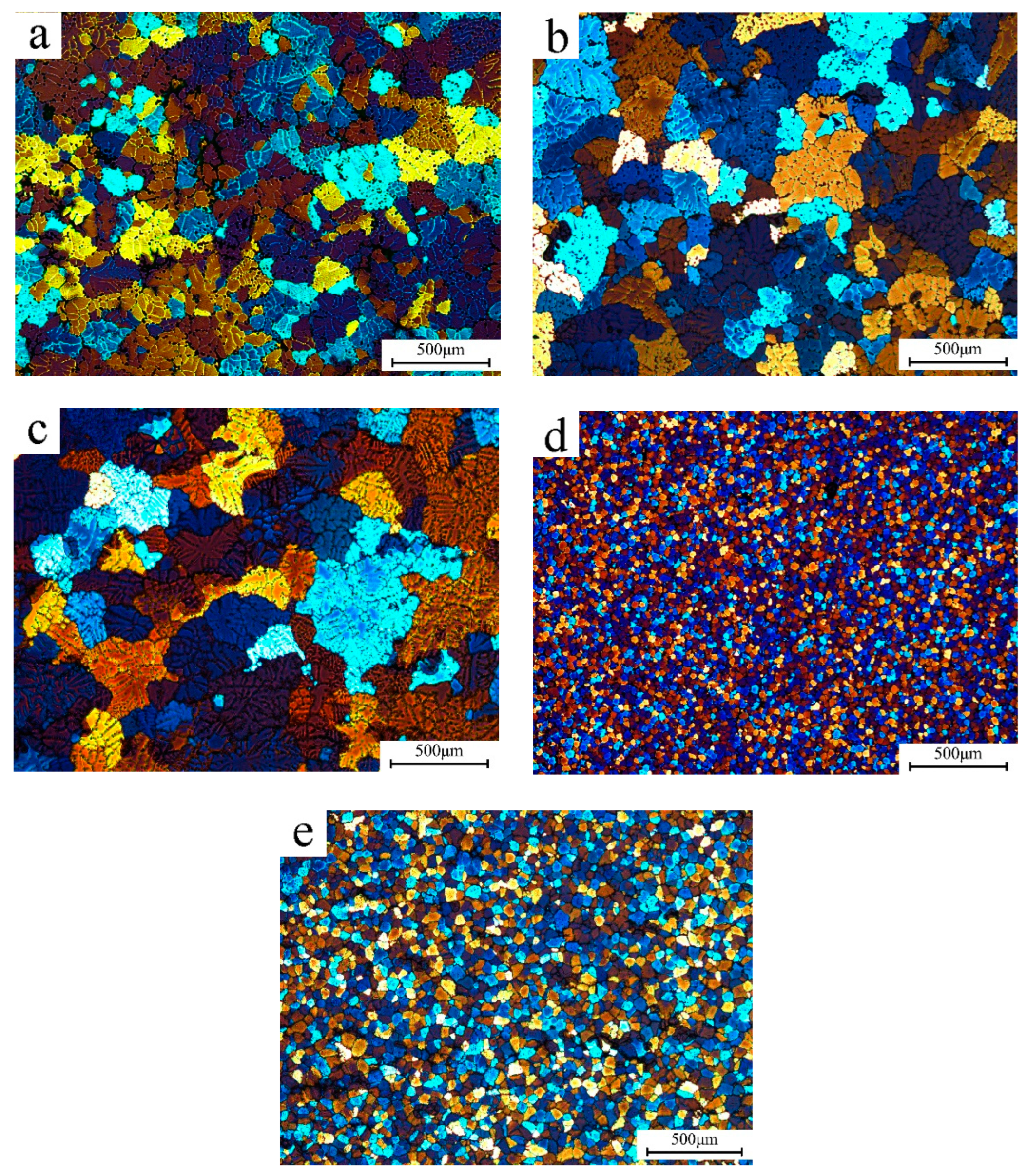
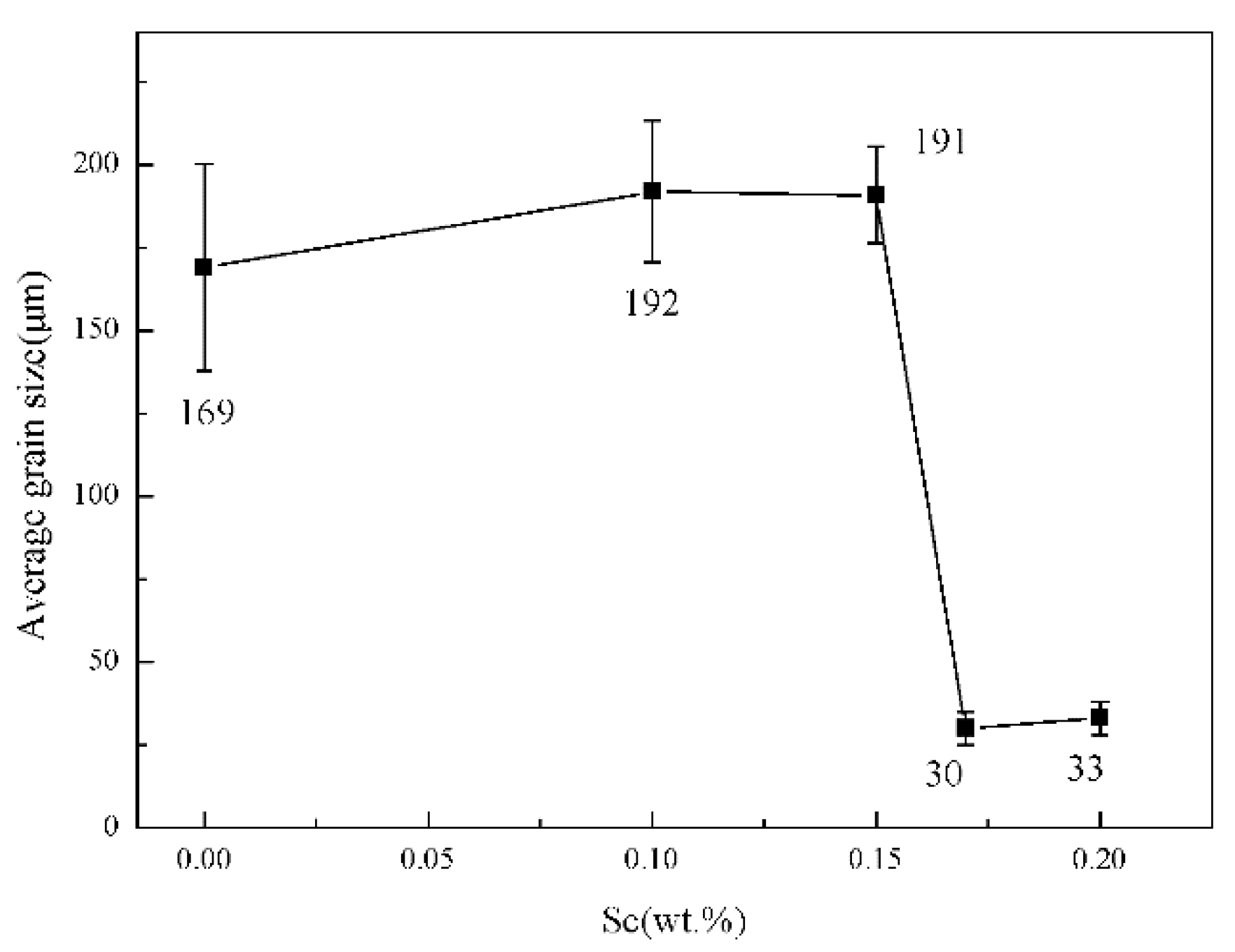
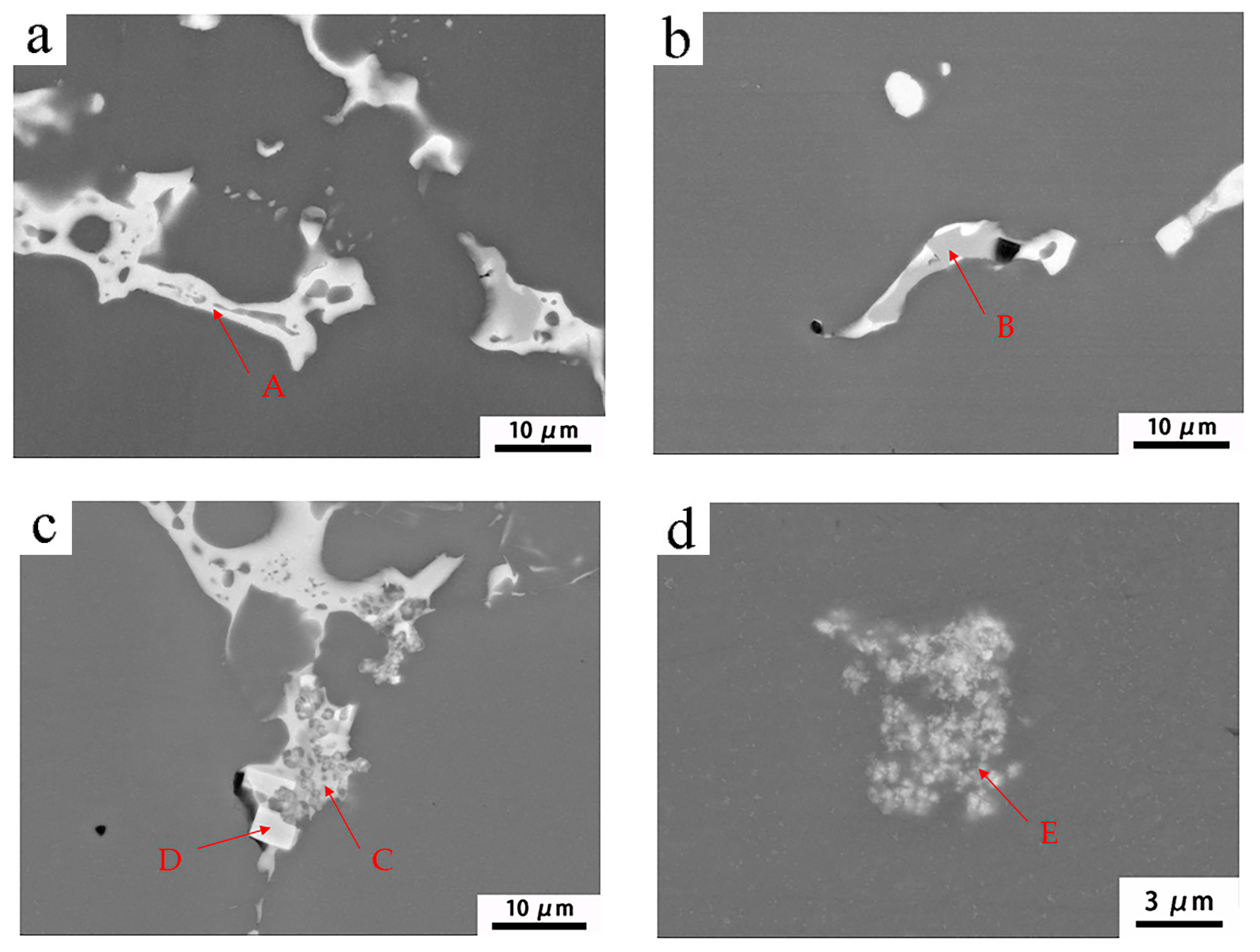


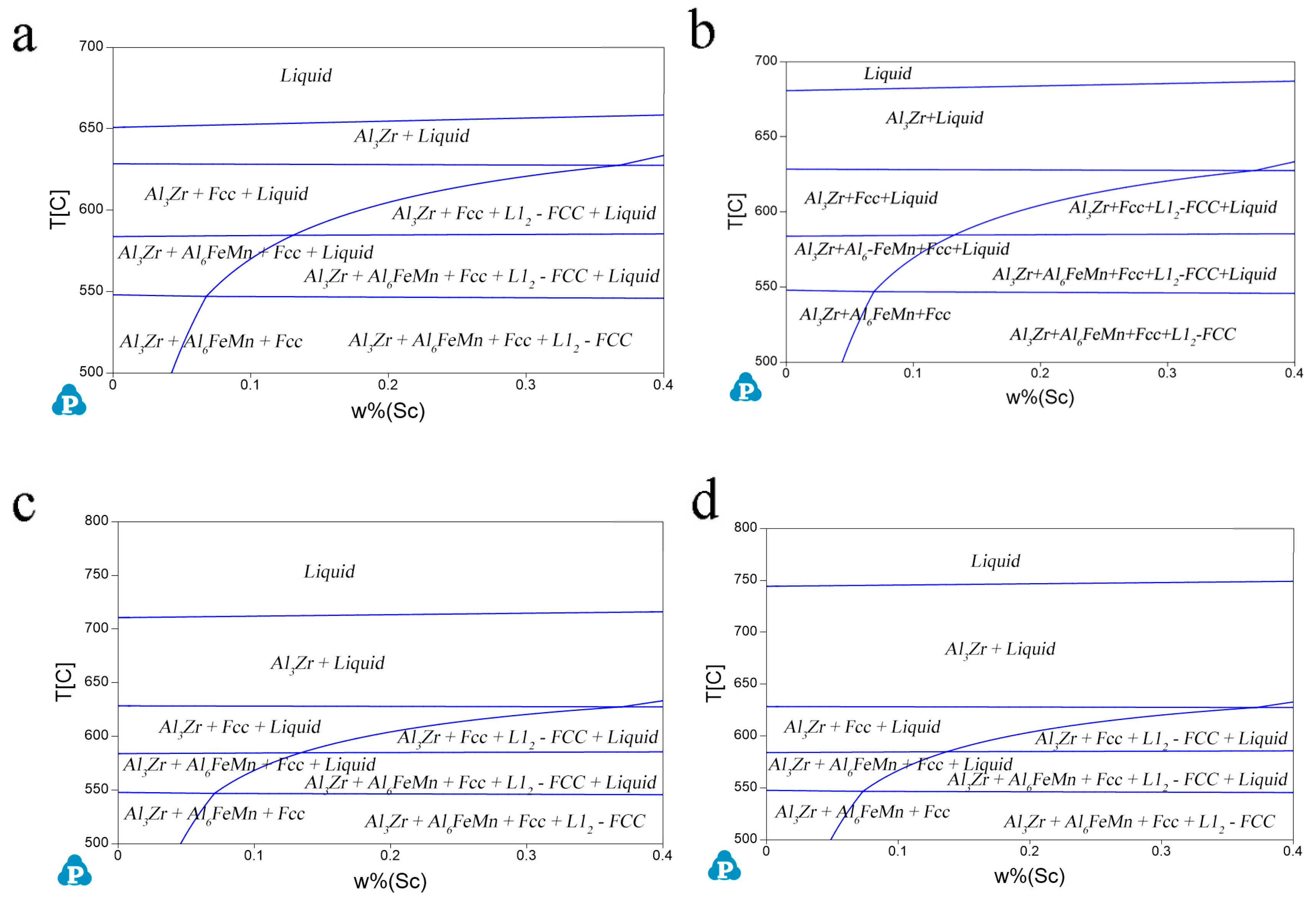
| Group Number | Alloy No. | Mg | Zn | Mn | Sc | Zr |
|---|---|---|---|---|---|---|
| I: single addition of Sc or Zr | Base alloy | 5.0 | 3.0 | 0.5 | 0 | 0 |
| 0.27Sc | 5.14 | 3.0 | 0.5 | 0.27 | 0 | |
| 0.27Zr | 5.1 | 3.0 | 0.5 | 0 | 0.27 | |
| II: addition of Sc only if mass fraction of Zr was 0.1 | 0Sc-0.1Zr | 5.1 | 2.93 | 0.54 | 0 | 0.1 |
| 0.1Sc-0.1Zr | 4.99 | 2.89 | 0.54 | 0.1 | 0.1 | |
| 0.15Sc-0.1Zr | 4.98 | 3.0 | 0.5 | 0.15 | 0.1 | |
| 0.17Sc-0.1Zr | 5.12 | 3.04 | 0.48 | 0.17 | 0.1 | |
| 0.2Sc-0.1Zr | 5.0 | 3.02 | 0.5 | 0.2 | 0.1 | |
| III: different Sc to Zr ratio only if the total mass fraction of Sc and Zr was 0.27 | 0.07Sc-0.2Zr | 5.0 | 3.0 | 0.52 | 0.07 | 0.20 |
| 0.1Sc-0.17Zr | 5.12 | 3.02 | 0.5 | 0.1 | 0.17 | |
| 0.13Sc-0.14Zr | 4.98 | 3.0 | 0.5 | 0.13 | 0.14 | |
| 0.2Sc-0.07Zr | 5.0 | 3.0 | 0.5 | 0.20 | 0.07 |
| Element | Al | Mg | Zn | Mn | Fe |
|---|---|---|---|---|---|
| A | 48.4 | 37.5 | 14.1 | - | - |
| B | 73.2 | 15.0 | 3.8 | 8.0 | - |
| C | 47.4 | 38.5 | 14.1 | - | |
| D | 75.7 | 5.8 | 1.4 | 3.5 | |
| E | 77.7 | 3.8 | 1.5 | 7.0 | 10.0 |
| Element | Al | Mg | Zn | Mn | Fe | Sc | Zr |
|---|---|---|---|---|---|---|---|
| A | 58.6 | 27.3 | 14.1 | - | - | - | |
| B | 70.9 | 15.3 | 4.6 | 9.3 | - | ||
| C | 75.7 | 5.8 | 1.4 | 3.5 | 9.1 | 3.7 | |
| D | 75.1 | 4.3 | 1.2 | 12.5 | 7.3 | - | - |
| E | 88.1 | 4.6 | 1.3 | - | 4.7 | 1.4 |
| Systems | Lattice Parameter (Å) | Lattice Misfit (%) |
|---|---|---|
| Al | 4.05 | - |
| Al3Sc | 4.103 | 1.304 |
| 4.106 [34] | - | |
| 4.047 [33] | - | |
| Al3Zr | 4.105 | 1.361 |
| 4.113 [35] | - | |
| 4.117 [36] | - | |
| Al24Sc2Zr6-1 | 4.098 | 1.361 |
| Al24Sc2Zr6-2 | 4.096 | 1.185 |
| Al24Sc2Zr6-3 | 4.097 | 1.132 |
| Al48Sc8Zr8-1 | 4.097 | 1.161 |
| Al48Sc8Zr8-2 | 4.096 | 1.161 |
| Al48Sc8Zr8-3 | 4.105 | 1.146 |
| Systems | Enthalpy of Formation (kJ/mol) | Bond | Bond Order |
|---|---|---|---|
| Al3Sc | −18.96 | Al-Sc | 0.236 |
| Al3Zr | −19.57 | Al-Zr | 0.318 |
| Al24Sc2Zr6-1 | −20.07 | Al-Sc | 0.231 |
| Al-Zr | 0.314 | ||
| Sc-Zr | 0.015 | ||
| Al24Sc2Zr6-2 | −19.88 | Al-Sc | 0.234 |
| Al-Zr | 0.323 | ||
| Sc-Zr | 0.015 | ||
| Al24Sc2Zr6-3 | −19.88 | Al-Sc | 0.236 |
| Al-Zr | 0.320 | ||
| Sc-Zr | 0.015 | ||
| Al48Sc8Zr8-1 | −19.59 | Al-Sc | 0.239 |
| Al-Zr | 0.328 | ||
| Sc-Zr | 0.016 | ||
| Al48Sc8Zr8-2 | −19.97 | Al-Sc | 0.234 |
| Al-Zr | 0.323 | ||
| Sc-Zr | 0.015 | ||
| Al48Sc8Zr8-3 | −19.97 | Al-Sc | 0.236 |
| Al-Zr | 0.324 | ||
| Sc-Zr | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yan, L.; Li, X.; Xiao, W.; Gao, G.; Li, Z.; Zhang, Y.; Xiong, B. Research on Grain Refinement of Sc and Zr Addition in an Al-Mg-Zn Alloy from Experiments and First-Principles Calculations. Metals 2023, 13, 519. https://doi.org/10.3390/met13030519
Zhang T, Yan L, Li X, Xiao W, Gao G, Li Z, Zhang Y, Xiong B. Research on Grain Refinement of Sc and Zr Addition in an Al-Mg-Zn Alloy from Experiments and First-Principles Calculations. Metals. 2023; 13(3):519. https://doi.org/10.3390/met13030519
Chicago/Turabian StyleZhang, Tianyou, Lizhen Yan, Xiwu Li, Wei Xiao, Guanjun Gao, Zhihui Li, Yongan Zhang, and Baiqing Xiong. 2023. "Research on Grain Refinement of Sc and Zr Addition in an Al-Mg-Zn Alloy from Experiments and First-Principles Calculations" Metals 13, no. 3: 519. https://doi.org/10.3390/met13030519
APA StyleZhang, T., Yan, L., Li, X., Xiao, W., Gao, G., Li, Z., Zhang, Y., & Xiong, B. (2023). Research on Grain Refinement of Sc and Zr Addition in an Al-Mg-Zn Alloy from Experiments and First-Principles Calculations. Metals, 13(3), 519. https://doi.org/10.3390/met13030519






