Clustering and Precipitation during Early-Stage Artificial Aging of Al–Si–Mg(–Cu) Foundry Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
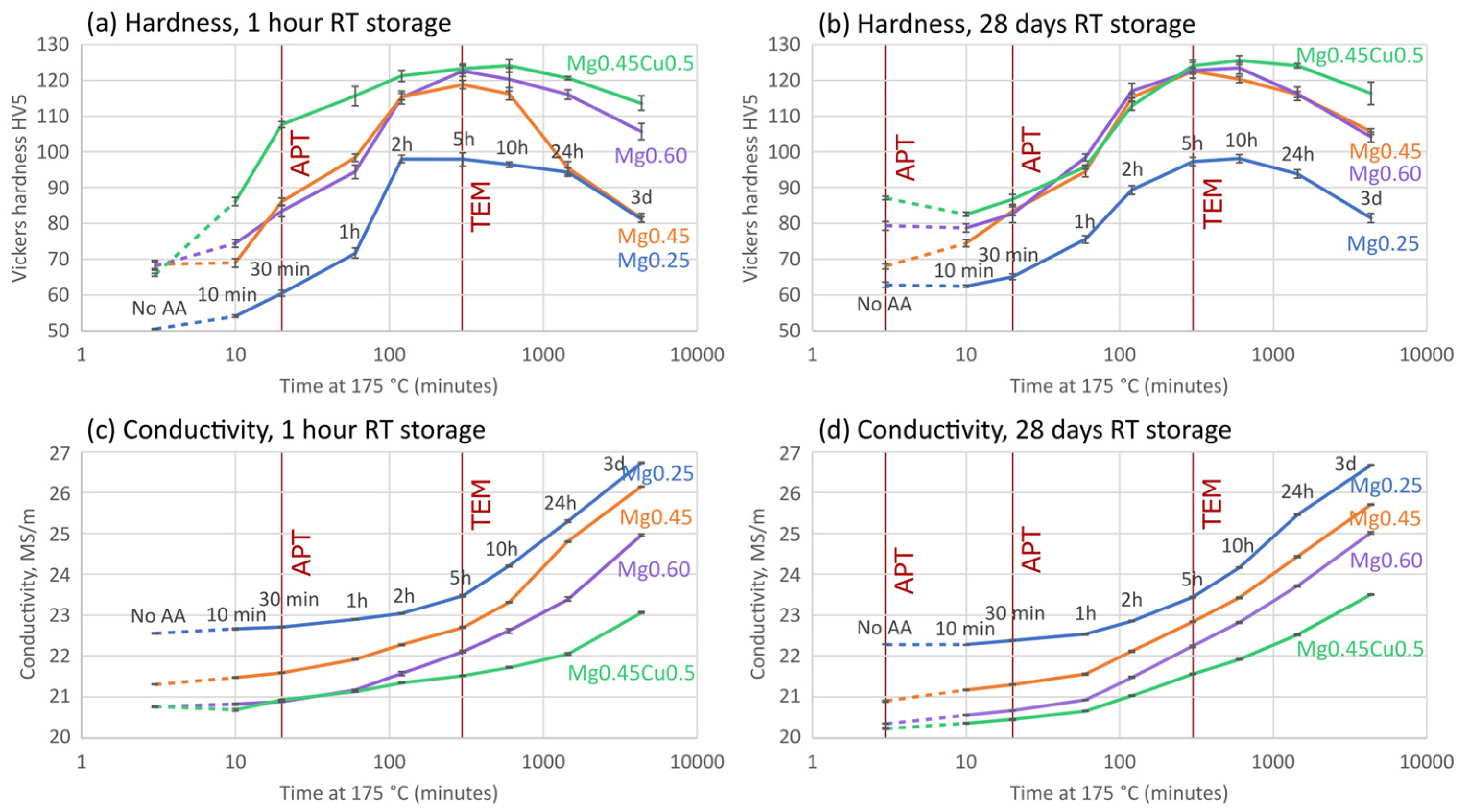
3.1. Artificial Aging Kinetics
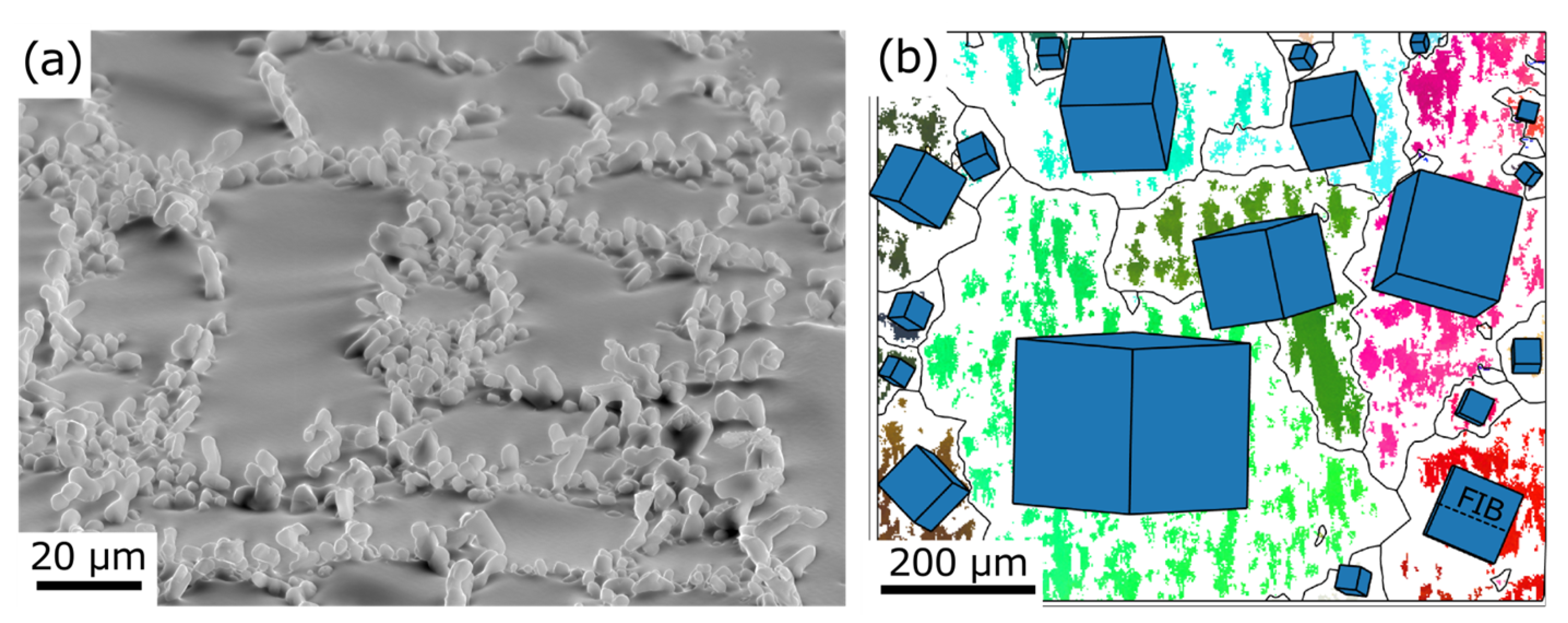
3.2. Microstructure Overview
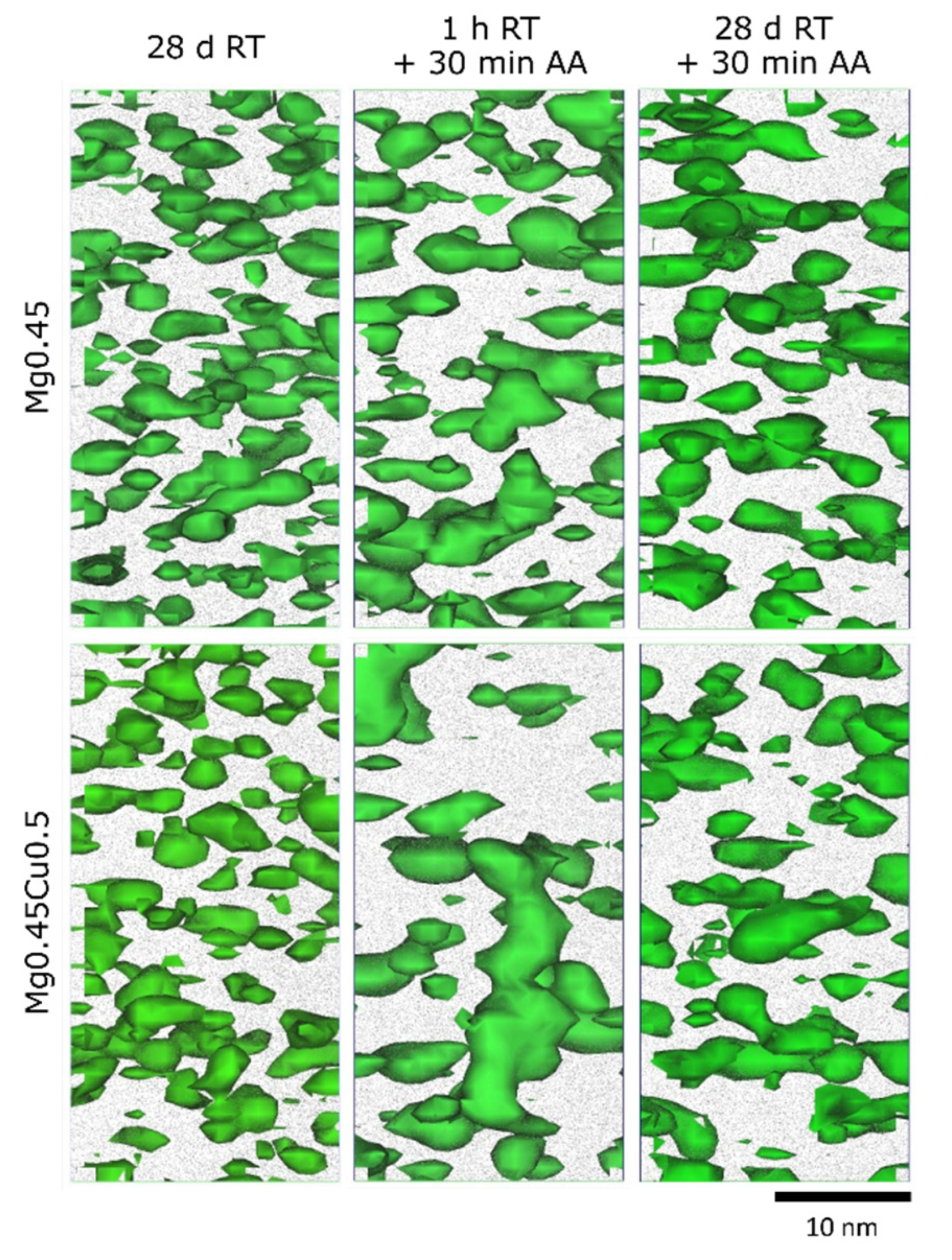
3.3. Early-Stage Precipitation
3.4. Precipitation at Peak Hardness
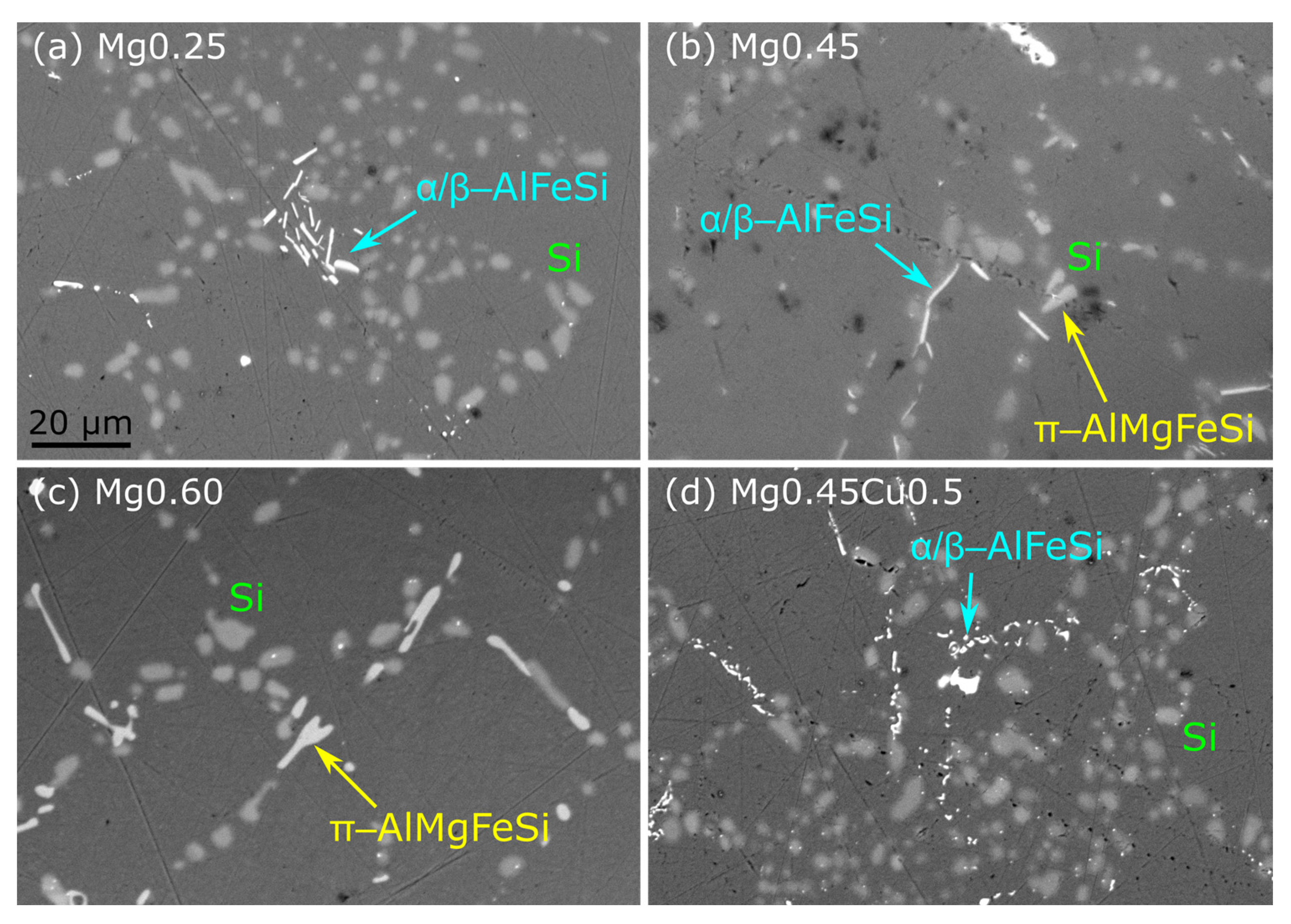
3.5. Influence of Eutectic Si Particles
4. Discussion
4.1. Solute Balance
4.2. Aging Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Sabatino, M.; Arnberg, L. Castability of Aluminium Alloys. Trans. Indian Inst. Met. 2010, 62, 321–325. [Google Scholar] [CrossRef]
- Yıldırım, M.; Özyürek, D. The Effect of Mg Amount on the Microstructure and Mechanical Properties of Al-Si-Mg Alloy. Mater. Des. 2013, 51, 767–774. [Google Scholar] [CrossRef]
- Triyono, T.; Surojo, E.; Utomo, V.T. Analysis of the Effect of Magnesium Addition on Al-Si Alloy Using Stirr Casting Method on Physical and Mechanical Properties. AIP Conf. Proc. 2020, 2217, 030177. [Google Scholar] [CrossRef]
- Dong, X.; Amirkhanlou, S.; Ji, S. Formation of Strength Platform in Cast Al–Si–Mg–Cu Alloys. Sci. Rep. 2019, 9, 9582. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, D.; Nagaumi, H.; Wang, R.; Zhang, X.; Li, X.; Zhang, H.; Zhang, B. Microstructural Evolution and Mechanical Properties of Al-Si-Mg-Cu Cast Alloys with Different Cu Contents. Metals 2023, 13, 98. [Google Scholar] [CrossRef]
- DuckerFrontier. Aluminium Content in European Passenger Cars; European Aluminium: Brussels, Belgium, 2019. [Google Scholar]
- Stadler, F.; Antrekowitsch, H.; Fragner, W.; Kaufmann, H.; Uggowitzer, P.J. Effect of Main Alloying Elements on Strength of Al–Si Foundry Alloys at Elevated Temperatures. Int. J. Cast Met. Res. 2012, 25, 215–224. [Google Scholar] [CrossRef]
- Pedersen, L.; Arnberg, L. The Effect of Solution Heat Treatment and Quenching Rates on Mechanical Properties and Microstructures in AlSiMg Foundry Alloys. Metall. Mater. Trans. A 2001, 32, 525–532. [Google Scholar] [CrossRef]
- Andersen, S.J.; Marioara, C.D.; Friis, J.; Wenner, S.; Holmestad, R. Precipitates in Aluminium Alloys. Adv. Phys. X 2018, 3, 1479984. [Google Scholar] [CrossRef]
- Dumitraschkewitz, P.; Gerstl, S.S.A.; Stephenson, L.T.; Uggowitzer, P.J.; Pogatscher, S. Clustering in Age-Hardenable Aluminum Alloys. Adv. Eng. Mater. 2018, 20, 1800255. [Google Scholar] [CrossRef]
- Banhart, J.; Lay, M.D.H.; Chang, C.S.T.; Hill, A.J. Kinetics of Natural Aging in Al-Mg-Si Alloys Studied by Positron Annihilation Lifetime Spectroscopy. Phys. Rev. B 2011, 83, 014101. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The Precipitation Sequence in Al–Mg–Si Alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-Precipitate Clusters and Precipitation Processes in Al–Mg–Si Alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Cao, L.; Rometsch, P.A.; Couper, M.J. Clustering Behaviour in an Al–Mg–Si–Cu Alloy during Natural Ageing and Subsequent under-Ageing. Mater. Sci. Eng. A 2013, 559, 257–261. [Google Scholar] [CrossRef]
- Wenner, S.; Marioara, C.D.; Andersen, S.J.; Holmestad, R. Effect of Room Temperature Storage Time on Precipitation in Al–Mg–Si(–Cu) Alloys with Different Mg/Si Ratios. Int. J. Mater. Res. 2012, 103, 948–954. [Google Scholar] [CrossRef]
- Kim, J.; Kobayashi, E.; Sato, T. Influence of Natural Aging Time on Two-Step Aging Behavior of Al-Mg-Si(-Cu) Alloys. Mater. Trans. 2015, 56, 1771–1780. [Google Scholar] [CrossRef]
- Martinsen, F.A.; Ehlers, F.J.H.; Torsæter, M.; Holmestad, R. Reversal of the Negative Natural Aging Effect in Al–Mg–Si Alloys. Acta Mater. 2012, 60, 6091–6101. [Google Scholar] [CrossRef]
- Banhart, J.; Chang, C.S.T.; Liang, Z.; Wanderka, N.; Lay, M.D.H.; Hill, A.J. Natural Aging in Al-Mg-Si Alloys—A Process of Unexpected Complexity. Adv. Eng. Mater. 2010, 12, 559–571. [Google Scholar] [CrossRef]
- Brenner, P.; Kostron, H. Über Die Vergütung Der Aluminium-Magnesium-Silizium-Legierungen (Pantal). Int. J. Mater. Res. 1939, 31, 89–97. [Google Scholar] [CrossRef]
- Chang, C.S.T.; Wieler, I.; Wanderka, N.; Banhart, J. Positive Effect of Natural Pre-Ageing on Precipitation Hardening in Al–0.44at% Mg–0.38at% Si Alloy. IFES 2008 2009, 109, 585–592. [Google Scholar] [CrossRef]
- Torsæter, M.; Hasting, H.S.; Lefebvre, W.; Marioara, C.D.; Walmsley, J.C.; Andersen, S.J.; Holmestad, R. The Influence of Composition and Natural Aging on Clustering during Preaging in Al–Mg–Si Alloys. J. Appl. Phys. 2010, 108, 073527. [Google Scholar] [CrossRef]
- Serizawa, A.; Hirosawa, S.; Sato, T. Three-Dimensional Atom Probe Characterization of Nanoclusters Responsible for Multistep Aging Behavior of an Al-Mg-Si Alloy. Metall. Mater. Trans. A 2008, 39, 243–251. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Takaki, Y.; Sato, T. Evaluation of Solute Clusters Associated with Bake-Hardening Response in Isothermal Aged Al-Mg-Si Alloys Using a Three-Dimensional Atom Probe. Metall. Mater. Trans. A 2014, 45, 5906–5913. [Google Scholar] [CrossRef]
- Engler, O.; Marioara, C.D.; Aruga, Y.; Kozuka, M.; Myhr, O.R. Effect of Natural Ageing or Pre-Ageing on the Evolution of Precipitate Structure and Strength during Age Hardening of Al–Mg–Si Alloy AA 6016. Mater. Sci. Eng. A 2019, 759, 520–529. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, J.; Zhao, J.; Yang, G.; Yang, B. Kinetic Monte Carlo Simulation of Clustering in an Al-Mg-Si-Cu Alloy. Materials 2021, 14, 4523. [Google Scholar] [CrossRef] [PubMed]
- Hayoune, A. Thermal Analysis of the Impact of RT Storage Time on the Strengthening of an Al-Mg-Si Alloy. Mater. Sci. Appl. 2012, 3, 460–466. [Google Scholar] [CrossRef]
- Jia, Z.; Ding, L.; Cao, L.; Sanders, R.; Li, S.; Liu, Q. The Influence of Composition on the Clustering and Precipitation Behavior of Al-Mg-Si-Cu Alloys. Metall. Mater. Trans. A 2017, 48, 459–473. [Google Scholar] [CrossRef]
- Weng, Y.; Jia, Z.; Ding, L.; Liu, M.; Wu, X.; Liu, Q. Combined Effect of Pre-Aging and Ag/Cu Addition on the Natural Aging and Bake Hardening in Al-Mg-Si Alloys. Prog. Nat. Sci. Mater. Int. 2018, 28, 363–370. [Google Scholar] [CrossRef]
- Zhu, S.; Shih, H.-C.; Cui, X.; Yu, C.-Y.; Ringer, S.P. Design of Solute Clustering during Thermomechanical Processing of AA6016 Al–Mg–Si Alloy. Acta Mater. 2021, 203, 116455. [Google Scholar] [CrossRef]
- Tweddle, D.; Johnson, J.A.; Kapoor, M.; Bikmukhametov, I.; Mileski, S.; Carsley, J.E.; Thompson, G.B. Atomic-Scale Clustering in a High-Strength Al-Mg-Si-Cu Alloy. Materialia 2022, 26, 101567. [Google Scholar] [CrossRef]
- Hell, C.M.; Søreide, H.-S.; Bjørge, R.; Marioara, C.D.; Li, Y.; Holmestad, R. Influence of Natural Aging and Ramping before Artificial Aging on the Microstructure of Two Different 6xxx Alloys. J. Mater. Res. Technol. 2022, 21, 4224–4240. [Google Scholar] [CrossRef]
- Yang, Z.; Erdle, I.; Liu, C.; Banhart, J. Clustering and Precipitation in Al-Mg-Si Alloys during Linear Heating. J. Mater. Sci. Technol. 2022, 120, 78–88. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Werinos, M.; Moszner, F.; Gerstl, S.S.A.; Francis, M.F.; Curtin, W.A.; Löffler, J.F.; Uggowitzer, P.J. Diffusion on Demand to Control Precipitation Aging: Application to Al-Mg-Si Alloys. Phys. Rev. Lett. 2014, 112, 225701. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Sun, B.; Wang, J.; Liu, Y.; Liu, C. High-Temperature Age-Hardening Behavior of Al–Mg–Si Alloys with Varying Sn Contents. J. Mater. Res. Technol. 2021, 14, 2165–2173. [Google Scholar] [CrossRef]
- Andersen, S.J.; Zandbergen, H.W.; Jansen, J.; TrÆholt, C.; Tundal, U.; Reiso, O. The Crystal Structure of the Β″ Phase in Al–Mg–Si Alloys. Acta Mater. 1998, 46, 3283–3298. [Google Scholar] [CrossRef]
- Wenner, S.; Jones, L.; Marioara, C.D.; Holmestad, R. Atomic-Resolution Chemical Mapping of Ordered Precipitates in Al Alloys Using Energy-Dispersive X-Ray Spectroscopy. Micron 2017, 96, 103–111. [Google Scholar] [CrossRef]
- Ehlers, F.J.H.; Wenner, S.; Andersen, S.J.; Marioara, C.D.; Lefebvre, W.; Boothroyd, C.B.; Holmestad, R. Phase Stabilization Principle and Precipitate-Host Lattice Influences for Al–Mg–Si–Cu Alloy Precipitates. J. Mater. Sci. 2014, 49, 6413–6426. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Stene, T.N.; Hasting, H.; Walmsley, J.; Van Helvoort, A.T.J.; Holmestad, R. The Effect of Cu on Precipitation in Al–Mg–Si Alloys. Philos. Mag. 2007, 87, 3385–3413. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. Phase Relations and Precipitation in Al–Mg–Si Alloys with Cu Additions. Festschr. Honor T B Massalski 2004, 49, 389–410. [Google Scholar] [CrossRef]
- Prosa, T.J.; Larson, D.J. Modern Focused-Ion-Beam-Based Site-Specific Specimen Preparation for Atom Probe Tomography. Microsc. Microanal. 2017, 23, 194–209. [Google Scholar] [CrossRef]
- Dumitraschkewitz, P.; Uggowitzer, P.J.; Gerstl, S.S.A.; Löffler, J.F.; Pogatscher, S. Size-Dependent Diffusion Controls Natural Aging in Aluminium Alloys. Nat. Commun. 2019, 10, 4746. [Google Scholar] [CrossRef]
- Tweddle, D.; Johnson, J.A.; Kapoor, M.; Mileski, S.; Carsley, J.E.; Thompson, G.B. Direct Observation of PFIB-Induced Clustering in Precipitation-Strengthened Al Alloys by Atom Probe Tomography. Microsc. Microanal. 2022, 28, 296–301. [Google Scholar] [CrossRef]
- Hatzoglou, C.; Rouland, S.; Radiguet, B.; Etienne, A.; Costa, G.; Sauvage, X.; Pareige, P.; Vurpillot, F. Preferential Evaporation in Atom Probe Tomography: An Analytical Approach. Microsc. Microanal. 2020, 26, 689–698. [Google Scholar] [CrossRef]
- Jägle, E.A.; Choi, P.-P.; Raabe, D. The Maximum Separation Cluster Analysis Algorithm for Atom-Probe Tomography: Parameter Determination and Accuracy. Microsc. Microanal. 2014, 20, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.M.; DaCosta, G.; Hatzoglou, C.; Weekes, H.; Radiguet, B.; Styman, P.D.; Vurpillot, F.; Pareige, C.; Etienne, A.; Bonny, G.; et al. Analysis of Radiation Damage in Light Water Reactors: Comparison of Cluster Analysis Methods for the Analysis of Atom Probe Data. Microsc. Microanal. 2017, 23, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Haley, D.; Marquis, E.A.; Smith, G.D.W.; Moody, M.P. Defining Clusters in APT Reconstructions of ODS Steels. IFES 2012 2013, 132, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.J. Quantification of the Mg2Si Β″ and Β′ Phases in AlMgSi Alloys by Transmission Electron Microscopy. Metall. Mater. Trans. A 1995, 26, 1931–1937. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Røyset, J.; Reiso, O.; Gulbrandsen-Dahl, S.; Nicolaisen, T.-E.; Opheim, I.-E.; Helgaker, J.F.; Holmestad, R. Improving Thermal Stability in Cu-Containing Al-Mg-Si Alloys by Precipitate Optimization. Metall. Mater. Trans. A 2014, 45, 2938–2949. [Google Scholar] [CrossRef]
- Mørtsell, E.A.; Qian, F.; Marioara, C.D.; Li, Y. Precipitation in an A356 Foundry Alloy with Cu Additions—A Transmission Electron Microscopy Study. J. Alloys Compd. 2019, 785, 1106–1114. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Birkeland, A.; Holmestad, R. Orientation of Silicon Particles in a Binary Al–Si Alloy. J. Mater. Sci. 2008, 43, 4962–4971. [Google Scholar] [CrossRef]
- Gazizov, M.; Marioara, C.D.; Friis, J.; Wenner, S.; Holmestad, R.; Kaibyshev, R. Precipitation Behavior in an Al–Cu–Mg–Si Alloy during Ageing. Mater. Sci. Eng. A 2019, 767, 138369. [Google Scholar] [CrossRef]
- Lervik, A.; Danbolt, T.; Furu, T.; Holmestad, R.; Lunder, O. Comparing Intergranular Corrosion in Al-Mg-Si-Cu Alloys with and without α-Al(Fe, Mn, Cu)Si Particles. Mater. Corros. 2021, 72, 575–584. [Google Scholar] [CrossRef]
- Ozawa, E.; Kimura, H. Excess Vacancies and the Nucleation of Precipitates in Aluminum-Silicon Alloys. Acta Metall. 1970, 18, 995–1004. [Google Scholar] [CrossRef]
- Rosenbaum, H.S.; Turnbull, D. On the Precipitation of Silicon out of a Supersaturated Aluminum-Silicon Solid Solution. Acta Metall. 1958, 6, 653–659. [Google Scholar] [CrossRef]
- Long, H.C.; Chen, J.H.; Liu, C.H.; Li, D.Z.; Li, Y.Y. The Negative Effect of Solution Treatment on the Age Hardening of A356 Alloy. Mater. Sci. Eng. A 2013, 566, 112–118. [Google Scholar] [CrossRef]
- Murray, J.L.; McAlister, A.J. The Al-Si (Aluminum-Silicon) System. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Feufel, H.; Gödecke, T.; Lukas, H.L.; Sommer, F. Investigation of the Al-Mg-Si System by Experiments and Thermodynamic Calculations. J. Alloys Compd. 1997, 247, 31–42. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Mg-Si (Aluminum-Magnesium-Silicon). J. Phase Equilibria Diffus. 2007, 28, 189–191. [Google Scholar] [CrossRef]
- Tian, N.; Wang, G.; Zhou, Y.; Liu, C.; Liu, K.; Zhao, G.; Zuo, L. Formation of Phases and Microstructures in Al-8Si Alloys with Different Mg Content. Materials 2021, 14, 762. [Google Scholar] [CrossRef]
- Taylor, J.A.; StJohn, D.H.; Barresi, J.; Couper, M.J. Influence of Mg Content on the Microstructure and Solid Solution Chemistry of Al-7%Si-Mg Casting Alloys During Solution Treatment. Mater. Sci. Forum 2000, 331–337, 277–282. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, J.; Wang, T.; Chen, J.; He, K. The Effects of Neodymium Addition on the Intermetallic Microstructure and Mechanical Properties of Al-7Si-0.3Mg-0.3Fe Alloys. J. Alloys Compd. 2018, 741, 161–173. [Google Scholar] [CrossRef]
- Hasting, H.S.; Frøseth, A.G.; Andersen, S.J.; Vissers, R.; Walmsley, J.C.; Marioara, C.D.; Danoix, F.; Lefebvre, W.; Holmestad, R. Composition of Β″ Precipitates in Al–Mg–Si Alloys by Atom Probe Tomography and First Principles Calculations. J. Appl. Phys. 2009, 106, 123527. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.J. Effect of Composition on Clustering Reactions in AlMgSi(Cu) Alloys. Scr. Mater. 2004, 50, 155–158. [Google Scholar] [CrossRef]
- Hatakeyama, D.; Nishimura, K.; Matsuda, K.; Namiki, T.; Lee, S.; Nunomura, N.; Aida, T.; Matsuzaki, T.; Holmestad, R.; Wenner, S.; et al. Effect of Copper Addition on the Cluster Formation Behavior of Al-Mg-Si, Al-Zn-Mg, and Al-Mg-Ge in the Natural Aging. Metall. Mater. Trans. A 2018, 49, 5871–5877. [Google Scholar] [CrossRef]
- Hu, Y.; Curtin, W.A. Modeling Peak-Aged Precipitate Strengthening in Al–Mg–Si Alloys. J. Mech. Phys. Solids 2021, 151, 104378. [Google Scholar] [CrossRef]
- Shishido, H.; Aruga, Y.; Murata, Y.; Marioara, C.D.; Engler, O. Evaluation of Precipitates and Clusters during Artificial Aging of Two Model Al–Mg–Si Alloys with Different Mg/Si Ratios. J. Alloys Compd. 2022, 927, 166978. [Google Scholar] [CrossRef]
- Christiansen, E.; Marioara, C.D.; Holmedal, B.; Hopperstad, O.S.; Holmestad, R. Nano-Scale Characterisation of Sheared β” Precipitates in a Deformed Al-Mg-Si Alloy. Sci. Rep. 2019, 9, 17446. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Orekhov, A.; Hu, Z.-Y.; Feng, M.; Jin, S.; Sha, G.; Li, K.; Samaee, V.; Song, M.; Du, Y.; et al. Shearing and Rotation of Β″ and Βʹ Precipitates in an Al-Mg-Si Alloy under Tensile Deformation: In-Situ and Ex-Situ Studies. Acta Mater. 2021, 220, 117310. [Google Scholar] [CrossRef]
- Poole, W.J.; Wang, X.; Lloyd, D.J.; Embury, J.D. The Shearable–Non-Shearable Transition in Al–Mg–Si–Cu Precipitation Hardening Alloys: Implications on the Distribution of Slip, Work Hardening and Fracture. Philos. Mag. 2005, 85, 3113–3135. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakaguchi, Y.; Miyata, Y.; Uetani, Y.; Sato, T.; Kamio, A.; Ikeno, S. Precipitation Sequence of Various Kinds of Metastable Phases in Al-1.0mass% Mg2Si-0.4mass% Si Alloy. J. Mater. Sci. 2000, 35, 179–189. [Google Scholar] [CrossRef]
- Li, Y.J.; Brusethaug, S.; Olsen, A. Influence of Cu on the Mechanical Properties and Precipitation Behavior of AlSi7Mg0.5 Alloy during Aging Treatment. Scr. Mater. 2006, 54, 99–103. [Google Scholar] [CrossRef]

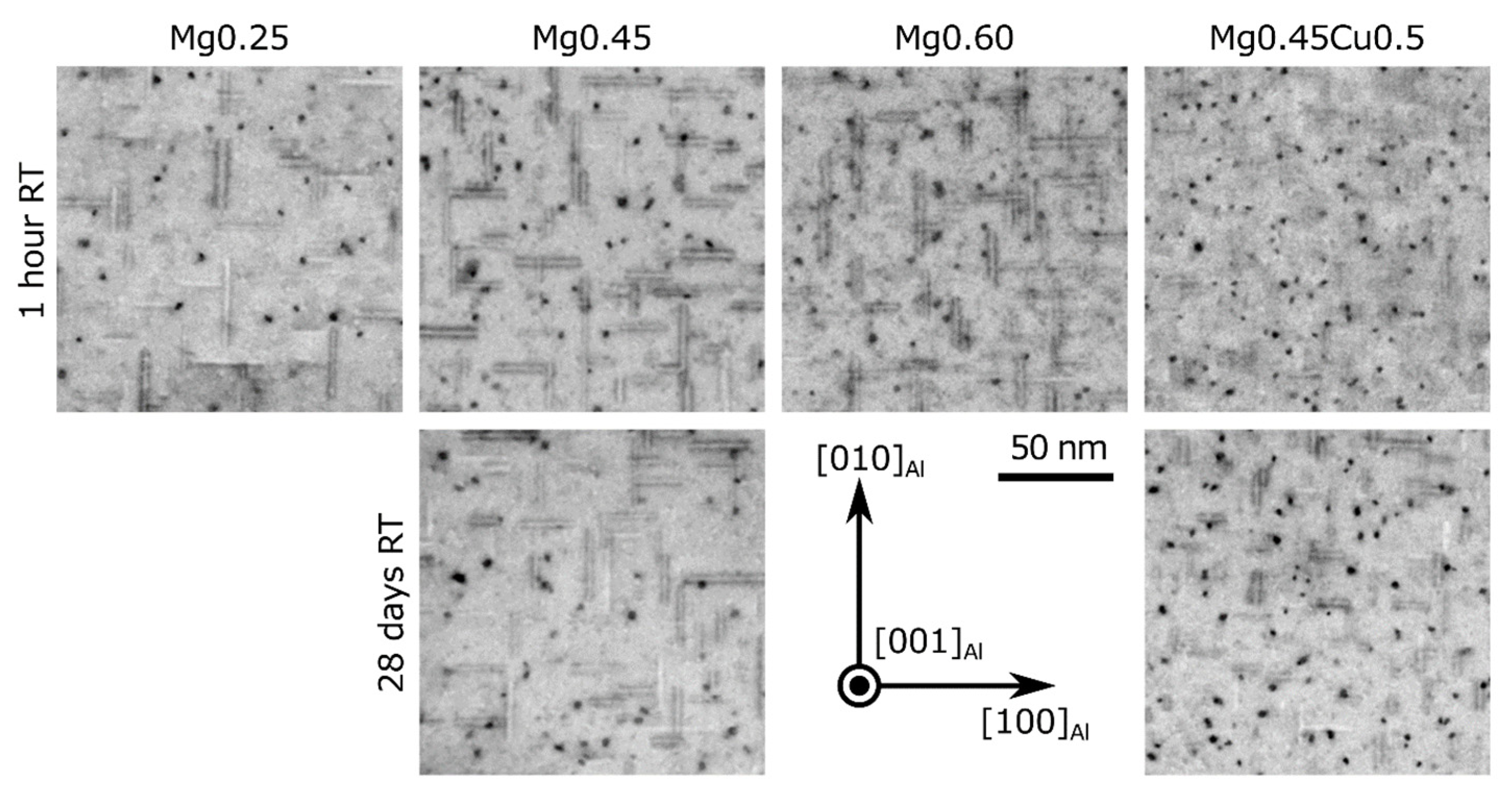
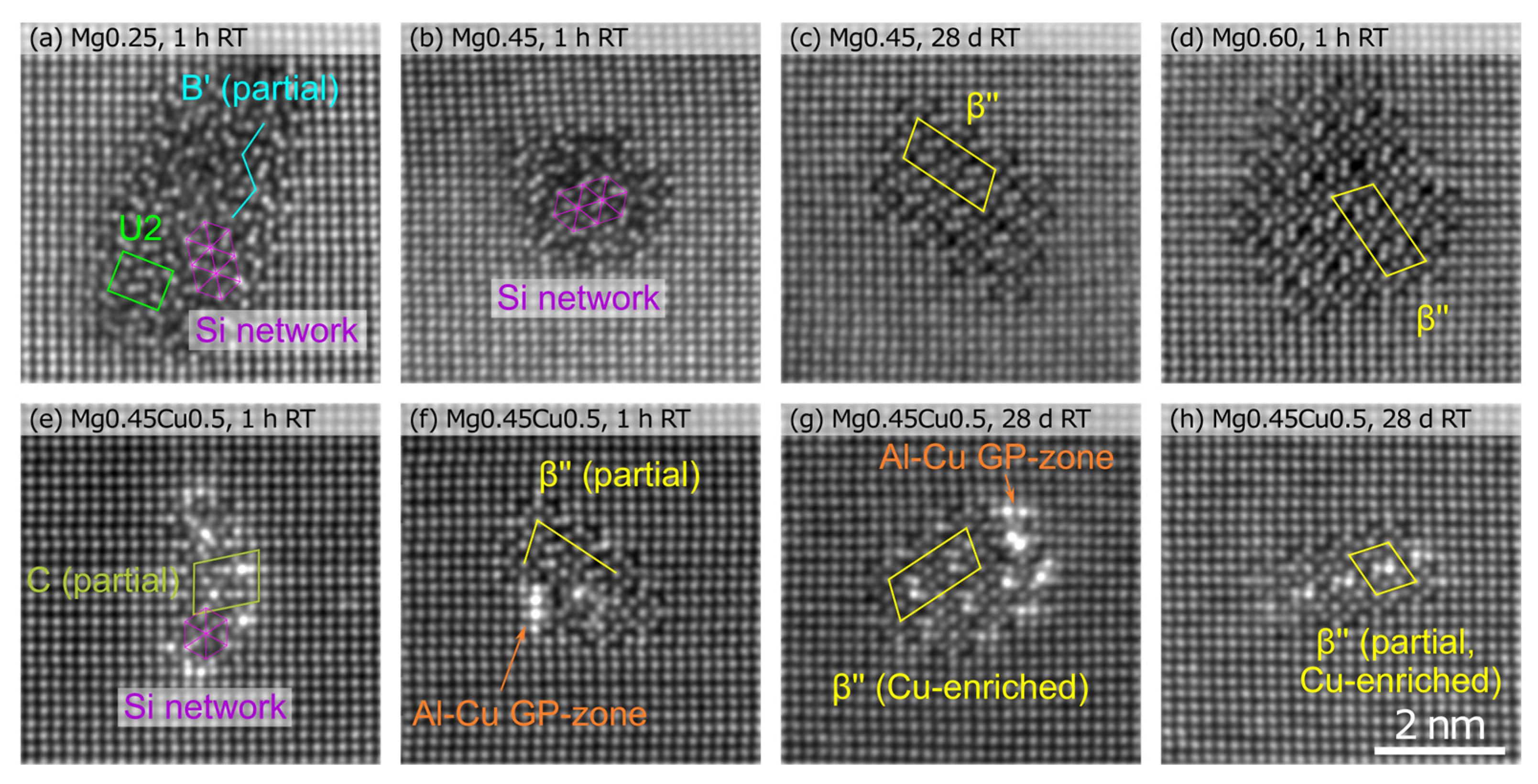

| Alloy | Si | Mg | Cu | Fe | Ti | Sr | Mn | |
|---|---|---|---|---|---|---|---|---|
| Mg0.25 | wt.% | 6.90 | 0.248 | <0.004 | 0.09 | 0.10 | 0.015 | <0.002 |
| at.% | 6.65 | 0.276 | 0.04 | 0.06 | 0.005 | |||
| Mg0.45 | wt.% | 6.97 | 0.447 | <0.004 | 0.12 | 0.10 | 0.015 | <0.002 |
| at.% | 6.72 | 0.498 | 0.06 | 0.06 | 0.005 | |||
| Mg0.60 | wt.% | 6.92 | 0.602 | <0.004 | 0.12 | 0.10 | 0.015 | <0.002 |
| at.% | 6.67 | 0.671 | 0.06 | 0.06 | 0.005 | |||
| Mg0.45Cu0.5 | wt.% | 6.90 | 0.399 | 0.535 | 0.10 | 0.12 | 0.021 | 0.072 |
| at.% | 6.67 | 0.446 | 0.229 | 0.06 | 0.07 | 0.007 | 0.036 |
| Alloy | Condition | Si (%) | Mg (%) | Cu (%) | Ti (%) |
|---|---|---|---|---|---|
| Mg0.45 | OES (bulk) | 6.718 | 0.498 | 0.057 | |
| APT: 28 days RT | 0.899 | 0.541 | 0.006 | ||
| APT: AA | 0.928 | 0.559 | 0.074 | ||
| APT: RT + AA | 0.857 | 0.559 | 0.066 | ||
| Mg0.45 Cu0.5 | OES (bulk) | 6.671 | 0.446 | 0.229 | 0.068 |
| APT: 28 days RT | 0.792 | 0.560 | 0.218 | 0.028 | |
| APT: AA | 0.755 | 0.547 | 0.194 | 0.075 | |
| APT: RT + AA | 0.760 | 0.535 | 0.207 | 0.067 |
| Alloy | Condition | Mg (%) | Si (%) | Cu (%) | Density (106/µm3) | Size (Atoms) | Clustered Atoms (%) | HV |
|---|---|---|---|---|---|---|---|---|
| Mg0.45 | 28 days RT | 23(8) | 29(9) | 2.90(26) | 75(43) | 0.45(4) | 81 | |
| AA | 21(8) | 26(11) | 1.81(8) | 136(171) | 0.52(2) | 86 | ||
| RT + AA | 24(7) | 25(9) | 1.63(8) | 100(67) | 0.34(2) | 80 | ||
| Mg0.45 Cu0.5 | 28 days RT | 19(6) | 23(8) | 4(3) | 2.53(18) | 86(47) | 0.46(3) | 87 |
| AA | 23(6) | 22(7) | 4(3) | 1.47(8) | 271(375) | 0.83(4) | 108 | |
| RT + AA | 20(7) | 22(8) | 4(2) | 2.00(9) | 120(112) | 0.50(2) | 87 |
| Alloy | Natural Aging | Particle Length (nm) | Particle Diameter (nm) | Particle Density (103 µm−3) | Volume Fraction (%) | HV |
|---|---|---|---|---|---|---|
| Mg0.25 | 1 h | 18.2(4) | 2.75(5) | 64(10) | 0.70(11) | 98 |
| Mg0.45 | 1 h | 17.8(5) | 2.91(4) | 99(15) | 1.17(20) | 119 |
| 28 d | 20.0(7) | 3.15(4) | 78(12) | 1.22(29) | 118 | |
| Mg0.60 | 1 h | 23.1(5) | 2.68(3) | 87(14) | 1.13(24) | 123 |
| Mg0.45Cu0.5 | 1 h | 14.6(6) | 2.97(3) | 196(30) | 1.97(51) | 123 |
| 28 d | 13.3(4) | 2.81(3) | 119(18) | 0.98(22) | 124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenner, S.; Hatzoglou, C.; Mørtsell, E.A.; Åsholt, P. Clustering and Precipitation during Early-Stage Artificial Aging of Al–Si–Mg(–Cu) Foundry Alloys. Metals 2023, 13, 557. https://doi.org/10.3390/met13030557
Wenner S, Hatzoglou C, Mørtsell EA, Åsholt P. Clustering and Precipitation during Early-Stage Artificial Aging of Al–Si–Mg(–Cu) Foundry Alloys. Metals. 2023; 13(3):557. https://doi.org/10.3390/met13030557
Chicago/Turabian StyleWenner, Sigurd, Constantinos Hatzoglou, Eva Anne Mørtsell, and Petter Åsholt. 2023. "Clustering and Precipitation during Early-Stage Artificial Aging of Al–Si–Mg(–Cu) Foundry Alloys" Metals 13, no. 3: 557. https://doi.org/10.3390/met13030557
APA StyleWenner, S., Hatzoglou, C., Mørtsell, E. A., & Åsholt, P. (2023). Clustering and Precipitation during Early-Stage Artificial Aging of Al–Si–Mg(–Cu) Foundry Alloys. Metals, 13(3), 557. https://doi.org/10.3390/met13030557






